Intake of Lactobacillus delbrueckii (pExu:hsp65) Prevents the Inflammation and the Disorganization of the Intestinal Mucosa in a Mouse Model of Mucositis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
2.2. DNA Vaccine Construction: Recombinant L. delbrueckii CIDCA 133 (pExu:hsp65)
2.3. Dairy Formulation
2.4. Mouse Handling and Experimental Design
2.5. Intestinal Permeability Evaluation
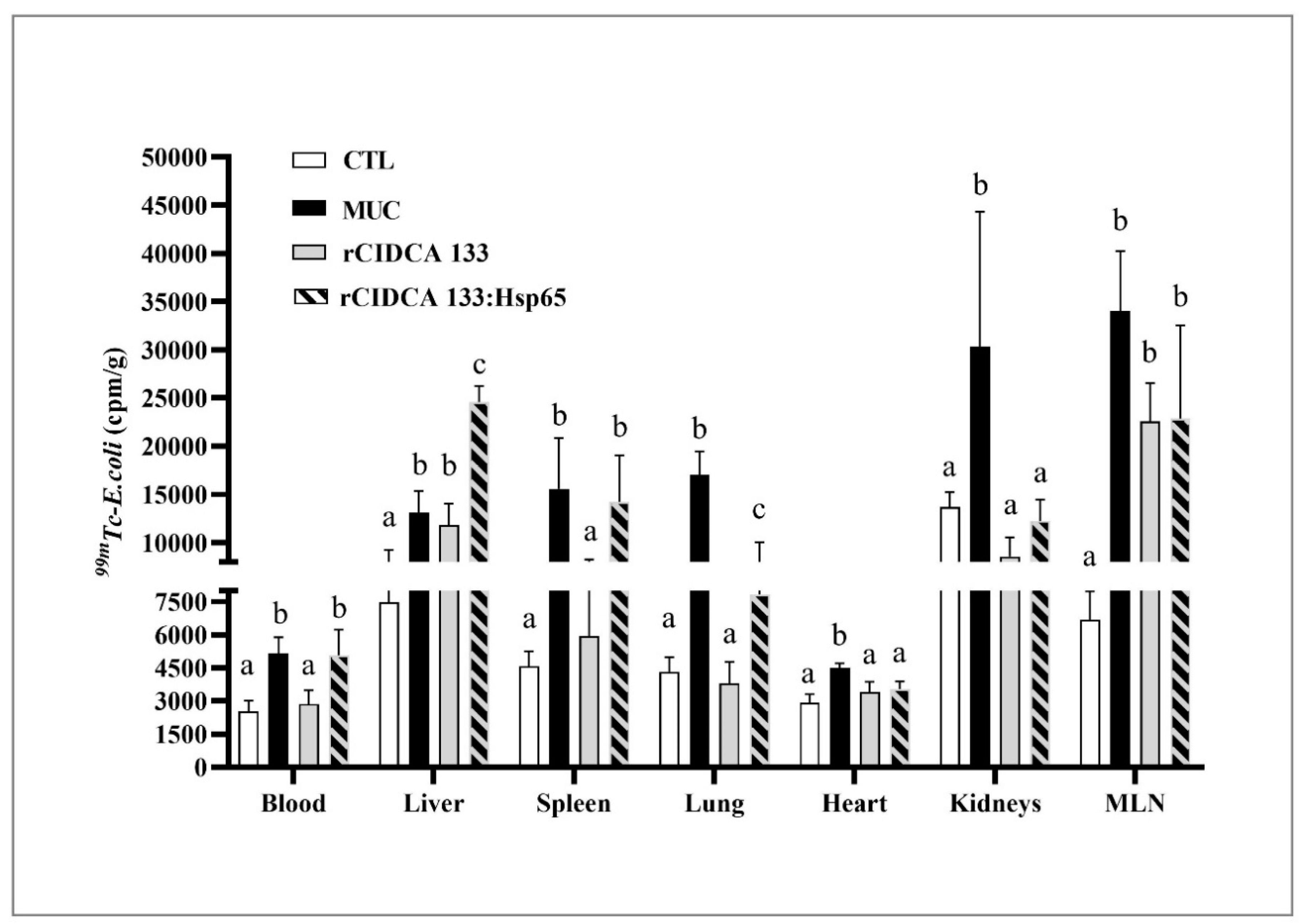
2.6. Bacterial Translocation Study
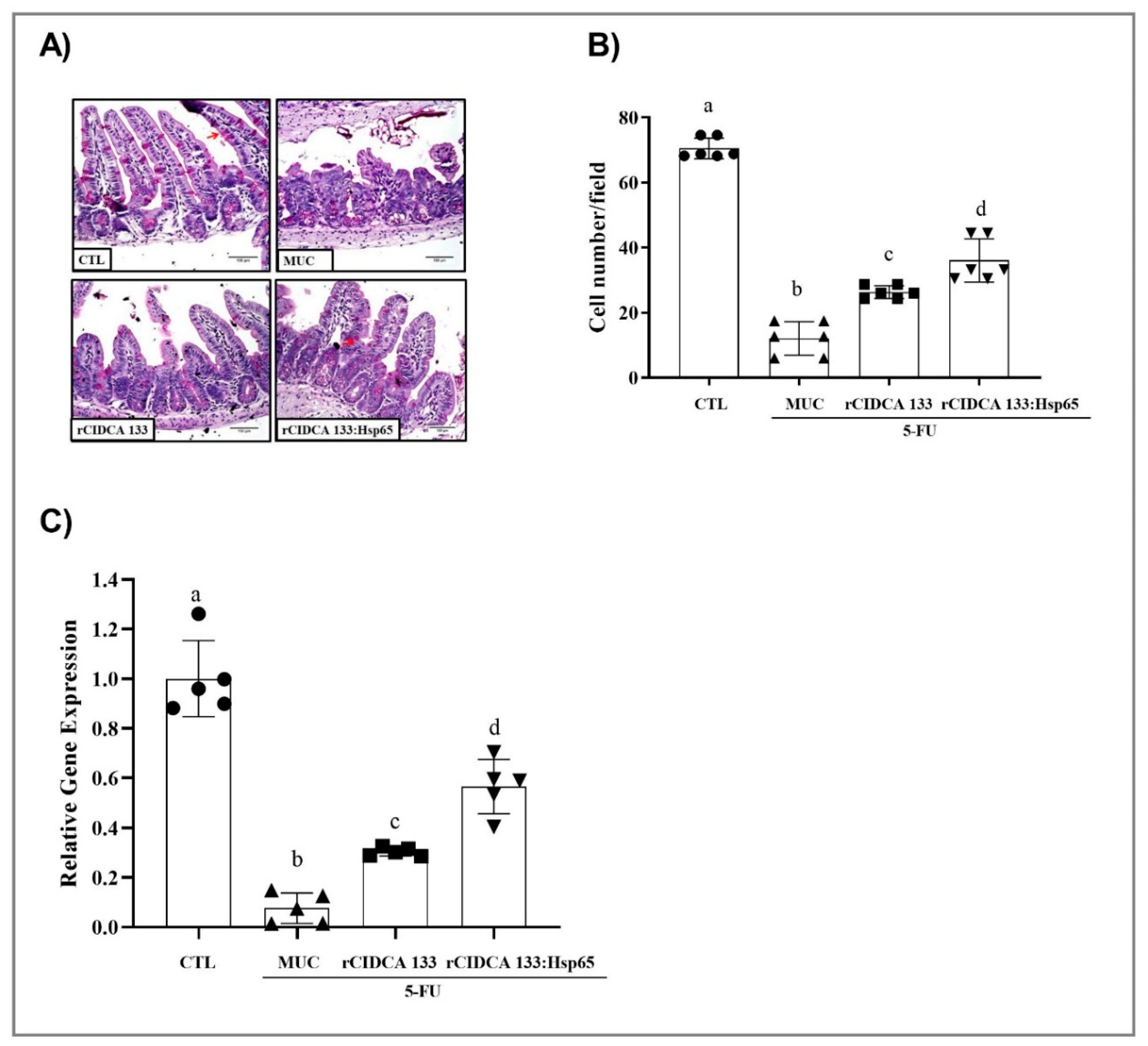
2.7. Histological, Morphological, and Goblet Cell Analyses
2.8. Leukocyte Count
2.9. Enzyme Assay: Intestinal Myeloperoxidase (MPO) and Eosinophil Peroxidase (EPO) Activity
2.10. Intestinal Secretory IgA (sIgA)
2.11. Transmission Electron Microscopy (MET)
2.12. RNA Extraction and Real-Time RT-qPCR of Ileum Section
2.13. Statistical Analysis
3. Results
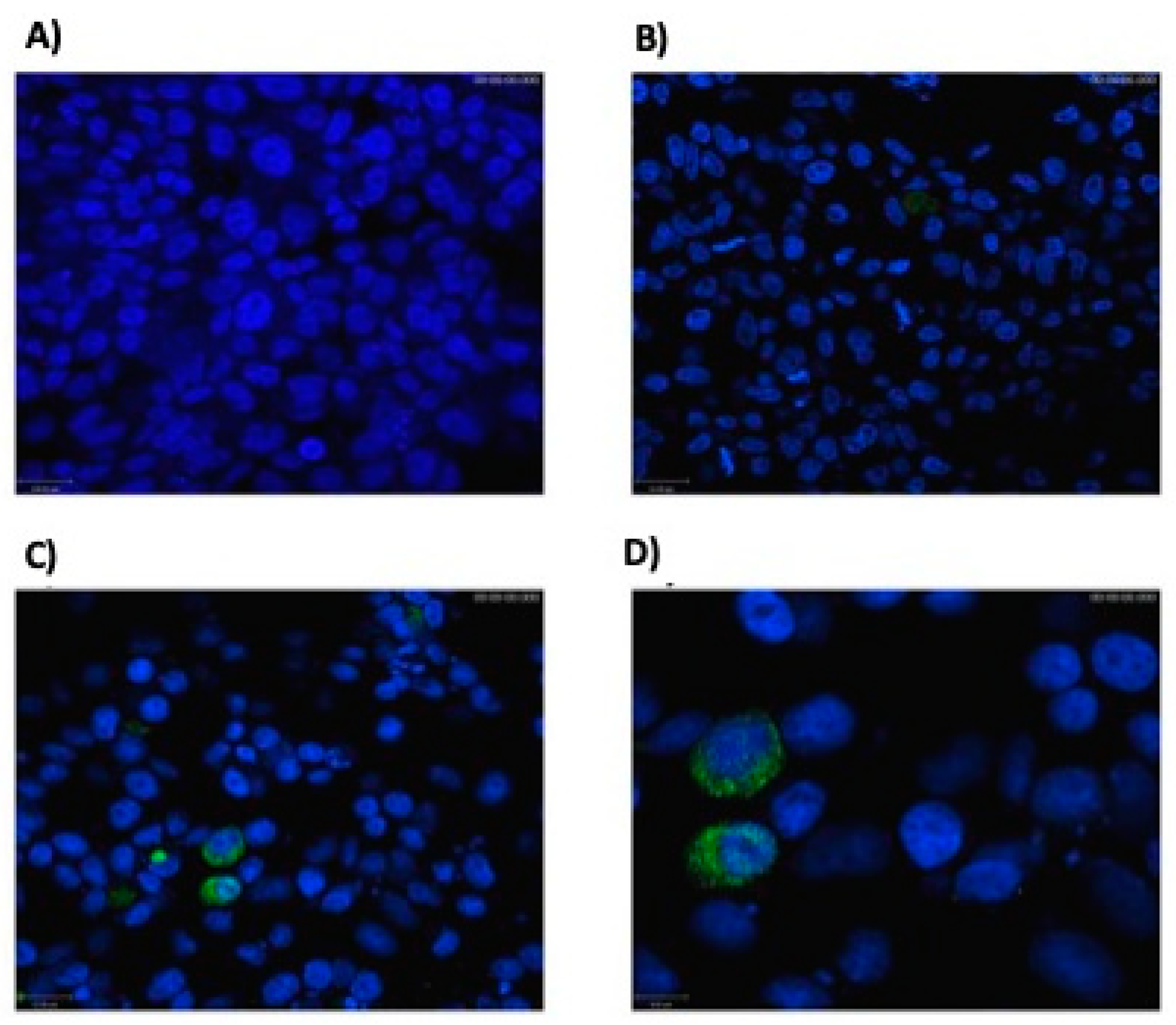
3.1. Eukaryotic Cells Can Express Hsp65 Protein
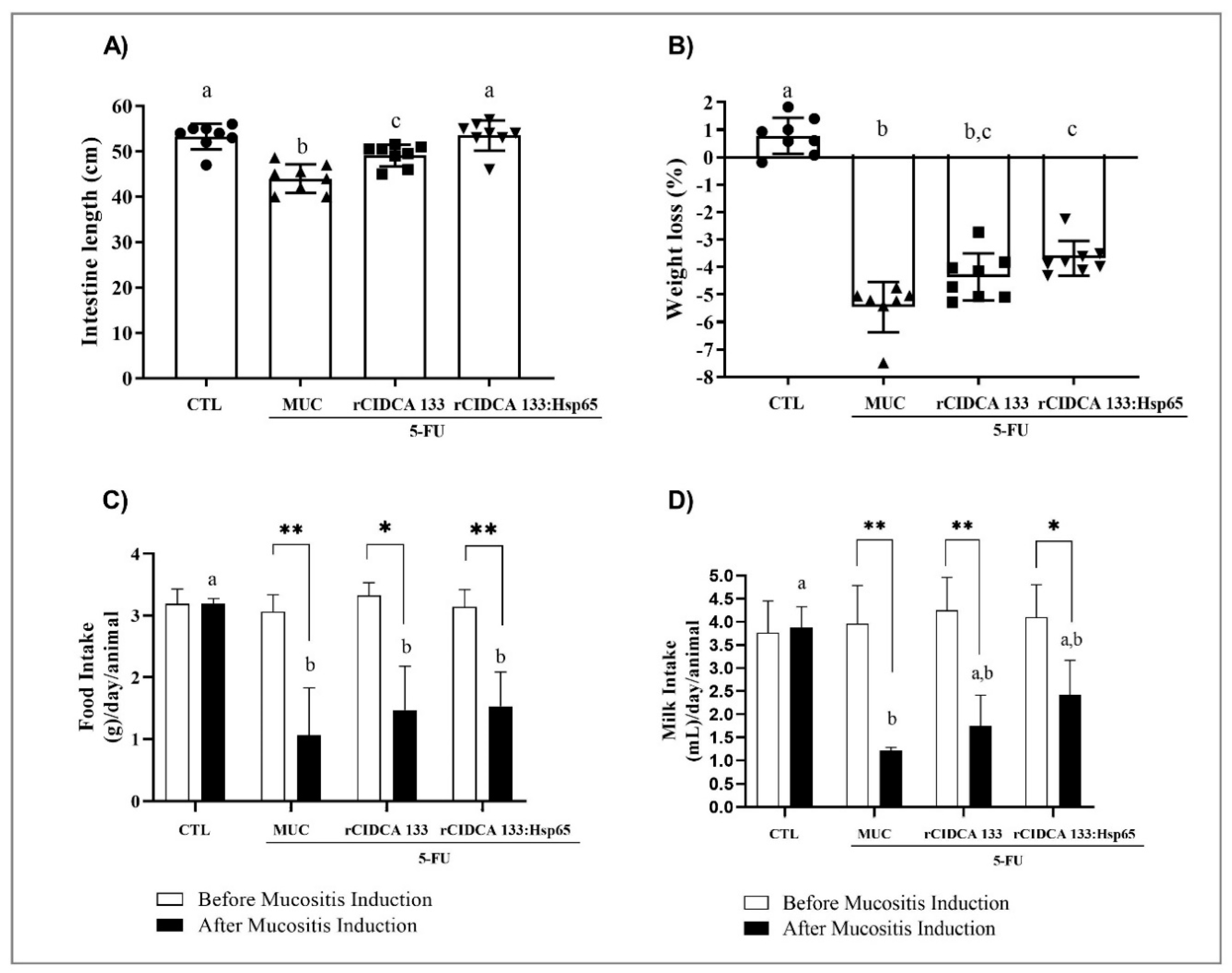
3.2. rCIDCA 133:Hsp65 Prevented Small Intestine Shortening and Decrease in Weight Loss
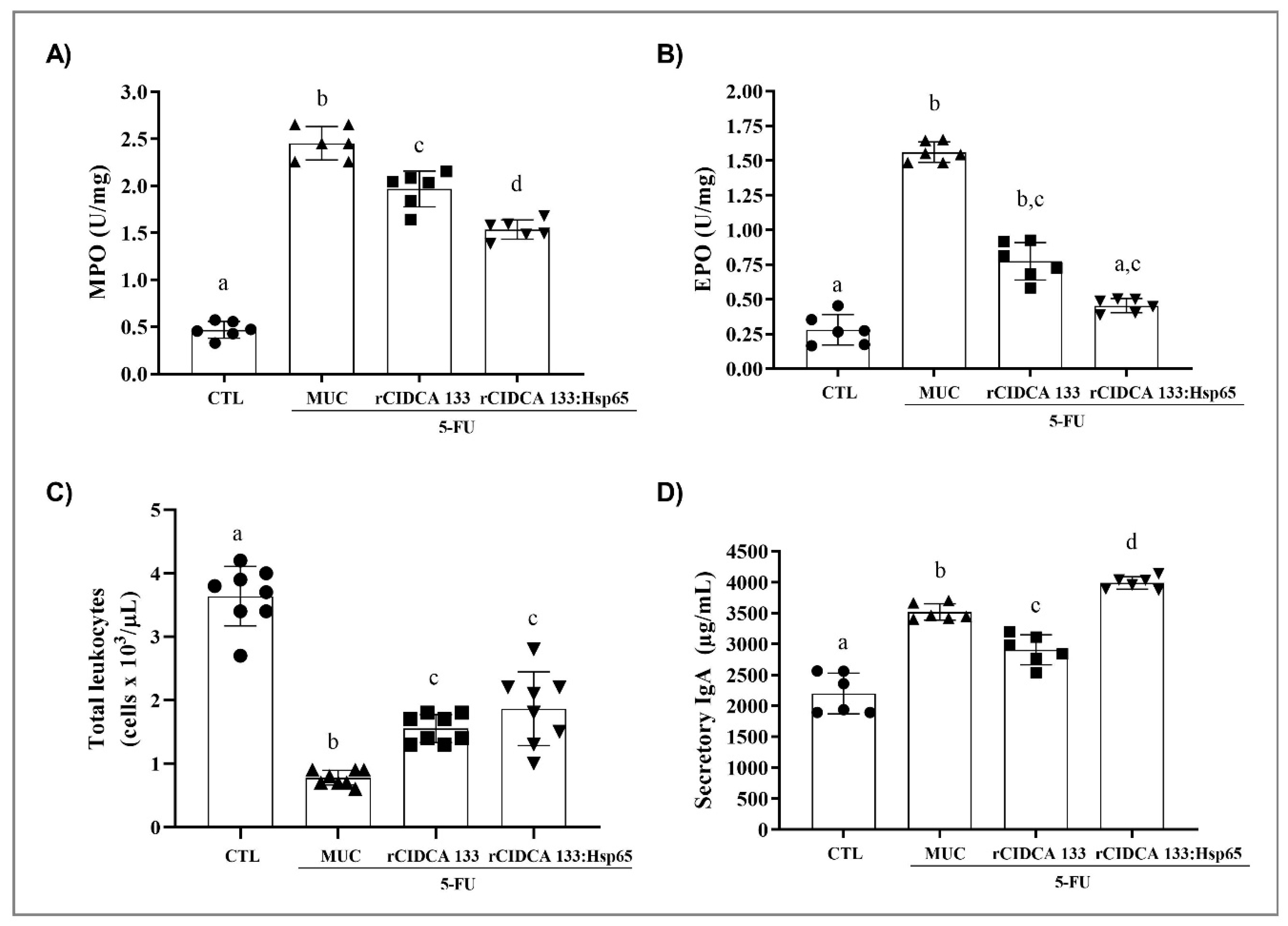
3.3. rCIDCA 133:Hsp65 Treatment Reduced Ileum Inflammatory Infiltrate and Increased sIgA Levels
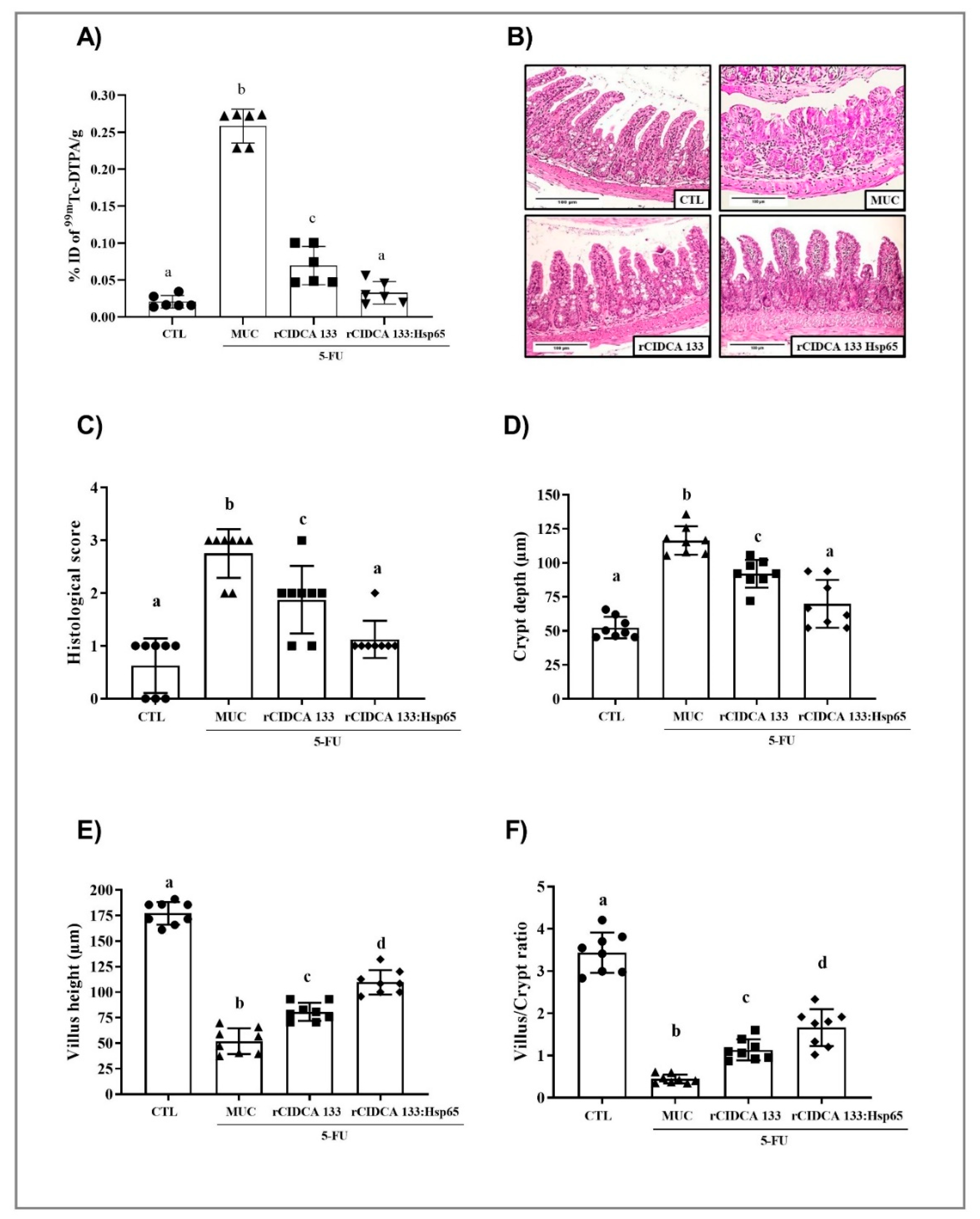
3.4. Reduction in Intestinal Permeability after Treatment with rCIDCA 133:Hsp65
3.5. rCIDCA 133:Hsp65 Reduced Both Mucosal Damage and Degeneration of Goblet Cells in 5-FU-Induced Intestinal Mucositis
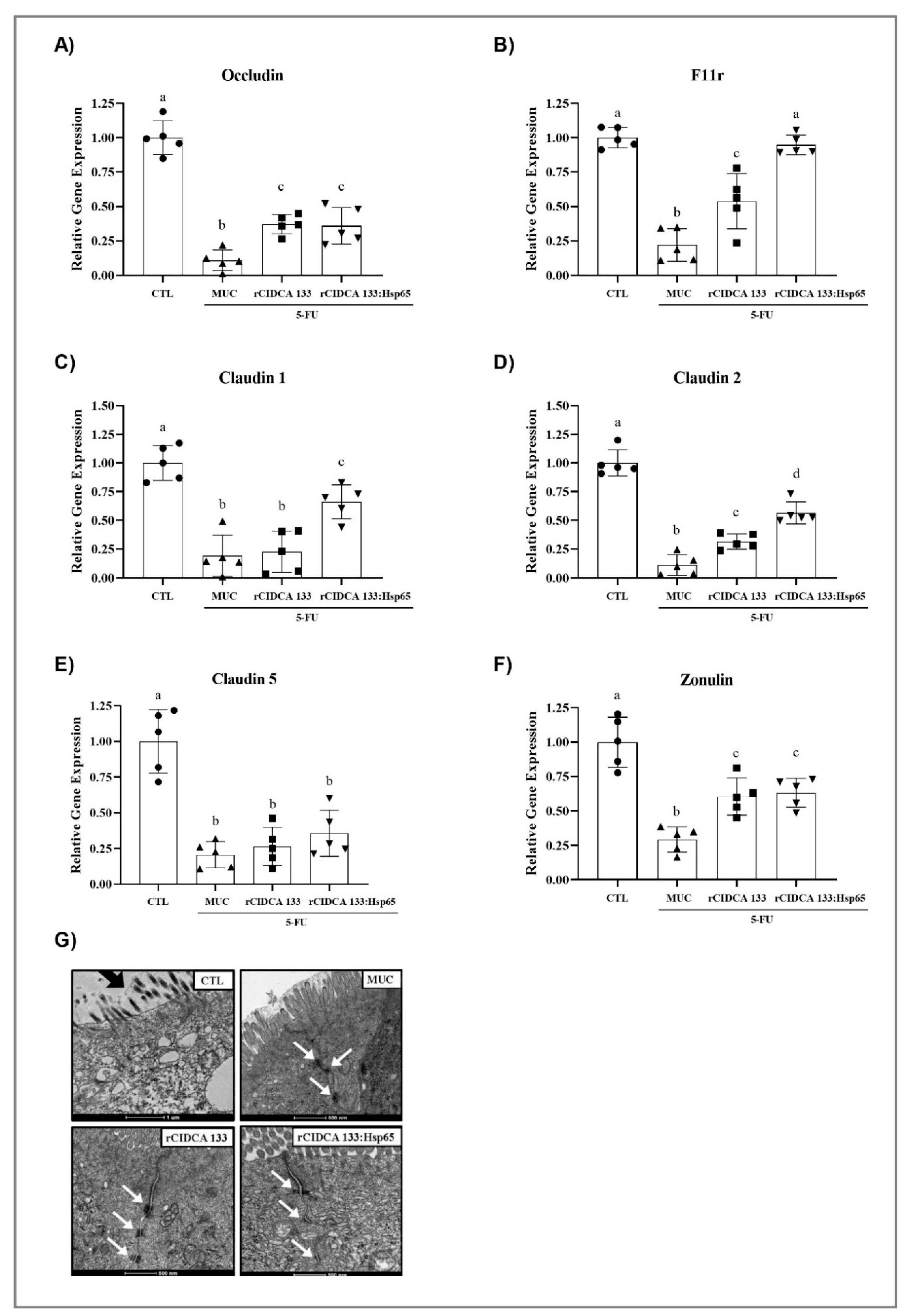
3.6. Tight Junctions Exhibit Up-Regulation after Oral Administration with Recombinant Strains
3.7. rCIDCA 133:Hsp65 Reduces Gene Expression of Pro-Inflammatory Molecules and Upregulates the IL10 Expression in Ileum of Inflamed Animals
3.8. Treatment with Recombinant Strains of Lactobacillus CIDCA 133 Reduces MYD88, NOS2, TLR2, and TLR4 Gene Expression
3.9. rCIDCA 133:Hsp65 Was Not Able to Reduce the Bacterial Translocation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Gifoni, M.; Lima, R.C.; Lima, A.A.M.; Facanha, A.; Callado, R.B.; Azevedo, C.; De Albuquerque Ribeiro, R. Clinical mucositis and intestinal permeability abnormalities in metastatic colorectal cancer patients treated with irinotecan and 5-fluoruracil. J. Clin. Oncol. 2012, 30, e14183. [Google Scholar] [CrossRef]
- Chang, C.-T.; Ho, T.-Y.; Lin, H.; Liang, J.-A.; Huang, H.-C.; Li, C.-C.; Lo, H.-Y.; Wu, S.-L.; Huang, Y.-F.; Hsiang, C.-Y. 5-Fluorouracil Induced Intestinal Mucositis via Nuclear Factor-κB Activation by Transcriptomic Analysis and In Vivo Bioluminescence Imaging. PLoS ONE 2012, 7, e31808. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.R.; Haddad, R.I. Novel agents for the treatment of mucositis. J. Support. Oncol. 2007, 5, 33–39. [Google Scholar] [PubMed]
- Nose, S.; Wasa, M.; Tazuke, Y.; Owari, M.; Fukuzawa, M. Cisplatin Upregulates Glutamine Transport in Human Intestinal Epithelial Cells. J. Parenter. Enter. Nutr. 2010, 34, 530–537. [Google Scholar] [CrossRef]
- Bodiga, V.L.; Bodiga, S.; Surampudi, S.; Boindala, S.; Putcha, U.; Nagalla, B.; Subramaniam, K.; Manchala, R. Effect of vitamin supplementation on cisplatin-induced intestinal epithelial cell apoptosis in Wistar/NIN rats. Nutrition 2012, 28, 572–580. [Google Scholar] [CrossRef]
- dos Santos Filho, E.X.; Ávila, P.H.M.; Bastos, C.C.C.; Batista, A.C.; Naves, L.N.; Marreto, R.N.; Lima, E.M.; Mendonça, E.F.; Valadares, M.C. Curcuminoids from Curcuma longa L. reduced intestinal mucositis induced by 5-fluorouracil in mice: Bioadhesive, proliferative, anti-inflammatory and antioxidant effects. Toxicol. Rep. 2016, 3, 55–62. [Google Scholar] [CrossRef]
- Koppelmann, T.; Pollak, Y.; Mogilner, J.; Bejar, J.; Coran, A.G.; Sukhotnik, I. Reversal of severe methotrexate-induced intestinal damage using enteral n -3 fatty acids. Br. J. Nutr. 2013, 109, 89–98. [Google Scholar] [CrossRef]
- Youmba, S.B.; Belmonte, L.; Galas, L.; Boukhettala, N.; Bôle-Feysot, C.; Déchelotte, P.; Coëffier, M. Methotrexate Modulates Tight Junctions Through NF-κB, MEK, and JNK Pathways. J. Pediatr. Gastroenterol. Nutr. 2012, 4, 463–470. [Google Scholar] [CrossRef]
- Thomas, C.M.; Saulnier, D.M.A.; Spinler, J.K.; Hemarajata, P.; Gao, C.; Jones, S.E.; Grimm, A.; Balderas, M.A.; Burstein, M.D.; Morra, C.; et al. FolC2-mediated folate metabolism contributes to suppression of inflammation by probiotic Lactobacillus reuteri. Microbiologyopen 2016, 5, 802–818. [Google Scholar] [CrossRef]
- Whitford, E.J.; Cummins, A.G.; Butler, R.N.; Prisciandaro, L.D.; Fauser, J.K.; Yazbeck, R.; Lawrence, A.; Cheah, K.Y.; Wright, T.H.; Lymn, K.A.; et al. Effects of Streptococcus thermophilus TH-4 on intestinal mucositis induced by the chemotherapeutic agent 5-Fluorouracil (5-FU). Cancer Biol. Ther. 2009, 8, 505–511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Llewellyn, A.; Foey, A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients 2017, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed]
- Santos Rocha, C.; Lakhdari, O.; Blottière, H.M.; Blugeon, S.; Sokol, H.; Bermu’dez-Humara’n, L.G.; Azevedo, V.; Miyoshi, A.; Doré, J.; Langella, P.; et al. Anti-inflammatory properties of dairy lactobacilli. Inflamm. Bowel Dis. 2012, 18, 657–666. [Google Scholar] [CrossRef]
- Luerce, T.; Gomes-Santos, A.; Rocha, C.; Moreira, T.; Cruz, D.; Lemos, L.; Sousa, A.; Pereira, V.; de Azevedo, M.; Moraes, K.; et al. Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis. Gut Pathog. 2014, 6, 33. [Google Scholar] [CrossRef]
- Trindade, L.M.; Martins, V.D.; Rodrigues, N.M.; Souza, E.L.S.; Martins, F.S.; Costa, G.M.F.; Almeida-Leite, C.M.; Faria, A.M.C.; Cardoso, V.N.; Maioli, T.U.; et al. Oral administration of Simbioflora® (synbiotic) attenuates intestinal damage in a mouse model of 5-fluorouracil-induced mucositis. Benef. Microbes 2018, 9, 477–486. [Google Scholar] [CrossRef]
- De Jesus, L.C.L.; Drumond, M.M.; de Carvalho, A.; Santos, S.S.; Martins, F.S.; Ferreira, Ê.; Fernandes, R.S.; de Barros, A.L.B.; do Carmo, F.L.R.; Perez, P.F.; et al. Protective effect of Lactobacillus delbrueckii subsp. Lactis CIDCA 133 in a model of 5 Fluorouracil-Induced intestinal mucositis. J. Funct. Foods 2019, 53, 197–207. [Google Scholar] [CrossRef]
- Del Carmen, S.; Zurita-Turk, M.; Lima, F.A.; Dos Santos, J.S.C.; Leclercq, S.Y.; Chatel, J.-M.; Azevedo, V.; De Moreno De Leblanc, A.; Miyoshi, A.; Leblanc, J.G. A Novel Interleukin-10 Dna Mucosal Delivery System Attenuates Intestinal Inflammation in a Mouse Model. Eur. J. Inflamm. 2013, 11, 641–654. [Google Scholar] [CrossRef]
- Zurita-Turk, M.; del Carmen, S.; Santos, A.C.; Pereira, V.B.; Cara, D.C.; Leclercq, S.Y.; de LeBlanc, A.dM.; Azevedo, V.; Chatel, J.-M.; LeBlanc, J.G.; et al. Lactococcus lactis carrying the pValac DNA expression vector coding for IL-10 reduces inflammation in a murine model of experimental colitis. BMC Biotechnol. 2014, 14, 73. [Google Scholar] [CrossRef]
- Brash, A.R.; Boeglin, W.E.; Chang, M.S. Discovery of a second 15S-lipoxygenase in humans. Proc. Natl. Acad. Sci. USA 1997, 94, 6148–6152. [Google Scholar] [CrossRef]
- Souza, B.M.; Preisser, T.M.; Pereira, V.B.; Zurita-Turk, M.; de Castro, C.P.; da Cunha, V.P.; de Oliveira, R.P.; Gomes-Santos, A.C.; de Faria, A.M.C.; Machado, D.C.C.; et al. Lactococcus lactis carrying the pValac eukaryotic expression vector coding for IL-4 reduces chemically-induced intestinal inflammation by increasing the levels of IL-10-producing regulatory cells. Microb. Cell Fact. 2016, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.D.; Breyner, N.; Menezes-Garcia, Z.; Rodrigues, N.M.; Lemos, L.; Maioli, T.U.; da Gloria Souza, D.; Carmona, D.; de Faria, A.M.C.; Langella, P.; et al. Secretion of biologically active pancreatitis-associated protein I (PAP) by genetically modified dairy Lactococcus lactis NZ9000 in the prevention of intestinal mucositis. Microb. Cell Fact. 2017, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Doimo, N.T.; Zárate-Bladés, C.R.; Rodrigues, R.F.; Tefé-Silva, C.; Trotte, M.N.; Souza, P.R.; Soares, L.S.; Rios, W.M.; Floriano, E.M.; Brandão, I.T.; et al. Immunotherapy of tuberculosis with Mycobacterium leprae Hsp65 as a DNA vaccine triggers cross-reactive antibodies against mammalian Hsp60 but not pathological autoimmunity. Hum. Vaccin. Immunother. 2014, 10, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Herrera Ramírez, J.C.; De la Mora, A.C.; De la Mora Valle, A.; Lopez-Valencia, G.; Hurtado, R.M.B.; Rentería Evangelista, T.B.; Rodríguez Castillo, J.L.; Rodríguez Gardea, A.; Gómez Gómez, S.D.; Medina-Basulto, G.E. Immunopathological evaluation of recombinant mycobacterial antigen Hsp65 expressed in Lactococcus lactis as a novel vaccine candidate. Iran. J. Vet. Res. 2017, 18, 197–202. [Google Scholar]
- Gomes-Santos, A.C.; de Oliveira, R.P.; Moreira, T.G.; Castro-Junior, A.B.; Horta, B.C.; Lemos, L.; de Almeida, L.A.; Rezende, R.M.; Cara, D.C.; Oliveira, S.C.; et al. Hsp65-Producing Lactococcus lactis Prevents Inflammatory Intestinal Disease in Mice by IL-10- and TLR2-Dependent Pathways. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Rezende, R.M.; Oliveira, R.P.; Medeiros, S.R.; Gomes-Santos, A.C.; Alves, A.C.; Loli, F.G.; Guimarães, M.A.F.; Amaral, S.S.; da Cunha, A.P.; Weiner, H.L.; et al. Hsp65-producing Lactococcus lactis prevents experimental autoimmune encephalomyelitis in mice by inducing CD4+LAP+ regulatory T cells. J. Autoimmun. 2013, 40, 45–57. [Google Scholar] [CrossRef]
- Baldon, E.; Marengo, E.; de Franco, M.; Starobinas, N.; Bueno, V.; Sant’Anna, O. Mycobacterium leprae Hsp65 administration reduces the lifespan of aged high antibody producer mice. Immun. Ageing 2014, 11, 6. [Google Scholar] [CrossRef]
- Harats, D.; Yacov, N.; Gilburd, B.; Shoenfeld, Y.; George, J. Oral tolerance with heat shock protein 65 attenuates mycobacterium tuberculosis-inducedand high-fat-diet-driven atherosclerotic lesions. J. Am. Coll. Cardiol. 2002, 40, 1333–1338. [Google Scholar] [CrossRef]
- Sakata, T.; Kojima, T.; Fujieda, M.; Miyakozawa, M.; Takahashi, M.; Ushida, K. Probiotic preparations dose-dependently increase net production rates of organic acids and decrease that of ammonia by pig cecal bacteria in batch culture. Dig. Dis. Sci. 1999, 44, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Walter, S. Structure and function of the GroE chaperone. Cell. Mol. Life Sci. 2002, 59, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.C.; Ramos, C.H.I. Protein folding assisted by chaperones. Protein Pept. Lett. 2005, 12, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U. Molecular chaperones in cellular protein folding. Nature 1996, 381, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.E. Polypeptide chain binding proteins: Catalysts of protein folding and related processes in cells. Cell 1989, 59, 591–601. [Google Scholar] [CrossRef]
- Mamalaki, C.; Murdjeva, M.; Tolaini, M.; Norton, T.; Chandler, P.; Townsend, A.; Simpson, E.; Kioussis, D. Tolerance in TCR/Cognate Antigen Double-Transgenic Mice Mediated by Incomplete Thymic Deletion and Peripheral Receptor Downregulation. Dev. Immunol. 1995, 4, 299–315. [Google Scholar] [CrossRef]
- Bukau, B.; Horwich, A.L. The Hsp70 and Hsp60 Chaperone Machines. Cell 1998, 92, 351–366. [Google Scholar] [CrossRef]
- Lindquist, S. The Heat-Shock Response. Annu. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G. Heat shock proteins as regulators of the immune response. Lancet 2003, 362, 469–476. [Google Scholar] [CrossRef]
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef]
- Lindquist, J.A. ER-60, a chaperone with thiol-dependent reductase activity involved in MHC class I assembly. EMBO J. 1998, 17, 2186–2195. [Google Scholar] [CrossRef]
- Tsan, M.-F.; Gao, B. Heat shock proteins and immune system. J. Leukoc. Biol. 2009, 85, 905–910. [Google Scholar] [CrossRef]
- Coelho, V.; Faria, A.M.C. HSP60: Issues and Insights on Its Therapeutic Use as an Immunoregulatory Agent. Front. Immunol. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Takemoto, S.; Takakura, Y. Heat shock protein derivatives for delivery of antigens to antigen presenting cells. Int. J. Pharm. 2008, 354, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.N.; Choi, B.; Lee, K.W.; Lee, D.J.; Kang, D.H.; Lee, J.Y.; Song, I.S.; Kim, H.I.; Lee, S.-H.; Kim, H.S.; et al. Cytosolic Hsp60 Is Involved in the NF-κB-Dependent Survival of Cancer Cells via IKK Regulation. PLoS ONE 2010, 5, e9422. [Google Scholar] [CrossRef] [PubMed]
- Lehner, T.; Bergmeier, L.A.; Wang, Y.; Tao, L.; Sing, M.; Spallek, R.; van der Zee, R. Heat shock proteins generate beta-chemokines which function as innate adjuvants enhancing adaptive immunity. Eur. J. Immunol. 2000, 30, 594–603. [Google Scholar] [CrossRef]
- Moré, S.H.; Breloer, M.; von Bonin, A. Eukaryotic heat shock proteins as molecular links in innate and adaptive immune responses: Hsp60-mediated activation of cytotoxic T cells. Int. Immunol. 2001, 13, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Niu, J.; Wang, K.; Xiao, Y.; Du, Y.; Zhou, L.; Duan, L.; Li, S.; Yang, G.; Chen, L.; et al. Heat Shock Factor 2 Levels Are Associated with the Severity of Ulcerative Colitis. PLoS ONE 2014, 9, e88822. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, M.J.; Chang, E.B. Do heat shock proteins play any role in gut inflammation? Inflamm. Bowel Dis. 2008, 14, S102–S103. [Google Scholar] [CrossRef]
- de Azevedo, M.S.P.; Rocha, C.S.; Electo, N.; Pontes, D.S.; Molfetta, J.B.; Gonçalves, E.D.C.; Azevedo, V.; Silva, C.L.; Miyoshi, A. Cytoplasmic and extracellular expression of pharmaceutical-grade mycobacterial 65-kDa heat shock protein in Lactococcus lactis. Genet. Mol. Res. 2012, 11, 1146–1157. [Google Scholar] [CrossRef]
- Mancha-Agresti, P.; Drumond, M.M.; do Carmo, F.L.R.; Santos, M.M.; dos Santos, J.S.C.; Venanzi, F.; Chatel, J.-M.; Leclercq, S.Y.; Azevedo, V. A New Broad Range Plasmid for DNA Delivery in Eukaryotic Cells Using Lactic Acid Bacteria: In Vitro and In Vivo Assays. Mol. Ther. Methods Clin. Dev. 2017, 4, 83–91. [Google Scholar] [CrossRef]
- Pelizon, A.C.; Martins, D.R.; Zorzella-Pezavento, S.F.G.; Seger, J.; Justulin Jr, L.A.; da Fonseca, D.M.; Santos Jr, R.R.; Masson, A.P.; Silva, C.L.; Sartori, A. Neonatal BCG Immunization Followed by DNAhsp65 Boosters: Highly Immunogenic but not Protective Against Tuberculosis - a Paradoxical Effect of the Vector? Scand. J. Immunol. 2010, 71, 63–69. [Google Scholar] [CrossRef]
- Green, M.R.; Hughes, H.; Sambrook, J.; MacCallum, P. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor: New York, NY, USA, 2012. [Google Scholar]
- Coelho-Rocha, N.D.; de Castro, C.P.; de Jesus, L.C.L.; Leclercq, S.Y.; de Cicco Sandes, S.H.; Nunes, A.C.; Azevedo, V.; Drumond, M.M.; Mancha-Agresti, P. Microencapsulation of Lactic Acid Bacteria Improves the Gastrointestinal Delivery and in situ Expression of Recombinant Fluorescent Protein. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- de Barros, P.A.V.; Rabelo Andrade, M.E.; de Vasconcelos Generoso, S.; Mendes Miranda, S.E.; dos Reis, D.C.; Lacerda Leocádio, P.C.; de Sales e Souza, É.L.; dos Santos Martins, F.; da Gama, M.A.S.; Cassali, G.D.; et al. Conjugated linoleic acid prevents damage caused by intestinal mucositis induced by 5-fluorouracil in an experimental model. Biomed. Pharmacother. 2018, 103, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Diniz, S.O.; Resende, B.; Nunan, E.; Simal, C.J.; Cardoso, V. 99mTechnetium labelled Escherichia coli. Appl. Radiat. Isot. 1999, 51, 33–36. [Google Scholar] [CrossRef]
- Soares, P.M.G.; Mota, J.M.S.C.; Gomes, A.S.; Oliveira, R.B.; Assreuy, A.M.S.; Brito, G.A.C.; Santos, A.A.; Ribeiro, R.A.; Souza, M.H.L.P. Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother. Pharmacol. 2008, 63, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of Cutaneous Inflammation: Estimation of Neutrophil Content with an Enzyme Marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.S.; Silva, A.A.; Vieira, A.T.; Barbosa, F.H.F.; Arantes, R.M.E.; Teixeira, M.M.; Nicoli, J.R. Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch. Microbiol. 2009, 191, 623–630. [Google Scholar] [CrossRef]
- Giulietti, A.; Overbergh, L.; Valckx, D.; Decallonne, B.; Bouillon, R.; Mathieu, C. An Overview of Real-Time Quantitative PCR: Applications to Quantify Cytokine Gene Expression. Methods 2001, 25, 386–401. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Volynets, V.; Rings, A.; Bárdos, G.; Ostaff, M.J.; Wehkamp, J.; Bischoff, S.C. Intestinal barrier analysis by assessment of mucins, tight junctions, and α-defensins in healthy C57BL/6J and BALB/cJ mice. Tissue Barriers 2016, 4, e1208468. [Google Scholar] [CrossRef]
- Song, M.-K.; Park, M.-Y.; Sung, M.-K. 5-Fluorouracil-Induced Changes of Intestinal Integrity Biomarkers in BALB/C Mice. J. Cancer Prev. 2013, 18, 322–329. [Google Scholar] [CrossRef]
- Chang, C.-W.; Lee, H.-C.; Li, L.-H.; Chiang Chiau, J.-S.; Wang, T.-E.; Chuang, W.-H.; Chen, M.-J.; Wang, H.-Y.; Shih, S.-C.; Liu, C.-Y.; et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 386. [Google Scholar] [CrossRef] [PubMed]
- Rha, Y.-H.; Taube, C.; Haczku, A.; Joetham, A.; Takeda, K.; Duez, C.; Siegel, M.; Aydintug, M.K.; Born, W.K.; Dakhama, A.; et al. Effect of Microbial Heat Shock Proteins on Airway Inflammation and Hyperresponsiveness. J. Immunol. 2002, 169, 5300–5307. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Takeda, K.; Shiraishi, Y.; Jeong, Y.Y.; Domenico, J.; Jia, Y.; Han, J.; Spallek, R.; Singh, M.; Lucas, J.J.; et al. Microbial Heat Shock Protein 65 Attenuates Airway Hyperresponsiveness and Inflammation by Modulating the Function of Dendritic Cells. J. Immunol. 2012, 189, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Kociubinski, G.L.; Pérez, P.F.; Añón, M.C.; DE Antoni, G.L. A Method of Screening for Highly Inhibitory Lactic Acid Bacteria. J. Food Prot. 1996, 59, 739–745. [Google Scholar] [CrossRef]
- Kociubinski, G.; Pérez, P.; DE Antoni, G. Screening of Bile Resistance and Bile Precipitation in Lactic Acid Bacteria and Bifidobacteria. J. Food Prot. 1999, 62, 905–912. [Google Scholar] [CrossRef]
- Hugo, A.A.; De Antoni, G.L.; Pérez, P.F. Lactobacillus delbrueckii subsp lactis strain CIDCA 133 inhibits nitrate reductase activity of Escherichia coli. Int. J. Food Microbiol. 2006, 111, 191–196. [Google Scholar] [CrossRef]
- Hugo, A.A.; Kakisu, E.; De Antoni, G.L.; Pérez, P.F. Lactobacilli antagonize biological effects of enterohaemorrhagic Escherichia coli in vitro. Lett. Appl. Microbiol. 2008, 46, 613–619. [Google Scholar] [CrossRef]
- Hugo, A.A.; De Antoni, G.L.; Pérez, P.F. Lactobacillus delbrueckii subsp lactis (strain CIDCA 133) resists the antimicrobial activity triggered by molecules derived from enterocyte-like Caco-2 cells. Lett. Appl. Microbiol. 2010, 50, 335–340. [Google Scholar] [CrossRef]
- Hugo, A.A.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Pérez, P.F. Effect of human defensins on lactobacilli and liposomes. J. Appl. Microbiol. 2012, 113, 1491–1497. [Google Scholar] [CrossRef]
- Hugo, A.A.; Rolny, I.S.; Romanin, D.; Pérez, P.F. Lactobacillus delbrueckii subsp. lactis (strain CIDCA 133) stimulates murine macrophages infected with Citrobacter rodentium. World J. Microbiol. Biotechnol. 2017, 33, 48. [Google Scholar] [CrossRef]
- Azevedo, O.G.R.; Oliveira, R.A.C.; Oliveira, B.C.; Zaja-Milatovic, S.; Araújo, C.V.; Wong, D.V.T.; Costa, T.B.; Lucena, H.B.M.; Lima-Júnior, R.C.P.; Ribeiro, R.A.; et al. Apolipoprotein E COG 133 mimetic peptide improves 5-fluorouracil-induced intestinal mucositis. BMC Gastroenterol. 2012, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.M.; Leonel, A.J.; Melo, M.A.; Santos, R.R.G.; Cara, D.C.; Cardoso, V.N.; Correia, M.I.T.D.; Alvarez-Leite, J.I. Oral Supplementation of Butyrate Reduces Mucositis and Intestinal Permeability Associated with 5-Fluorouracil Administration. Lipids 2012, 47, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.M.G.; Mota, J.M.S.C.; Souza, E.P.; Justino, P.F.C.; Franco, A.X.; Cunha, F.Q.; Ribeiro, R.A.; Souza, M.H.L.P. Inflammatory intestinal damage induced by 5-fluorouracil requires IL-4. Cytokine 2013, 61, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Justino, P.F.C.; Melo, L.F.M.; Nogueira, A.F.; Costa, J.V.G.; Silva, L.M.N.; Santos, C.M.; Mendes, W.O.; Costa, M.R.; Franco, A.X.; Lima, A.A.; et al. Treatment with Saccharomyces boulardii reduces the inflammation and dysfunction of the gastrointestinal tract in 5-fluorouracil-induced intestinal mucositis in mice. Br. J. Nutr. 2014, 111, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Aranow, J.S.; Fink, M.P. Determinants of intestinal barrier failure in critical illness. Br. J. Anaesth. 1996, 77, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.-E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef]
- Cordeiro, B.F.; Oliveira, E.R.; da Silva, S.H.; Savassi, B.M.; Acurcio, L.B.; Lemos, L.; de Alves, J.L.; Carvalho Assis, H.; Vieira, A.T.; Faria, A.M.C.; et al. Whey Protein Isolate-Supplemented Beverage, Fermented by Lactobacillus casei BL23 and Propionibacterium freudenreichii 138, in the Prevention of Mucositis in Mice. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Schmucker, D.L.; Owen, R.L.; Outenreath, R.; Thoreux, K. Basis for the Age-related Decline in Intestinal Mucosal Immunity. Clin. Dev. Immunol. 2003, 10, 167–172. [Google Scholar] [CrossRef]
- Nagayoshi, H.; Fukatsu, K.; Ueno, C.; Hara, E.; Maeshima, Y.; Omata, J.; Hiraide, H.; Mochizuki, H. 5-Fluorouracil Infusion Reduces Gut-Associated Lymphoid Tissue Cell Number and Mucosal Immunoglobulin A Levels. J. Parenter. Enter. Nutr. 2005, 29, 395–400. [Google Scholar] [CrossRef]
- Lycke, N.Y.; Bemark, M. The regulation of gut mucosal IgA B-cell responses: Recent developments. Mucosal Immunol. 2017, 10, 1361–1374. [Google Scholar] [CrossRef]
- Berg, R. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995, 3, 149–154. [Google Scholar] [CrossRef]
- Lichtman, S.M. Baterial Translocation in Humans. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- MacFie, J.; Reddy, B.S.; Gatt, M.; Jain, P.K.; Sowdi, R.; Mitchell, C.J. Bacterial translocation studied in 927 patients over 13 years. Br. J. Surg. 2006, 93, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Mainous, M.R. Studies of the Route, Magnitude, and Time Course of Bacterial Translocation in a Model of Systemic Inflammation. Arch. Surg. 1991, 126, 33. [Google Scholar] [CrossRef]
- O’Hara, S.P.; Karlsen, T.H.; LaRusso, N.F. Cholangiocytes and the environment in primary sclerosing cholangitis: Where is the link? Gut 2017, 66, 1873–1877. [Google Scholar] [CrossRef]
- Brandl, K.; Kumar, V.; Eckmann, L. Gut-liver axis at the frontier of host-microbial interactions. Am. J. Physiol. Liver Physiol. 2017, 312, G413–G419. [Google Scholar] [CrossRef]
- Stringer, A.M.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Yeoh, A.S.J.; Hamilton, J.; Keefe, D.M.K. Gastrointestinal Microflora and Mucins May Play a Critical Role in the Development of 5-Fluorouracil-Induced Gastrointestinal Mucositis. Exp. Biol. Med. 2009, 234, 430–441. [Google Scholar] [CrossRef]
- Carvalho, R.; Vaz, A.; Pereira, F.L.; Dorella, F.; Aguiar, E.; Chatel, J.-M.; Bermudez, L.; Langella, P.; Fernandes, G.; Figueiredo, H.; et al. Gut microbiome modulation during treatment of mucositis with the dairy bacterium Lactococcus lactis and recombinant strain secreting human antimicrobial PAP. Sci. Rep. 2018, 8, 15072. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U.; Gwee, K.-A.; Ng, S.C.; Quigley, E.M.M. The Gut Microbiota and Irritable Bowel Syndrome: Friend or Foe? Int. J. Inflam. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Stringer, A.M.; Al-Dasooqi, N.; Bowen, J.M.; Tan, T.H.; Radzuan, M.; Logan, R.M.; Mayo, B.; Keefe, D.M.K.; Gibson, R.J. Biomarkers of chemotherapy-induced diarrhoea: A clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support. Care Cancer 2013, 21, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, C. Translocation of gut flora and its role in sepsis. Indian J. Med. Microbiol. 2013, 31, 334. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.M. “The stress of dying”: The role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 2004, 117, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- van Eden, W.; van der Zee, R.; Prakken, B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat. Rev. Immunol. 2005, 5, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Hauet-Broere, F.; Wieten, L.; Guichelaar, T.; Berlo, S.; van der Zee, R.; Van Eden, W. Heat shock proteins induce T cell regulation of chronic inflammation. Ann. Rheum. Dis. 2006, 65, iii65–iii68. [Google Scholar] [CrossRef]
- Wilson, J.; Winter, M.; Shasby, D.M. Oxidants, ATP depletion, and endothelial permeability to macromolecules. Blood 1990, 76, 2578–2582. [Google Scholar] [CrossRef]
- Choudhry, M.A.; Fazal, N.; Goto, M.; Gamelli, R.L.; Sayeed, M.M. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am. J. Physiol. Liver Physiol. 2002, 282, G937–G947. [Google Scholar] [CrossRef]
- Berg, R.D. Immunosuppression and Intestinal Bacterial Overgrowth Synergistically Promote Bacterial Translocation. Arch. Surg. 1988, 123, 1359. [Google Scholar] [CrossRef]
- MacFie, J. Current status of bacterial translocation as a cause of surgical sepsis. Br. Med. Bull. 2005, 71, 1–11. [Google Scholar] [CrossRef]
- Wiest, R.; Lawson, M.; Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef]
- Andrade, M.E.R.; Araújo, R.S.; de Barros, P.A.V.; Soares, A.D.N.; Abrantes, F.A.; de Generoso, S.V.; Fernandes, S.O.A.; Cardoso, V.N. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin. Nutr. 2015, 34, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W.; Boyce, S.T.; Babcock, G.F.; Gianotti, L.; Peck, M.D.; Dunn, D.L.; Pyles, T.; Childress, C.P.; Ash, S.K. The process of microbial translocation. Ann. Surg. 1990, 212, 496–510, discussion 511–512. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef]
- Lépine, A.F.P.; de Wit, N.; Oosterink, E.; Wichers, H.; Mes, J.; de Vos, P. Lactobacillus acidophilus Attenuates Salmonella-Induced Stress of Epithelial Cells by Modulating Tight-Junction Genes and Cytokine Responses. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Blackwood, B.P.; Yuan, C.Y.; Wood, D.R.; Nicolas, J.D.; Grothaus, J.S.; Hunter, C.J. Probiotic Lactobacillus Species Strengthen Intestinal Barrier Function and Tight Junction Integrity in Experimental Necrotizing Enterocolitis. J. Probiotics Heal. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Logan, R.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. Nuclear factor-κB (NF-κB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007, 43, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Sultani, M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J. Anti-Inflammatory Cytokines: Important Immunoregulatory Factors Contributing to Chemotherapy-Induced Gastrointestinal Mucositis. Chemother. Res. Pract. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Hall, P.D.; Benko, H.; Hogan, K.R.; Stuart, R.K. The influence of serum tumor necrosis factor-alpha and interleukin-6 concentrations on nonhematologic toxicity and hematologic recovery in patients with acute myelogenous leukemia. Exp. Hematol. 1995, 23, 1256–1260. [Google Scholar] [PubMed]
- Sonis, S. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support. Oncol. 2007, 5, 3–11. [Google Scholar] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Q.; Han, X.-D.; Wang, Y.; Yuan, K.-L.; Jin, Z.-M.; Di, J.-Z.; Yan, J.; Pan, Y.; Zhang, P.; Huang, X.-Y.; et al. Interleukin-1 receptor antagonist reduced apoptosis and attenuated intestinal mucositis in a 5-fluorouracil chemotherapy model in mice. Cancer Chemother. Pharmacol. 2011, 68, 87–96. [Google Scholar] [CrossRef]
- Turner, J.R. Molecular Basis of Epithelial Barrier Regulation. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.-J.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef]
- Logan, R.M.; Gibson, R.J.; Bowen, J.M.; Stringer, A.M.; Sonis, S.T.; Keefe, D.M.K. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: Implications for the pathobiology of mucositis. Cancer Chemother. Pharmacol. 2008, 62, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, C.J.; Rose-John, S.; Lissilaa, R.; Ferlin, W.; Ernst, M.; Hertzog, P.J.; Mansell, A.; Jenkins, B.J. IL-6 Trans -Signaling Modulates TLR4-Dependent Inflammatory Responses via STAT3. J. Immunol. 2011, 186, 1199–1208. [Google Scholar] [CrossRef]
- Cario, E. Toll-like receptors in the pathogenesis of chemotherapy-induced gastrointestinal toxicity. Curr. Opin. Support. Palliat. Care 2016, 10, 157–164. [Google Scholar] [CrossRef]
- Fukata, M.; Michelsen, K.S.; Eri, R.; Thomas, L.S.; Hu, B.; Lukasek, K.; Nast, C.C.; Lechago, J.; Xu, R.; Naiki, Y.; et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Liver Physiol. 2005, 288, G1055–G1065. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N. An induced proximity model for NF-kappaB activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem. 2000. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S. Signaling to NF- B. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004, 127, 224–238. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Sanders, C.J.; Taylor, R.T.; Kumar, A.; Aitken, J.D.; Sitaraman, S.V.; Neish, A.S.; Uematsu, S.; Akira, S.; Williams, I.R.; et al. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Investig. 2007. [Google Scholar] [CrossRef]
- Ahrne, S.; Johansson Hagslatt, M.-L. Effect of Lactobacilli on Paracellular Permeability in the Gut. Nutrients 2011, 3, 104–117. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, Y.; Huang, Z.; Dong, W.; Deng, Y.; Wang, F.; Li, M.; Yuan, J. Administration of probiotic mixture DM#1 ameliorated 5-fluorouracil–induced intestinal mucositis and dysbiosis in rats. Nutrition 2017, 33, 96–104. [Google Scholar] [CrossRef]
- Rubtsov, Y.P.; Rasmussen, J.P.; Chi, E.Y.; Fontenot, J.; Castelli, L.; Ye, X.; Treuting, P.; Siewe, L.; Roers, A.; Henderson, W.R.; et al. Regulatory T Cell-Derived Interleukin-10 Limits Inflammation at Environmental Interfaces. Immunity 2008, 28, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Lacki, J.K.; Wiktorowicz, K.E. Biological properties of interleukin 10. Postepy Hig. Med. Dosw. 1994, 48, 363–370. [Google Scholar] [PubMed]
- Asadullah, K.; Sterry, W.; Volk, H.D. Interleukin-10 Therapy—Review of a New Approach. Pharmacol. Rev. 2003, 55, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Bruyns, C.; Marchant, A.; Abramowicz, D.; Vandenabeele, P.; Delvaux, A.; Fiers, W.; Goldman, M.; Velu, T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J. Exp. Med. 1993, 177, 547–550. [Google Scholar] [CrossRef]
- Clarke, C.J.P.; Hales, A.; Hunt, A.; Foxwell, B.M.J. IL-10-mediated suppression of TNF-α production is independent of its ability to inhibit NFκB activity. Eur. J. Immunol. 1998, 28, 1719–1726. [Google Scholar] [CrossRef]

| Gene | Foward Primer | Reverse Primer | Reference |
|---|---|---|---|
| Actb | GCTGAGAGGGAAATCGTGCGTG | CCAGGGAGGAAGAGGATGCGG | [60] |
| Gapdh | TCACCACCATGGAGAAGGC | GCTAAGCAGTTGGTGGTGCA | [58] |
| Il6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA | [58] |
| Il10 | GGTTGCCAAGCCTTATCGGA | ACCTGCTCCACTGCCTTGCT | [58] |
| Il12p40 | GGAAGCACGGCAGCAGAATA | AACTTGAGGGAGAAGTAGGAATGG | [58] |
| Tnf | ACGTGGAACTGGCAGAAGAG | CTCCTCCACTTGGTGGTTTG | [61] |
| Il1b | CTCCATGAGCTTTGTACAAGG | TGCTGATGTACCAGTTGGGG | [61] |
| Muc2 | GATGGCACCTACCTCGTTGT | GTCCTGGCACTTGTTGGAAT | [60] |
| Myd88 | ATCGCTGTTCTTGAACCCTCG | CTCACGGTCTAACAAGGCCAG | [62] |
| Tlr2 | ACAATAGAGGGAGACGCCTTT | AGTGTCTGGTAAGGATTTCCCAT | [62] |
| Tlr4 | ATGGCATGGCTTACACCACC | GAGGCCATTTTTGTCTCCACA | [62] |
| Nos2 | CAGCTGGGCTGTACAAACCTT | CATTGGAAGTGAAGCGTTTCG | [58] |
| Cldn1 | TCCTTGCTGAATCTGAACA | AGCCATCCACATCTTCTG | [60] |
| Cldn2 | GTCATCGCCCATCAGAAGAT | ACTGTTGGACAGGGAACCAG | [60] |
| Cldn5 | GCTCTCAGAGTCCGTTGACC | CTGCCCTTTCAGGTTAGCAG | [60] |
| Occludin | ACTCCTCCAATGGACAAGTG | CCCCACCTGTCGTGTAGTCT | [60] |
| Zonulin | CCACCTCTGTCCAGCTCTTC | CACCGGAGTGATGGTTTTCT | [60] |
| F11r | CACCTTCTCATCCAGTGGCATC | CTCCACAGCATCCATGTGTGC | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso, F.A.L.; de Jesus, L.C.L.; de Castro, C.P.; Batista, V.L.; Ferreira, Ê.; Fernandes, R.S.; de Barros, A.L.B.; Leclerq, S.Y.; Azevedo, V.; Mancha-Agresti, P.; et al. Intake of Lactobacillus delbrueckii (pExu:hsp65) Prevents the Inflammation and the Disorganization of the Intestinal Mucosa in a Mouse Model of Mucositis. Microorganisms 2021, 9, 107. https://doi.org/10.3390/microorganisms9010107
Barroso FAL, de Jesus LCL, de Castro CP, Batista VL, Ferreira Ê, Fernandes RS, de Barros ALB, Leclerq SY, Azevedo V, Mancha-Agresti P, et al. Intake of Lactobacillus delbrueckii (pExu:hsp65) Prevents the Inflammation and the Disorganization of the Intestinal Mucosa in a Mouse Model of Mucositis. Microorganisms. 2021; 9(1):107. https://doi.org/10.3390/microorganisms9010107
Chicago/Turabian StyleBarroso, Fernanda Alvarenga Lima, Luís Cláudio Lima de Jesus, Camila Prosperi de Castro, Viviane Lima Batista, Ênio Ferreira, Renata Salgado Fernandes, André Luís Branco de Barros, Sophie Yvette Leclerq, Vasco Azevedo, Pamela Mancha-Agresti, and et al. 2021. "Intake of Lactobacillus delbrueckii (pExu:hsp65) Prevents the Inflammation and the Disorganization of the Intestinal Mucosa in a Mouse Model of Mucositis" Microorganisms 9, no. 1: 107. https://doi.org/10.3390/microorganisms9010107
APA StyleBarroso, F. A. L., de Jesus, L. C. L., de Castro, C. P., Batista, V. L., Ferreira, Ê., Fernandes, R. S., de Barros, A. L. B., Leclerq, S. Y., Azevedo, V., Mancha-Agresti, P., & Drumond, M. M. (2021). Intake of Lactobacillus delbrueckii (pExu:hsp65) Prevents the Inflammation and the Disorganization of the Intestinal Mucosa in a Mouse Model of Mucositis. Microorganisms, 9(1), 107. https://doi.org/10.3390/microorganisms9010107







