Fungicide Resistance in Powdery Mildew Fungi
Abstract
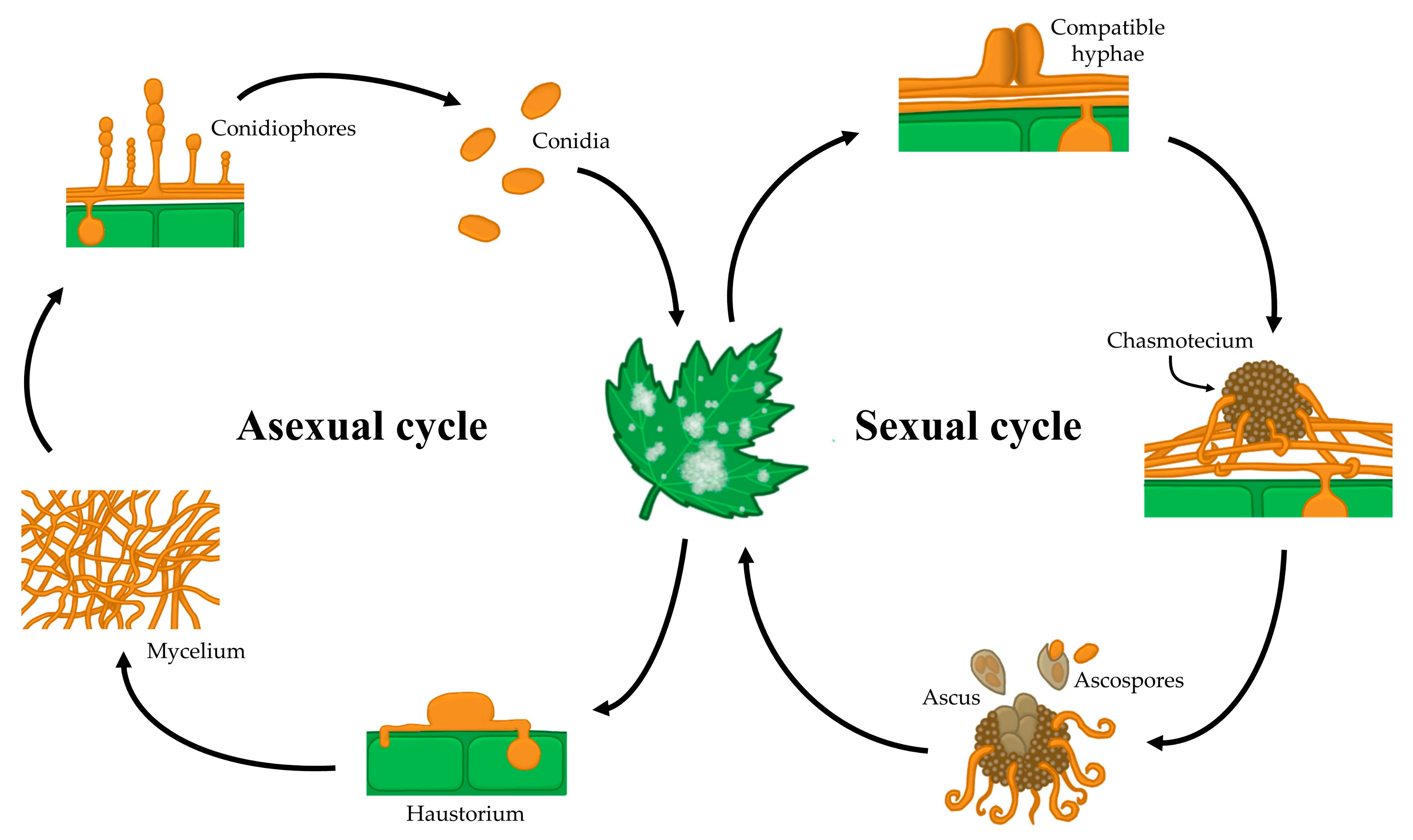
1. Introduction
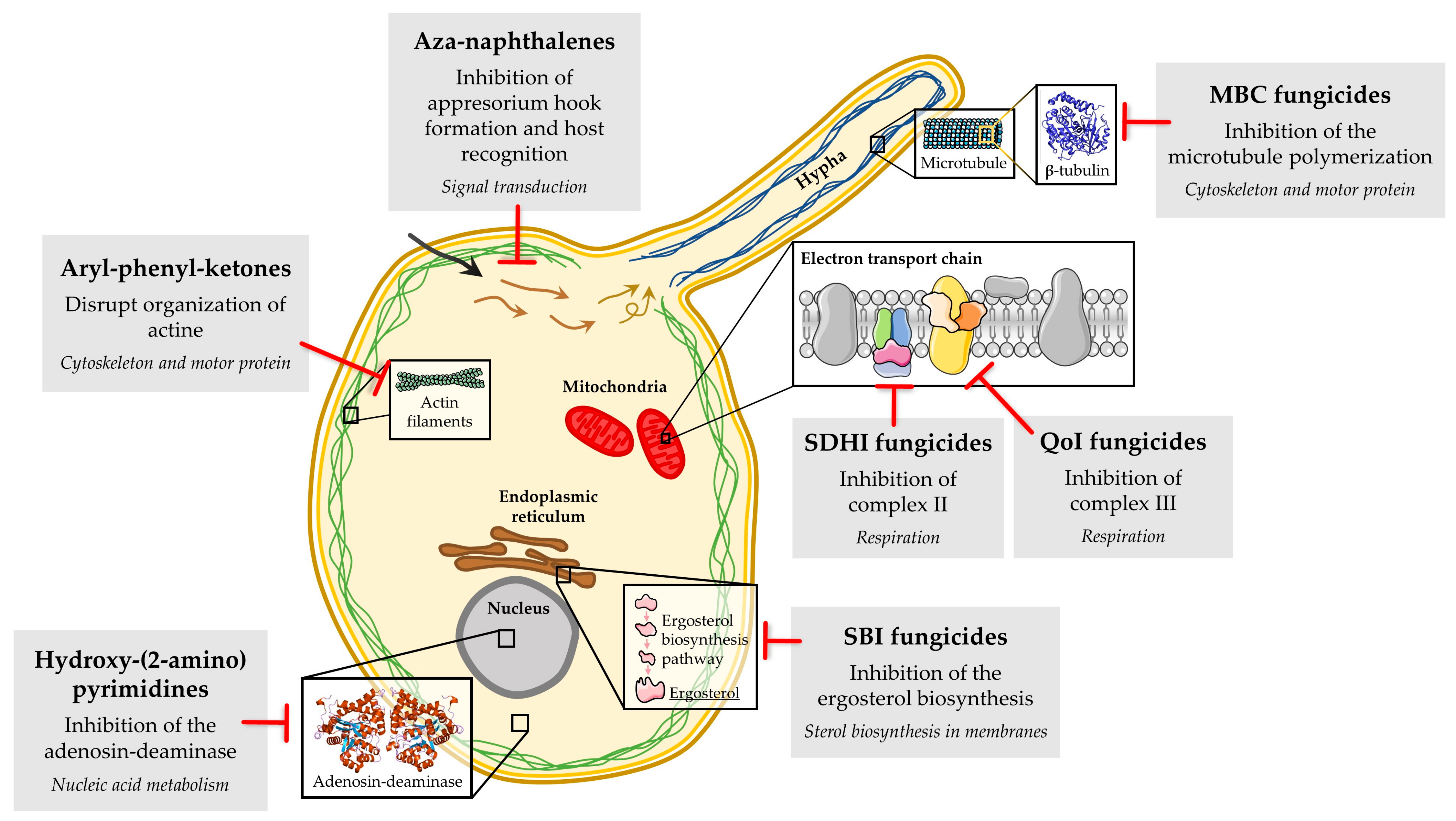
2. Fungicides, the Main Anti-Powdery Mildew Tools
3. Current Fungicide Resistance Status in Powdery Mildew Fungi
3.1. Resistance to Aryl-Phenyl-Ketones in B. graminis f. sp. hordei, B. graminis f. sp. tritici, E. necator and P. xanthii
3.2. Resistance to Aza-Naphthalenes in B. graminis f. sp. hordei, B. graminis f. sp. tritici, E. necator and P. xanthii
3.3. Resistance to Hydroxy-(2-amino) Pyrimidines in B. graminis f. sp. hordei, Golovinomyces cichoracearum and P. xanthii
3.4. Resistance to MBC Fungicides in E. necator, G. cichoracearum, P. xanthii and Sphaerotheca pannosa
3.5. Resistance to Phosphorothiolates in P. xanthii
3.6. Resistance to QoIs in B. graminis f. sp. hordei, B. graminis f. sp. tritici, Erysiphe betae, E. necator, Leveillula taurica, Podosphaera leucotricha and P. xanthii
3.7. Resistance to SBI Fungicides, Amines and DMIs in B. graminis f. sp. hordei, Blumeria graminis f. sp. tritici, E. necator, G. cichoracearum, Podosphaera aphanis and P. xanthii
3.8. Resistance to SDHIs in E. necator and P. xanthii
3.9. Resistance to Fungicides with an Unknown Mode of Action in P. xanthii
4. Conclusions and Recommendations
- Use IPM that includes non-chemical methods.
- Do not use a single active ingredient; it is highly recommended to use mixtures or more than one fungicide in the fungicide programme.
- Do not exclusively use a group of fungicides with the same mode of action. It is recommended to use fungicides with at least three different mechanisms of action.
- In the event that a high-risk fungicide must be used, use it only once per crop, preferably in a mixture with organic multi-site fungicides and other alternatives such as inorganic fungicides (copper and sulphur), host plant defence inductors or biologicals products with multiple modes of actions.
- Restrict the number of fungicide sprays applied per season and apply them only when strictly necessary, employing different fungicides before and after the season.
- Use the recommended field doses labelled in the commercial product. Sublethal doses, even in mixtures, favour the appearance of resistance.
- Achieve good spray coverage (reduces populations exposed to selection).
Funding
Conflicts of Interest
References
- Braun, U. The current systematics and taxonomy of the powdery mildews (Erysiphales): An overview. Mycoscience 2011, 52, 210–212. [Google Scholar] [CrossRef]
- Green, J.R.; Carver, T.L.W.; Gurr, S.J. The formation and function of infection and feeding structures. In The Powdery Mildews: A Comprehensive Treatise; Bélanger, R.R., Bushnell, W.R., Dik, A.J., Carver, T.L.W., Eds.; APS Press: St. Paul, MN, USA, 2002; pp. 66–82. [Google Scholar]
- Vogel, J.P.; Raab, T.K.; Somerville, C.R.; Somerville, S.C. Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 2004, 40, 968–978. [Google Scholar] [CrossRef]
- Eichmann, R.; Hückelhoven, R. Accommodation of powdery mildew fungi in intact plant cells. J. Plant Physiol. 2008, 165, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2002, 39, 385–417. [Google Scholar] [CrossRef] [PubMed]
- Heffer, V.; Johnson, K.B.; Powelson, M.L.; Shishkoff, N. Identification of powdery mildew fungi anno 2006. Plant Health Instr. 2006. [Google Scholar] [CrossRef]
- Sidhu, G.S. Genetics of plant pathogenic fungi. In Advances in Plant Pathology; Ingram, D.S., Williams, P.H., Eds.; Academic Press: Cambridge, MA, USA, 1988; Volume 6. [Google Scholar]
- Pérez-García, A.; Romero, D.; Fernández-Ortuño, D.; López-Ruiz, F.; De Vicente, A.; Torés, J.A. The powdery mildew fungus Podosphaera fusca (synonym Podosphaera xanthii), a constant threat to cucurbits. Mol. Plant Pathol. 2009, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine powdery mildew (Erysiphe necator): A fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Saharan, G.S.; Mehta, N.K.; Meena, P.D. Infection, pathogenesis and disease cycle. In Powdery Mildew Disease of Crucifers: Biology, Ecology and Disease Management; Saharan, G.S., Mehta, N.K., Meena, P.D., Eds.; Springer: Singapore, 2019; pp. 95–130. [Google Scholar]
- Jarvis, W.R.; Gubler, W.D.; Grove, G.G. Epidemiology of powdery mildews in agricultural pathosystems. In The Powdery Mildews: A Comprehensive Treatise; Bélanger, R.R., Bushnell, W.R., Dik, A.J., Carver, T.L.W., Eds.; APS Press: St. Paul, MN, USA, 2002; pp. 169–200. [Google Scholar]
- Pearson, R.C.; Gadoury, D.M. Cleistothecia, the source of primary inoculum for grape powdery mildew in New York. Phytopathology 1987, 77, 1509–1514. [Google Scholar] [CrossRef]
- Pirondi, A.; Vela-Corcía, D.; Dondini, L.; Brunelli, A.; Pérez-García, A.; Collina, M. Genetic diversity analysis of the cucurbit powdery mildew fungus Podosphaera xanthii suggests a clonal population structure. Fungal Biol. 2015, 119, 791–801. [Google Scholar] [CrossRef]
- Tetteh, A.Y.; Wehner, T.C.; Davis, A.R. Identifying resistance to powdery mildew race 2W in the USDA-ARS Watermelon Germplasm Collection. Crop Sci. 2010, 50, 933–939. [Google Scholar] [CrossRef]
- Davis, A.R.; Levi, A.; Tetteh, A.; Wehner, T.C.; Russo, V.M.; Pitrat, M. Evaluation of watermelon and related species for resistance to race 1W powdery mildew. J. Am. Soc. Hort. Sci. 2007, 132, 790–795. [Google Scholar] [CrossRef]
- Romero, D.; de Vicente, A.; Zeriouh, H.; Cazorla, F.M.; Fernández-Ortuño, D.; Torés, J.A.; Pérez-García, A. Evaluation of biological control agents for managing cucurbit powdery mildew on greenhouse-grown melon. Plant Pathol. 2007, 56, 976–986. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D. Fungicide Resistance in Crop Pathogens. How Can It Be Managed? 2nd ed.; FRAC: Brussels, Belgium, 2007. [Google Scholar]
- Oliver, R.; Hewitt, H.G. Fungicides in Crop Protection, 2nd ed.; CABI International: Wallingford, UK, 2014. [Google Scholar]
- De Miccolis Angelini, R.M.; Pollastro, S.; Faretra, F. Genetics of fungicide resistance. In Fungicide Resistance in Plant Pathogens, 1st ed.; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokio, Japan, 2015; pp. 13–34. [Google Scholar] [CrossRef]
- Hu, W.; Yan, L.; Ma, Z. Cloning and expression analysis of a putative ABC transporter gene BgABC1 from the biotrophic pathogenic fungus Blumeria graminis f. sp. tritici. J. Phytopathol. 2008, 156, 120–124. [Google Scholar] [CrossRef]
- Frenkel, O.; Cadle-Davidson, L.; Wilcox, W.F.; Milgroom, M.G. Mechanisms of resistance to an azole fungicide in the grapevine powdery mildew fungus, Erysiphe necator. Phytopathology 2015, 105, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Rallos, L.E.E.; Baudoin, A.B. Co-occurrence of two allelic variants of CYP51 in Erysiphe necator and their correlation with over-expression for DMI resistance. PLoS ONE 2016, 11, e0148025. [Google Scholar] [CrossRef] [PubMed]
- Waxman, M.F. The Agrochemical and Pesticides Safety Handbook; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Schmitt, M.R.; Carzaniga, R.; Cotter, H.V.T.; O’Connell, R.; Hollomon, D. Microscopy reveals disease control through novel effects on fungal development: A case study with an early-generation benzophenone fungicide. Pest Manag. Sci. 2006, 62, 383–392. [Google Scholar] [CrossRef]
- Gilbert, S.R.; Cools, H.J.; Fraaije, B.A.; Bailey, A.M.; Lucas, J.A. Impact of proquinazid on appressorial development of the barley powdery mildew fungus Blumeria graminis f. sp. hordei. Pestic. Biochem. Physiol. 2009, 94, 127–132. [Google Scholar] [CrossRef]
- FRAC Pathogen Risk List 2019. Available online: https://www.frac.info/docs/default-source/publications/pathogen-risk/frac-pathogen-list-2019.pdf?sfvrsn=caf3489a_2 (accessed on 27 July 2020).
- Felsenstein, F.; Semar, M.; Stammler, G. Sensitivity of wheat powdery mildew (Blumeria graminis f. sp. tritici) towards metrafenone. Gesunde Pflanz 2010, 62, 29–33. [Google Scholar] [CrossRef]
- Stammler, G.; Semar, M.; Strobel, D. Resistance management of metrafenone in powdery mildews. In Modern fungicides and antifungal compounds VII. In Proceedings of the 17th International Reinhardsbrunn Symposium, Friedrichroda, Germany, 21–25 April 2013; Volume 2, pp. 179–184. [Google Scholar]
- Mikaberidze, A.; McDonald, B.A. Fitness cost of resistance: Impact on management. In Fungicide Resistance in Plant Pathogens, 1st ed.; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokio, Japan, 2015; pp. 77–89. [Google Scholar] [CrossRef]
- Anderson, J.B.; Sirjusingh, C.; Ricker, N. Haploidy, Diploidy and Evolution of Antifungal Drug Resistance in Saccharomyces cerevisiae. Genetics 2004, 168, 1915–1923. [Google Scholar] [CrossRef][Green Version]
- Graf, S. Characterisation of Metrafenone and Succinate Dehydrogenase Inhibitor Resistant Isolates of the Grapevine Powdery Mildew Erysiphe necator. Ph.D. Thesis, Technische Universität Kaiserslautern, Kaiserslautern, Germany, 19 October 2017. [Google Scholar]
- Kunova, A.; Pizzatti, C.; Bonaldi, M.; Cortesi, P. Metrafenone resistance in a population of Erysiphe necator in northern Italy. Pest Manag. Sci. 2016, 72, 398–404. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hayashi, K.; Ogawara, T. First report of the occurrence of multiple resistance to Flutianil and Pyriofenone in field isolates of Podosphaera xanthii, the causal fungus of cucumber powdery mildew. Eur. J. Plant Pathol. 2020, 156, 953–963. [Google Scholar] [CrossRef]
- Opalski, K.S.; Tresch, S.; Kogel, K.-H.; Grossmann, K.; Köhle, H.; Hückelhoven, R. Metrafenone: Studies on the mode of action of a novel cereal powdery mildew fungicide. Pest Manag. Sci. 2006, 62, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Selby, T.P.; Sternberg, C.G.; Bereznak, J.F.; Coats, R.A.; Marshall, E.A. The Discovery of Proquinazid: A New and Potent Powdery Mildew Control Agent. In Synthesis and Chemistry of Agrochemicals VII; Lyga, J.W., Theodoridis, G., Eds.; American Chemical Society: Washington, DC, USA, 2007; Volume 948, pp. 209–222. [Google Scholar]
- Corran, A.; Knauf-Beiter, G.; Zeun, R. Fungicides Acting on Signal Transduction. In Modern Crop Protection Compounds; Jeschke, P., Witschel, M., Krämer, W., Schirmer, U., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 761–783. [Google Scholar]
- Bernhard, U.; Leader, A.; Longhurst, C.; Felsenstein, F.G. Quinoxyfen—Resistance management and sensitivity monitoring in wheat: 1995–2000. Pest Manag. Sci. 2002, 58, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.S.; Wilhelm, E.; Leroux, P. Observatoire des resistances aux fongicides chez l’Oidium du ble: Resultats du groupe de travail AFPP 2001–2005. In Proceedings of the 8ème conférence internationale sur les maladies des plantes, Tours, France, 5–6 December 2006; pp. 533–544. [Google Scholar]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The Evolution of Fungicide Resistance. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 90, pp. 29–92. [Google Scholar]
- Genet, J.L.; Jaworska, G. Baseline sensitivity to proquinazid in Blumeria graminis f. sp tritici and Erysiphe necator and cross-resistance with other fungicides. Pest Manag. Sci. 2009, 65, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Hollomon, D.W.; Wheeler, I.; Dixon, K.; Longhurst, C.; Skylakakis, G. Defining the resistance risk of the new powdery mildew fungicide quinoxyfen. Pestic. Sci. 1997, 51, 347–351. [Google Scholar] [CrossRef]
- Green, E.; Duriatti, A. Sensitivity of Uncinula necator isolates to quinoxyfen: Baseline studies, validation of baseline method, and targeted sensitivity monitoring after several years of commercial use. In Proceedings of the BCPC International Congress Crop Science or Technology 2003, Glasgow, UK, 10–12 November 2003; pp. 163–168. [Google Scholar]
- Wilcox, W.F.; Riegel, D.G. Evaluation of Fungicide Programs for Control of Grapevine Powdery Mildew, 2011; Rep6SMF044; Plant Management Network: New York, NY, USA, 2012. [Google Scholar]
- Wilcox, W.F.; Riegel, D.G. Evaluation of Fungicide Programs for Control of Powdery Mildew on Chardonnay Grapes, 2010; Rep6SMF049; Plant Management Network: New York, NY, USA, 2012. [Google Scholar]
- Wilcox, W.F.; Riegel, D.G. Evaluation of Fungicide Programs for Control of Grapevine Powdery Mildew, 2010; Rep6SMF048; Plant Management Network: New York, NY, USA, 2012. [Google Scholar]
- Colcol, J.F.; Baudoin, A.B. Sensitivity of Erysiphe necator and Plasmopara viticola in Virginia to QoI fungicides, boscalid, quinoxyfen, thiophanate methyl, and mefenoxam. Plant Dis. 2016, 100, 337–344. [Google Scholar] [CrossRef]
- Feng, X.; Nita, M.; Baudoin, A.B. Evaluation of quinoxyfen resistance of Erysiphe necator (grape powdery mildew) in a single Virginia vineyard. Plant Dis. 2018, 102, 2586–2594. [Google Scholar] [CrossRef]
- Davey, J.; McGrath, M.T. Baseline sensitivity of cucurbit powdery mildew (Podosphaera xanthii) to the fungicide quinoxyfen in New York State. Resist. Pest Manag. Newsl. 2006, 15, 47–49. [Google Scholar]
- Davey, J.F.; McGrath, M.T. Sensitivity to the fungicide quinoxyfen of powdery mildew isolates collected from pumpkin in New York in 2004. Phytopathology 2006, 96, 177. [Google Scholar]
- Miazzi, M.; McGrath, M.T. Sensitivity of Podosphaera xanthii to registered fungicides and experimental in GA and NY, USA, in 2007. J. Plant Pathol. 2008, 90, 90. [Google Scholar]
- McGrath, M.T. Efficacy of fungicides with resistance risk for cucurbit powdery mildew and fungicide sensitivity of Podosphaera xanthii in New York (Abstr.). Phytopathology 2013, 103, 93. [Google Scholar]
- McGrath, M.T. First report of resistance to quinoxyfen in Podosphaera xanthii, causal agent of cucurbit powdery mildew, in the United States. Plant Health Prog. 2017, 18, 94. [Google Scholar] [CrossRef]
- Wheeler, I.E.; Hollomon, D.W.; Gustafson, G.; Mitchell, J.C.; Longhurst, C.; Zhang, Z.; Gurr, S.J. Quinoxyfen perturbs signal transduction in barley powdery mildew (Blumeria graminis f.sp. hordei). Mol. Plant Pathol. 2003, 4, 177–186. [Google Scholar] [CrossRef]
- Lee, S.; Gustafson, G.; Skamnioti, P.; Baloch, R.; Gurr, S. Host perception and signal transduction studies in wild-type Blumeria graminis f. sp. hordei and a quinoxyfen-resistant mutant implicate quinoxyfen in the inhibition of serine esterase activity. Pest. Manag. Sci. 2008, 64, 544–555. [Google Scholar] [CrossRef]
- Bent, K.J.; Cole, A.M.; Turner, J.A.W.; Woolner, M. Resistance of cucumber powdery mildew to dimethirimol. In Proceedings of the 6th British Insecticide and Fungicide Conference, Brighton, UK, 15–18 November 1971; Volume 1, pp. 274–282. [Google Scholar]
- Schepers, H.T.A.M. Persistence of resistance to fungicides in Sphaerotheca fuliginea. Neth. J. Plant Pathol. 1984, 90, 165–171. [Google Scholar] [CrossRef]
- Hollomon, D.W. Behaviour of a barley powdery mildew strain tolerant to ethirimol. In Proceedings of the 8th British Insecticide and Fungicide Conference, Brighton, UK, 17–20 November 1975; Volume 1, pp. 51–58. [Google Scholar]
- Walmsley-Woodward, D.J.; Laws, F.A.; Whittington, W.J. The characteristics of isolates of Erysiphe graminis f. sp. hordei varying in response to tridemorph and ethirimol. Ann. Appl. Biol. 1979, 92, 211–219. [Google Scholar] [CrossRef]
- Hollomon, D.W. Competitive ability and ethirimol sensitivity in strains of barley powdery mildew. Ann. Appl. Biol. 1978, 90, 195–204. [Google Scholar] [CrossRef]
- Malathrakis, N.E. Fungicide resistance in diseases of vegetables growing under plastic in Crete. EPPO Bull. 1986, 16, 429–435. [Google Scholar] [CrossRef]
- O’Brien, R.; Vawdrey, L.; Glass, R. Fungicide resistance in cucurbit powdery mildew (Sphaerotheca fuliginea) and its effect on field control. Aust. J. Exp. Agric. 1988, 28, 417. [Google Scholar] [CrossRef]
- Moustafa, M.S.H.; Abd-el-Shahid, Y.A.; Ez-Eldin, I.; Anwar, H.M. Occurrence of acquired resistance to fungicides in Erysiphe cichoracearum, the causal organism of cucumber powdery mildew. Agric. Res. Rev. 1990, 68, 513–520. [Google Scholar]
- Moustafa, M.S.H.; Abd-el-Shahid, Y.A.; Ez-Eldin, I.; Anwar, H.M. Factors affecting acquired resistance in Erysiphe cichoracearum the causal organism of cucumber powdery mildew. Agric. Res. Rev. 1990, 68, 521–528. [Google Scholar]
- Bellón-Gómez, D.; Vela-Corcía, D.; Pérez-García, A.; Torés, J.A. Sensitivity of Podosphaera xanthii populations to anti-powdery-mildew fungicides in Spain. Pest. Manag. Sci. 2015, 71, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Hollomon, D.W. Evidence that ethirimol may interfere with adenine metabolism during primary infection of barley powdery mildew. Pestic. Biochem. Physiol. 1979, 10, 181–189. [Google Scholar] [CrossRef]
- Schroeder, W.T.; Provvidenti, R. Systemic control of powdery mildew on cucurbits with fungicide 1991 applied as soil drenches and seed treatments. Plant Dis. Rep. 1968, 52, 630–632. [Google Scholar]
- McGrath, M.T. Increased resistance to triadimefon and to benomyl in Sphaerotheca fuliginea populations following fungicide usage over one season. Plant Dis. 1996, 80, 633. [Google Scholar] [CrossRef]
- McGrath, M.T.; Shishkoff, N. Fungicide sensitivity of Sphaerotheca fuliginea populations in the United States. Plant Dis. 1996, 80, 697–703. [Google Scholar] [CrossRef]
- O’Brien, R.G. Fungicide resistance in populations of cucurbit powdery mildew (Sphaerotheca fuliginea). N. Z. J. Crop Hortic. Sci. 1994, 22, 145–149. [Google Scholar] [CrossRef]
- Sedláková, B.; Lebeda, A. Resistance to fungicides in cucurbit powdery mildew populations in the Czech Republic. Acta Fytotech. Zootech. 2004, 7, 269–271. [Google Scholar]
- Sedláková, B.; Lebeda, A. Fungicide resistance in Czech populations of cucurbit powdery mildews. Phytoparasitica 2008, 36, 272–289. [Google Scholar] [CrossRef]
- Sedláková, B.; Lebeda, A.; Jerabkova, H.; Paulik, R.; Vajdova, M. Resistance to fenarimol, dinocap, benomyl, thiophanate-methyl and azoxystrobin in cucurbit powdery mildew populations in the Czech Republic. Acta Fytotech. Zootech. 2012, 15, 46–49. [Google Scholar]
- Lebeda, A.; Sedláková, B.; Pejchar, M.; Jeřábková, H. Variation for fungicide resistance among cucurbit powdery mildew populations in the Czech Republic. Acta Hortic. 2010, 871, 465–476. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Monitoring Methyl Benzimidazole Carbamate-resistant isolates of the cucurbit powdery mildew pathogen, Podosphaera xanthii, using loop-mediated isothermal amplification. Plant Dis. 2019, 103, 1515–1524. [Google Scholar] [CrossRef]
- Jarvis, W.R.; Slingsby, K. Tolerance of Botrytis cinerea and rose powdery mildew to benomyl. Can. Plant Dis. Surv. 1975, 55, 44. [Google Scholar]
- Pearson, R.C.; Taschenberg, E.F. Benomyl-resistant strains of Uncinula necator on grapes. Plant Dis. 1980, 64, 677–680. [Google Scholar] [CrossRef]
- Ypema, H.L.; Ypema, M.; Gubler, W.D. Sensitivity of Uncinula necator to benomyl, triadimefon, myclobutanil, and fenarimol in California. Plant Dis. 1997, 81, 293–297. [Google Scholar] [CrossRef]
- FRAC Code List 2020. Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2020-final.pdf?sfvrsn=8301499a_2 (accessed on 27 July 2020).
- Hawkins, N.J.; Fraaije, B.A. Predicting resistance by mutagenesis: Lessons from 45 Years of MBC resistance. Front. Microbiol. 2016, 7, 1814. [Google Scholar] [CrossRef]
- Vela-Corcía, D.; Bellón-Gómez, D.; López-Ruiz, F.; Torés, J.A.; Pérez-García, A. The Podosphaera fusca TUB2 gene, a molecular “Swiss Army knife” with multiple applications in powdery mildew research. Fungal Biol. 2014, 118, 228–241. [Google Scholar] [CrossRef]
- Dekker, J.; Gielink, A.J. Decreased sensitivity to pyrazophos of cucumber and gherkin powdery mildew. Neth. J. Plant Pathol. 1979, 85, 137–142. [Google Scholar] [CrossRef]
- Ishii, H.; Fraaije, B.A.; Sugiyama, T.; Noguchi, K.; Nishimura, K.; Takeda, T.; Amano, T.; Hollomon, D.W. Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology 2001, 91, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Heaney, S.P.; Hall, A.A.; Davies, S.A.; Olaya, G. Resistance to fungicides in the Qol-STAR cross-resistance group: Current perspectives. In Proceedings of the BCPC Conference: Pests and Diseases, Brighton, UK, 13–16 November 2000; Volume 2, pp. 755–762. [Google Scholar]
- Fernández-Ortuño, D.; Pérez-García, A.; López-Ruiz, F.; Romero, D.; de Vicente, A.; Torés, J.A. Occurrence and distribution of resistance to QoI fungicides in populations of Podosphaera fusca in south central Spain. Eur. J. Plant Pathol. 2006, 115, 215–222. [Google Scholar] [CrossRef]
- McGrath, M.T.; Shishkoff, N. First report of the cucurbit powdery mildew fungus (Podosphaera xanthii) resistant to strobilurin fungicides in the United States. Plant Dis. 2003, 87, 1007. [Google Scholar] [CrossRef]
- Ishii, H.; Yano, K.; Date, H.; Furuta, A.; Sagehashi, Y.; Yamaguchi, T.; Nishimura, K.; Hasama, W. Molecular Characterization and Diagnosis of QoI Resistance in Cucumber and Eggplant Fungal Pathogens. Phytopathology 2007, 97, 1458–1466. [Google Scholar] [CrossRef]
- Chin, K.M.; Chavaillaz, D.; Kaesbohrer, M.; Staub, T.; Felsenstein, F.G. Characterizing resistance risk of Erysiphe graminis f.sp. tritici to strobilurins. Crop Prot. 2001, 20, 87–96. [Google Scholar] [CrossRef]
- Bäumler, S.; Felsenstein, F.G.; Schwarz, G. CAPS and DHPLC analysis of a single nucleotide polymorphism in the cytochrome b gene conferring resistance to strobilurins in field isolates of Blumeria graminis f. sp. hordei. J. Phytopathol. 2003, 151, 149–152. [Google Scholar] [CrossRef]
- Wong, F.P.; Wilcox, W.F. Sensitivity to azoxystrobin among isolates of Uncinula necator: Baseline distribution and relationship to myclobutanil sensitivity. Plant Dis. 2002, 86, 394–404. [Google Scholar] [CrossRef][Green Version]
- Miller, T.C.; Gubler, W.D. Sensitivity of California isolates of Uncinula necator to trifloxystrobin and spiroxamine, and update on triadimefon sensitivity. Plant Dis. 2004, 88, 1205–1212. [Google Scholar] [CrossRef][Green Version]
- Miles, L.A.; Miles, T.D.; Kirk, W.W.; Schilder, A.M.C. Strobilurin (QoI) resistance in populations of Erysiphe necator on grapes in Michigan. Plant Dis. 2012, 96, 1621–1628. [Google Scholar] [CrossRef]
- Baudoin, A.; Olaya, G.; Delmotte, F.; Colcol, J.F.; Sierotzki, H. QoI Resistance of Plasmopara viticola and Erysiphe necator in the Mid-Atlantic United States. Plant Health Prog. 2008, 9, 25. [Google Scholar] [CrossRef]
- Ghule, S.B.; Sawant, I.S.; Sawant, S.D.; Saha, S.; Devarumath, R.M. Detection of G143A mutation in Erysiphe necator and its implications for powdery mildew management in grapes. Indian J. Hortic. 2018, 75, 434. [Google Scholar] [CrossRef]
- Beresford, R.M.; Wright, P.J.; Wood, P.N.; Agnew, R.H. Sensitivity of grapevine powdery mildew (Erysiphe necator) to demethylation inhibitor and quinone outside inhibitor fungicides in New Zealand. N. Z. Plant Prot. 2016, 69, 1–10. [Google Scholar] [CrossRef]
- Rallos, L.E.E.; Johnson, N.G.; Schmale, D.G.; Prussin, A.J.; Baudoin, A. Fitness of Erysiphe necator with G143A-based resistance to quinone outside Inhibitors. Plant Dis. 2014, 98, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.D.; Neher, O.T. First report of QoI-insensitive powdery mildew (Erysiphe polygoni) on sugar beet in the United States. Plant Dis. 2014, 98, 1004. [Google Scholar] [CrossRef]
- Heick, T.M.; Hansen, A.L.; Justesen, A.F.; Jørgensen, L.N. QoI resistance in sugar beet powdery mildew (Erysiphe betae) in Scandinavia. Plant Health Prog. 2019, 20, 179. [Google Scholar] [CrossRef]
- Lesemann, S.S.; Schimpke, S.; Dunemann, F.; Deising, H.B. Mitochondrial heteroplasmy for the cytochrome b gene controls the level of strobilurin resistance in the apple powdery mildew fungus Podosphaera leucotricha (Ell. & Ev.) E.S. Salmon. J. Plant Dis. Prot. 2006, 113, 259–266. [Google Scholar] [CrossRef]
- Mosquera, S.; Chen, L.-H.; Aegerter, B.; Miyao, E.; Salvucci, A.; Chang, T.-C.; Epstein, L.; Stergiopoulos, I. Cloning of the cytochrome b gene from the tomato powdery mildew fungus Leveillula taurica reveals high levels of allelic variation and heteroplasmy for the G143A mutation. Front. Microbiol. 2019, 10, 663. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A. Mechanisms of resistance to QoI fungicides in phytopathogenic fungi. Int. Microbiol. 2008, 11, 1–9. [Google Scholar] [CrossRef]
- Lucas, J. Resistance to Qol fungicides: Implications for cereal disease management in Europe. Pestic. Outlook 2003, 14, 268. [Google Scholar] [CrossRef]
- Sierotzki, H.; Wullschleger, J.; Gisi, U. Point Mutation in Cytochrome b Gene Conferring Resistance to Strobilurin Fungicides in Erysiphe graminis f. sp. tritici Field Isolates. Pestic. Biochem. Physiol. 2000, 68, 107–112. [Google Scholar] [CrossRef]
- Fraaije, B.A.; Butters, J.A.; Coelho, J.M.; Jones, D.R.; Hollomon, D.W. Following the dynamics of strobilurin resistance in Blumeria graminis f. sp. tritici using quantitative allele-specific real-time PCR measurements with the fluorescent dye SYBR Green, I. Plant Pathol. 2002, 51, 45–54. [Google Scholar] [CrossRef]
- Gisi, U.; Sierotzki, H.; Cook, A.; McCaffery, A. Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Manag. Sci. 2002, 58, 859–867. [Google Scholar] [CrossRef]
- Robinson, H.L.; Ridout, C.J.; Sierotzki, H.; Gisi, U.; Brown, J.K.M. Isogamous, hermaphroditic inheritance of mitochondrion-encoded resistance to Qo inhibitor fungicides in Blumeria graminis f. sp. tritici. Fungal Genet. Biol. 2002, 36, 98–106. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; Bellón-Gómez, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Heteroplasmy for the Cytochrome b Gene in Podosphaera xanthii and its Role in Resistance to QoI Fungicides in Spain. Plant Dis. 2018, 102, 1599–1605. [Google Scholar] [CrossRef]
- Koch, A.; Felsenstein, F.; Stammler, G. No evidence of QoI resistance in apple powdery mildew (Podosphaera leucotricha). J. Phytopathol. 2015, 163, 178–184. [Google Scholar] [CrossRef]
- Stevenson, K.L.; McGrath, M.T.; Wyenandt, C.A. Fungicide Resistance in North America, 2nd ed.; American Phytopathological Society Press: St. Paul, MN, USA, 2019. [Google Scholar]
- Brown, J.K.M.; Slater, S.E.; See, K.A. Sensitivity of Erysiphe graminis f.sp. hordei to morpholine and piperidine fungicides. Crop Prot. 1991, 10, 445–454. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Wolfe, M.S. Insensitivity of Erysiphe graminis f. sp. hordei to triadimefon, triadimenol and other fungicides. In 1981 British Crop Protection Conference: Pests and Diseases, Proceedings of the 11th British Insecticide and Fungicide Conference, Brighton, UK, 16–19 November 1981; British Crop Production Council: Alton, UK, 1981. [Google Scholar]
- Limpert, E.; Schwarzbach, E. Virulence analysis of powdery mildew of barley in different European regions in 1979 and 1980. In Barley Genetics IV: Proceedings of the Fourth International Barley Genetics Symposium, Proceedings of the Fourth International Barley Genetics Symposium, Edinburgh, UK, 22–29 July 1981; Edinburgh University Press: Edinburgh, UK, 1981. [Google Scholar]
- Buchenauer, H.; Hellwald, K.H. Resistance of Erysiphe graminis on barley and wheat to sterol C-14-demethylation inhibitors. EPPO Bull. 1985, 15, 459–466. [Google Scholar] [CrossRef]
- Robertson, S.; Gilmour, J.; Newman, D.; Lennard, J.H. Sensitivity of barley powdery mildew isolates to morpholine fungicides. In Brighton Crop Protection Conference, Pests and Diseases; British Crop Protection Council: Alton, UK, 1990; Volume 3, pp. 1159–1162. [Google Scholar]
- Brown, J.K.M.; Wolfe, M.S. Levels of resistance of Erysiphe graminis f. sp. hordei to the systemic fungicide triadimenol. Neth. J. Plant Pathol. 1991, 97, 251–263. [Google Scholar] [CrossRef]
- Sykora, M.; Miklovicova, M.; Krippel, E. The effect of selected fungicides on the sensitivity of the population of powdery mildew in spring barley in 1992. Agrochemia 1993, 33, 263–265. [Google Scholar]
- Sykora, M.; Krippel, E.; Miklovičová, M.; Plesník, S. Sensitivity of barley powdery mildew (Erysiphe graminis f. sp. hordei) populations against some fungicidal substances in central. J. Plant Dis. Prot. 1995, 102, 211–214. [Google Scholar]
- Sykora, M.; Krippel, E. Virulence genes analysis and fungicide sensitivity of barley powdery mildews population (Erysiphe graminis f. sp. hordei) in Hungary in 1993. Novtermel. Hung. 1994, 43, 379–385. [Google Scholar]
- Blatter, R.H.E.; Brown, J.K.M.; Wolfe, M. Genetic control of the resistance of Erysiphe graminis f.sp. hordei to five triazole fungicides. Plant Pathol. 1998, 47, 570–579. [Google Scholar] [CrossRef]
- Napier, B.A.S.; Bayles, R.A.; Stigwood, P.L.; Burnett, F.J. Sensitivity of powdery mildew and yellow rust to DMI, morpholine and strobilurin fungicides in England and Scotland. In The BCPC Conference: Pests and Disease, Proceedings of the BCPC Conference: Pests and Diseases, Brighton, UK, 13–16 November 2000; British Crop Protection Council: Alton, UK, 2000; Volume 1, pp. 427–434. [Google Scholar]
- Reis, E.M.; Zanatta, M.; Brustolin, F.; Danelli, A.L.D. Sensitivity reduction in Blumeria graminis f. sp. hordei to triadimenol fungicide applied as barley seed treatment. Summa Phytopathol. 2013, 39, 276–280. [Google Scholar] [CrossRef][Green Version]
- Waard, M.A.; Kipp, E.M.C.; Horn, N.M.; Nistelrooy, J.G.M. Variation in sensitivity to fungicides which inhibit ergosterol biosynthesis in wheat powdery mildew. Neth. J. Plant Pathol. 1986, 92, 21–32. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, Y.L.; Duan, X.Y.; Liu, X.M. Monitoring of Blumeria graminis f. sp. tritici isolates to triadimefon in 2002 and establishment of pathogen baseline sensitivity to kresoxim-methyl. Acta Phytopathol. Sin. 2002, 35, 74–78. [Google Scholar]
- Al-Mughrabi, K.I.; Gray, A.B. Build-up of resistance to triadimefon for isolates of Erysiphe graminis f.sp. tritici from Nova Scotia, Canada. Can. Plant Dis. Surv. 1996, 76, 9–14. [Google Scholar]
- Svec, M.; Miklovičová, M.; Sýkora, M.; Krippel, E. Fungicide sensitivity of populations of wheat powdery mildew (Erysiphe graminis f.sp. tritici) in Central Europe in 1993. Pestic. Sci. 1995, 43, 47–52. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, W.; Zhou, Y.; Duan, X.; Zhang, Y.; Ding, K. Monitoring of resistance of Blumeria graminis f.sp. tritici isolates to triadimefon in 2007. Acta Phytopathol. Sin. 2008, 35, 74–78. [Google Scholar]
- Reis, E.M.; Basso, D.F.; Zanatta, M. Loss of sensitivity of Blumeria graminis f. sp. tritici to triadimenol applied as seed treatment. Trop. Plant Pathol. 2013, 38, 55–57. [Google Scholar] [CrossRef][Green Version]
- Schepers, H.T.A.M. Decreased sensitivity of Sphaerotheca fuliginea to fungicides which inhibit ergosterol biosynthesis. Neth. J. Plant Pathol. 1983, 89, 185–187. [Google Scholar] [CrossRef]
- Schepers, H.T.A.M. Changes during a three-year period in the sensitivity to ergosterol biosynthesis inhibitors of Sphaerotheca fuliginea in the Netherlands. Neth. J. Plant Pathol. 1985, 91, 105–118. [Google Scholar] [CrossRef]
- Huggenberger, F.; Collins, M.A.; Skylakakis, G. Decreased sensitivity of Sphaerotheca fuliginea to fenarimol and other ergosterol-biosynthesis inhibitors. Crop Prot. 1984, 3, 137–149. [Google Scholar] [CrossRef]
- McGrath, M.T. Reduced effectiveness of triadimefon for controlling cucurbit powdery mildew associated with fungicide resistance in Sphaerotheca fuliginea. Phytopathology 1991, 81, 1191. [Google Scholar]
- Ohtsuka, N.; Sou, K.; Amano, T.; Ojima, M.; Nakazawa, Y.; Yamada, Y. Decreased sensitivity of cucumber powdery mildew (Sphaerotheca fuliginea) to ergosterol biosynthesis inhibitors. Jpn. J. Phytopathol. 1988, 54, 629–632. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hong, S.S.; Lim, J.W.; Park, K.Y.; Kim, H.G. Screening of fungicide resistance of cucumber powdery mildew pathogen, Sphaerotheca fusca in Gyeonggi province. Res. Plant Dis. 2008, 14, 95–101. [Google Scholar] [CrossRef][Green Version]
- López-Ruiz, F.J.; Pérez-García, A.; Fernández-Ortuño, D.; Romero, D.; García, E.; de Vicente, A.; Brown, J.K.; Torés, J.A. Sensitivities to DMI fungicides in populations of Podosphaera fusca in south central Spain. Pest Manag. Sci. 2010, 66, 801–808. [Google Scholar] [CrossRef]
- McGrath, M.T. Fungicide sensitivity in Podosphaera xanthii and efficacy for cucurbit powdery mildew in NY, USA, in 2003–2006. J. Plant Pathol. 2008, 90, 90. [Google Scholar]
- McGrath, M.T.; Shishkoff, N. Resistance to triadimefon and benomyl: Dynamics and impact on managing cucurbit powdery mildew. Plant Dis. 2001, 85, 147–154. [Google Scholar] [CrossRef]
- Schepers, H.T.A.M. Fitness of isolates of Sphaerotheca fuliginea resistant or sensitive to fungicides which inhibit ergosterol biosynthesis. Neth. J. Plant Pathol. 1985, 91, 65–76. [Google Scholar] [CrossRef]
- McGrath, M.T. Podosphaera xanthii strains with resistance and reduced sensitivity to several fungicides may challenge control of cucurbit powdery mildew. Phytopathology 2016, 106, 74. [Google Scholar]
- Gubler, W.D.; Ypema, H.L.; Ouimette, D.G.; Bettiga, L.J. Occurrence of resistance in Uncinula necator to triadimefon, myclobutanil, and fenarimol in California grapevines. Plant Dis. 1996, 80, 902–909. [Google Scholar] [CrossRef]
- Steva, H.; Clerjeau, M. Cross resistance to sterol biosynthesis inhibitor fungicides in strains of Uncinula necator isolated in France and Portugal. Meded. Fac. Mededelingen Faculteit Landbouwwetenschappen Rijksuniversiteit Gent 1990, 55, 983–988. [Google Scholar]
- Erickson, E.O.; Wilcox, W.F. Distributions of sensitivities to three sterol Demethylation Inhibitor fungicides among populations of Uncinula necator sensitive and resistant to triadimefon. Phytopathology 1997, 87, 784–791. [Google Scholar] [CrossRef]
- Halleen, F.; Holz, G.; Pringle, K.L. Resistance in Uncinula necator to Triazole Fungicides in South African Grapevines. S. Afr. J. Enol. Vitic. 2000, 21. [Google Scholar] [CrossRef]
- Savocchia, S.; Stummer, B.E.; Wicks, T.J.; van Heeswijck, R.; Scott, E.S. Reduced sensitivity of Uncinula necator to sterol demethylation inhibiting fungicides in southern Australian vineyards. Australas. Plant Pathol. 2004, 33, 465. [Google Scholar] [CrossRef]
- Steinkellner, S.; Reld, H. Sensitivity of Uncinula necator populations following DMI-fungicide usage in Austrian vineyards. Bond 2001, 52, 213–219. [Google Scholar]
- Northover, J.; Homeyer, C.A. Detection and management of myclobutanil-resistant grapevine powdery mildew (Uncinula necator) in Ontario. Can. J. Plant Pathol. 2001, 23, 337–345. [Google Scholar] [CrossRef]
- Ghule, S.B.; Sawant, I.S.; Sawant, S.D.; Saha, S.; Devarumath, R.M. Detection of resistance to demethylation inhibitor fungicides in Erysiphe necator from tropical India by biological and molecular assays. Indian Phytopathol. 2019, 72, 53–61. [Google Scholar] [CrossRef]
- Hajian Shahri, M.; Abbaspoor, M.; Gazanchian, A.; Mokhtarian, A. Occurrence of resistance in grapevine powdery mildew (Erysiphe necator) to penconazole and hexaconazole in Khorasan Razavi province. J. Plant Prot. Agric. Sci. Technol. 2012, 26, 55–63. [Google Scholar]
- Colcol, J.F.; Rallos, L.E.; Baudoin, A.B. Sensitivity of Erysiphe necator to Demethylation Inhibitor fungicides in Virginia. Plant Dis. 2012, 96, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Sombardier, A.; Dufour, M.C.; Blancard, D.; Corio-Costet, M.-F. Sensitivity of Podosphaera aphanis isolates to DMI fungicides: Distribution and reduced cross-sensitivity. Pest Manag. Sci. 2010, 66, 35–43. [Google Scholar] [CrossRef]
- Délye, C.; Bousset, L.; Corio-Costet, M.F. PCR cloning and detection of point mutations in the eburicol 14 α-demethylase (CYP51) gene from Erysiphe graminis f. sp. hordei, a “recalcitrant” fungus. Curr. Genet. 1998, 34, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Wyand, R.A.; Brown, J.K.M. Sequence variation in the CYP51 gene of Blumeria graminis associated with resistance to sterol demethylase inhibiting fungicides. Fungal Genet. Biol. 2005, 42, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.A.; Lopez-Ruiz, F.; Jayasena, K.; Oliver, R.P. Origin of fungicide-resistant barley powdery mildew in Western Australia: Lessons to be learned. In Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokyo, Japan, 2015; pp. 329–340. [Google Scholar]
- Tucker, M.A.; Lopez-Ruiz, F.; Cools, H.J.; Mullins, J.G.L.; Jayasena, K.; Oliver, R.P. Analysis of mutations in West Australian populations of Blumeria graminis f. sp. hordei CYP51 conferring resistance to DMI fungicides. Pest Manag. Sci. 2019. [Google Scholar] [CrossRef]
- Zulak, K.G.; Cox, B.A.; Tucker, M.A.; Oliver, R.P.; Lopez-Ruiz, F.J. Improved detection and monitoring of fungicide resistance in Blumeria graminis f. sp. hordei with high-throughput genotype quantification by digital PCR. Front. Microbiol. 2018, 9, 706. [Google Scholar] [CrossRef]
- Délye, C.; Laigret, F.; Corio-Costet, M.F. A mutation in the 14α-Demethylase gene of Uncinula necator that correlates with resistance to a sterol biosynthesis inhibitor. Appl. Environ. Microbiol. 1997, 63, 2966–2970. [Google Scholar] [CrossRef]
- Pintye, A.; Németh, M.Z.; Molnár, O.; Horváth, Á.N.; Spitzmüller, Z.; Szalóki, N.; Pál, K.; Váczy, K.Z.; Kovács, G.M. Improved DNA extraction and quantitative real-time PCR for genotyping Erysiphe necator and detecting the DMI fungicide resistance marker A495T, using single ascocarps. Phytopathol. Mediterr. 2020, 59, 97–106. [Google Scholar] [CrossRef]
- Sierotzki, H.; Scalliet, G. A Review of Current Knowledge of Resistance Aspects for the Next-Generation Succinate Dehydrogenase Inhibitor Fungicides. Phytopathology 2013, 103, 880–887. [Google Scholar] [CrossRef]
- McGrath, M.T.; Wyenandt, C.A. First detection of boscalid resistance in Podosphaera xanthii in the United States associated with failure to control cucurbit powdery mildew in New York and New Jersey in 2009. Plant Health Prog. 2017, 18, 93. [Google Scholar] [CrossRef]
- Keinath, A.P.; Rennberger, G.; Kousik, C.S. First report of resistance to boscalid in Podosphaera xanthii, cucurbit powdery mildew, in South Carolina. Plant Health Prog. 2018, 19, 220–221. [Google Scholar] [CrossRef]
- Miyamoto, T.; Ishii, H.; Tomita, Y. Occurrence of boscalid resistance in cucumber powdery mildew in Japan and molecular characterization of the iron–sulfur protein of succinate dehydrogenase of the causal fungus. J. Gen. Plant Pathol. 2010, 76, 261–267. [Google Scholar] [CrossRef]
- Ishii, H.; Miyamoto, T.; Ushio, S.; Kakishima, M. Lack of cross-resistance to a novel succinate dehydrogenase inhibitor, fluopyram, in highly boscalid-resistant isolates of Corynespora cassiicola and Podosphaera xanthii. Pest. Manag. Sci. 2011, 67, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Hayashi, K.; Okada, R.; Wari, D.; Ogawara, T. Resistance to succinate dehydrogenase inhibitors in field isolates of Podosphaera xanthii on cucumber: Monitoring, cross-resistance patterns and molecular characterization. Pestic. Biochem. Phys. 2020. [Google Scholar] [CrossRef]
- Cherrad, S.; Hernandez, C.; Vacher, S.; Steva, H. First Detection of Boscalid-Resistant Strains of Erysiphe necator in French Vineyards: Biological and Molecular Characterization. In Modern Fungicides and Antifungal Compounds; Deising, H.B., Fraaije, B., Mehl, A., Oerke, E.C., Sierotzki, H., Stammler, G., Eds.; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2017; Volume 8, pp. 211–216. [Google Scholar]
- Ruprecht, J.; Yankovskaya, V.; Maklashina, E.; Iwata, S.; Cecchini, G. Structure of Escherichia coli Succinate:Quinone Oxidoreductase with an Occupied and Empty Quinone-binding Site. J. Biol. Chem. 2009, 284, 29836–29846. [Google Scholar] [CrossRef]
- Hägerhäll, C. Succinate: Quinone oxidoreductases. Biochim. Biophys. Acta BBA—Bioenerg. 1997, 1320, 107–141. [Google Scholar] [CrossRef]
- Cherrad, S.; Charnay, A.; Hernandez, C.; Steva, H.; Belbahri, L.; Vacher, S. Emergence of boscalid-resistant strains of Erysiphe necator in French vineyards. Microbiol. Res. 2018, 216, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J. Recently introduced powdery mildew fungicides. In Modern Crop Protection Compounds; Krämer, W., Schrimer, U., Eds.; Wiley-VCH: Weinheim, Germany, 2007; Volume 2, pp. 727–738. [Google Scholar]
- Pirondi, A.; Nanni, I.M.; Brunelli, A.; Collina, M. First report of resistance to cyflufenamid in Podosphaera xanthii, causal agent of powdery mildew, from melon and zucchini fields in Italy. Plant Dis. 2014, 98, 1581. [Google Scholar] [CrossRef]
- Mike, A.; Quesada-Ocampo, L.M. Evaluation of fungicides for control of powdery mildew of winter squash, Cleveland 2016. Plant Dis. Manag. Rep. 2017, 11, 112. [Google Scholar]
- McGrath, M.T.; Sexton, Z.F. Poor control of cucurbit powdery mildew associated with first detection of resistance to cyflufenamid in the causal agent, Podosphaera xanthii, in the United States. Plant Health Prog. 2018, 19, 222–223. [Google Scholar] [CrossRef]
- Wise, K.A.; Smith, D.; Freije, A.; Mueller, D.S.; Kandel, Y.; Allen, T.; Bradley, C.A.; Byamukama, E.; Chilvers, M.; Faske, T.; et al. Meta-analysis of yield response of foliar fungicide-treated hybrid corn in the United States and Ontario, Canada. PLoS ONE 2019, 14, e0217510. [Google Scholar] [CrossRef] [PubMed]
- Hollomon, D.W. Fungicide Resistance: 40 Years on and Still a Major Problem. In Fungicide Resistance in Plant Pathogens, 1st ed.; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokio, Japan, 2015; pp. 3–11. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance: The Assessment of Risk, 2nd ed.; Global Crop Protection Federation: Brussels, Belgium, 1998; pp. 1–48. [Google Scholar]
- Corio-Costet, M.F. Monitoring resistance in obligate pathogens by bioassays relating to field use: Grapevine powdery and downy mildews. In Fungicide Resistance in Plant Pathogens, 1st ed.; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokio, Japan, 2015; pp. 251–279. [Google Scholar] [CrossRef]
- Niessen, L. Current state and future perspectives of loop-mediated isothermal amplification (LAMP)-based diagnosis of filamentous fungi and yeasts. Appl. Microbiol. Biotechnol. 2015, 99, 553–574. [Google Scholar] [CrossRef] [PubMed]

| Mode of Action | Target | Group Name | Active Ingredient | Authorized Countries 1 | FRAC Code |
|---|---|---|---|---|---|
| 1- MULTI-SITE INHIBITORS | |||||
| Multi-site activity | Multi-site contact activity | Chloronitriles | Chlorothalonil | BG, BR, CA, CL, CO, CR, CZ, EE, FR, IE, IL, JP, LV, LT, MA, MX, NL, PL, RS, RU, SI, SK, TN, TR, UA, UY, VN | M05 |
| Dithiocarbamates and relatives | Mancozeb | BR, CO, HR, EC, IN, JP, NL, PE, PK, TR, UA, UZ, VN | M03 | ||
| Metiram | BG, BR, CZ, FR, HR, IT, MD, PT, RO, SI, SK, TR, UA, ZA | ||||
| Propineb | KZ, VN | ||||
| Zineb | VN | ||||
| Ziram | AR | ||||
| Phthalimides | Captan | MX, UA | M04 | ||
| Folpet | AR, BE, BR, BG, CA, CH, CL, DK, EE, ES, FR, GR, HR, IT, LT, MD, MX, NL, PL, PT, RO, RS, SK, TR, UA, UY, VN | ||||
| 2- SITE-SPECIFIC FUNGICIDES | |||||
| Amino acids and protein synthesis | Methionine biosynthesis (proposed cgs gene) | Anilinopyrimidines | Cyprodinil | AT, BE, BG, CA, CH, CL, CZ, DE, FR, IE, JP, NL, PE, PL, PT, RU, SK, UA, ZA | 9 |
| Mepanipyrim | BE, CL, JP, NL | ||||
| Pyrimethanil | CA, CL, MD, PE, RU, UA | ||||
| Cytoskeleton and motor protein | Actin/myosin/fimbrin function | Aryl-phenyl-ketones | Metrafenone | AT, BE, BG, BY, CA, CH, CL, CZ, DE, EC, EE, ES, FR, GR, HR, IN, IE, IL, IT, LT, LV, MD, MA, NL, PE, PL, PT, RO, RU, SK, SI, TN, TR, UA, ZA | 50 |
| Pyriofenone | AT, BE, BG, CA, CL, DE, EE, ES, FI, FR, GR, HR, HU, IE, IL, IT, JP, LV, LT, MD, NL, PE, PL, PT, RS, SI, TR | ||||
| ß-tubulin assembly in mitosis | Methyl-Benzimidazole Carbamates (MBC fungicides) | Benomyl | CL, CR, JP, MX, PE, RU, UA, UZ, ZA | 1 | |
| Carbendazim | AR, BR, BY, CL, CR, IN, MX, PE, PK, PY, RU, TN, UA, VN, ZA | ||||
| Fuberidazole | DE | ||||
| Thiabendazole | BY, MD, PE, RU, UA, UY | ||||
| Thiophanate | PK | ||||
| Thiophanate-methyl | AR, BG, BR, BY, CL, CZ, ES, HR, FR, ID, IN, IT, JP, LT, LV, MA, MD, MX, NL, PE, PK, PL, RO, RS, RU, SI, SK, TN, TR, TZ, UA, UY, ZA | ||||
| Membrane integrity or function | Phospholipid biosynthesis, methyltransferase | Dithiolanes | Isoprothiolane | PE | 6 |
| Nucleic acid metabolism | Adenosin-deaminase | Hydroxy-(2-amino) pyrimidines | Bupirimate | CH, EC, ES, FR, GR, HR, IL, IT, MA, NL, PK, PE, PT, TN, TR, ZA | 8 |
| Respiration | Complex II: succinate-dehydrogenase (SdhB, SdhC and SdhD) | Succinate dehydrogenase inhibitors (SDHIs) | Benzovindiflupyr | CA, CH | 7 |
| Bixafen | AT, BE, BR, CH, CL, CZ, DE, EE, ES, FI, FR, IE, IT, LT, LV, MD, NL, PL, PT, SI, SK, TR, UA, ZA | ||||
| Boscalid | AR, AT, BE, BG, CA, CH, CL, CZ, DE, DK, EC, EE, ES, FR, HR, IE, IL, IN, IT, LT, LV, MA, MD, NL, NZ, PE, PL, PT, SI, SK, TN, TR, UA, UY, ZA | ||||
| Carboxin | BY, UA | ||||
| Fluopyram | AT, BE, BG, BY, CA, CH, CL, CR, CZ, DE, EE, ES, FI, FR, GR, HR, HU, IE, IL, IN, IT, JP, LT, LV, MA, MD, NL, PE, PL, PT, RS, RU, SI, SK, TN, TR, UA, ZA | ||||
| Fluxapyroxad | AT, BE, BY, BG, CA, CH, CL, CZ, DE, EE, ES, FR, GR, IE, IT, LT, LV, MD, NL, NZ, PL, PT, RU, RS, SI, SK, TN, TR, UA, ZA | ||||
| Isofetamid | JP | ||||
| Isopyrazam | AT, BE, BG, CL, CZ, DE, ES, GR, HR, IE, IT, JP, NL, PL, PT, RS, RU, SI, SK, TR, UA | ||||
| Penthiopyrad | BG, BY, CH, CZ, GR, JP, NL, PL, RU, UA | ||||
| Pydiflumetofen | NZ | ||||
| Pyraziflumid | JP | ||||
| Sedaxane | UK | ||||
| Complex III: cytochrome bc1 at Qo site (Cytb) | Quinone outside inhibitors (QoIs) | Azoxystrobin | AR, AT, BE, BR, BG, BY, CA, CH, CL, CZ, DE, EC, EE, ES, FI, FR, DE, GR, HR, HU, IN, IE, IL, IN, IT, JP, LT, LV, MA, MD, MY, MX, NL, PE, PL, PT, RU, RS, SK, SI, TN, TR, UA, UY, ZA | 11 | |
| Dimoxystrobin | PL, SK | ||||
| Famoxadone | BG | ||||
| Fluoxastrobin | AT, BE, BY, CA, CH, CL, CZ, DE, EC, EE, FR, IE, LT, LV, NL, PL, RU, SK, TR, UA | ||||
| Kresoxim-methyl | AR, AT, BE, BG, BR, BY, CH, CL, CZ, DE, EC, EE, ES, FR, GR, HR, IE, IL, IN, IT, JP, LT, LV, MA, MD, MY, NL, PE, PL, PT, PY, RU, RS, SI, SK, TN, TR, UA, UY, VN, ZA | ||||
| Mandestrobin | CA, JP | ||||
| Metominostrobin | IL, PE | ||||
| Picoxystrobin | BR, BY, CA, CH, IL, IN, RU, TR, UA, ZA | ||||
| Pyraclostrobin | AR, AT, BE, BG, BO, BR, BY, CA, CH, CL, CZ, DE, EE, ES, FI, FR, GR, HR, IE, IL, IN, IT, LT, LV, MD, NL, NZ, PE, PL, PT, RO, RS, RU, SI, SK, TN, TR, UA, UY, ZA | ||||
| Trifloxystrobin | AR, AT, BE, BG, BR, CA, CH, CL, CR, CZ, DE, EC, ES, FR, GR, HR, HU, IE, IL, IN, IT, MA, MD, MX, NL, PE, PK, PL, PT, PY, RS, RU, SI, SK, TN, TR, UA, UY, VN, ZA | ||||
| Uncouplers of oxidative phosphorylation | 2,6-Dinitro-anilines | Fluazinam | BR, CH | 29 | |
| Dinitrophenyl-crotonates | Dinocap | IN, MX | |||
| Meptyldinocap | AT, CL, CZ, ES, FR, GR, HR, IL, IT, MA, PE, PT, RS, SK, SI, TN, TR | ||||
| Signal transduction | Signal transduction (mechanism unknown) | Aza-naphthalenes | Quinoxyfen | AR, CA, CH, CL, ES, IL, MA, NZ, PE, PT, RS, TR, UA, ZA | 13 |
| Proquinazid | AT, BG, BY, CL, CH, CZ, DE, EE, ES, FI, FR, GR, HR, IE, IL, IT, LT, LV, MA, MD, PE, PL, PT, RO, RU, SE, SI, SK, TN, TR, UA, ZA | ||||
| MAP/Histidine-Kinase in osmotic signal transduction (Os-2, HOG1) | Phenylpyrroles (PP-fungicides) | Fludioxonil | BG, CL, FR, LT, PT, RU, UA, ZA | 12 | |
| Sterol biosynthesis in membranes | Δ14-reductase and Δ8→Δ7-isomerase (Erg24, Erg2) | Amines (“morpholines”) | Fenpropidin | AT, BE, BG, BY, CH, CL, CZ, DE, EE, FR, IE, IT, LT, LV, MD, NL, PL, SK, UA | 5 |
| Fenpropimorph | AT, BE, BG, BY, CH, CL, CZ, DE, EE, FR, IE, LT, LV, MD, NL, PL, RO, RU, SI, SK, TR, UA | ||||
| Spiroxamine | AT, BE, BG, BY, CA, CH, CL, CZ, DE, EC, EE, ES, FR, HR, HU, IE, IL, IT, LT, LV, MD, NL, PE, PL, PT, RO, RS, RU, SI, SK, TN, TR, UA, ZA | ||||
| Tridemorph | PK | ||||
| C14- demethylase (Erg11/Cyp51) | Demethylation inhibitors (DMI-fungicides) | Bromuconazole | AT, BE, CZ, DE, NL, PE, PL, TN, TR, UA | 3 | |
| Cyproconazole | AR, AT, BE, BG, BO, BR, BY, CH, CL, CZ, DE, EE, ES, FI, FR, GR, HR, IL, IT, LT, LV, MA, MD, NL, NZ, PE, PL, PY, RS, RU, SI, SK, TN, TR, UA, UY, UZ, ZA | ||||
| Difenoconazole | AR, AT, BE, BR, BG, BY, CA, CH, CL, CZ, DE, EC, EE, ES, FR, GR, HR, IN, IL, IT, JP, LT, LV, MA, MD, NL, NZ, PK, PE, PL, PT, RU, RS, SI, SK, TN, TR, UA, UY, UZ, ZA | ||||
| Diniconazole | PE | ||||
| Epoxiconazole | AR, AT, BE, BG, BO, BR, BY, CH, CL, CZ, DE, DK, EE, ES, FR, GR, HR, IE, IT, LT, LV, MD, NL, PL, PY, RO, RS, RU, SI, SK, TN, TR, UA, UY, ZA | ||||
| Fenarimol | CL, JP, PK, PE | ||||
| Fenbuconazole | BG, CL, HR, FR, GR, IL, IT, JP, PT, ES, TR | ||||
| Fluquinconazole | CL | ||||
| Flusilazole | BG, TR, ZA | ||||
| Flutriafol | BR, BG, BY, CA, CL, CZ, ES, FR, GR, HR, IE, IT, MD, MX, PY, PE, PL, PT, RU, RS, TN, UA, UY, UZ, ZA | ||||
| Hexaconazole | CO, IN, MA, MX, PK, PE, VN, ZA | ||||
| Imazalil | BE, BY, DE, MD, NL, RU, UA | ||||
| Imibenconazole | BR, JP, VN | ||||
| Ipconazole | UA | ||||
| Mefentrifluconazole | AT, CA, CZ, DE, EE, FR, LT, LV, PE, PL, TR | ||||
| Metconazole | AR, AT, BE, BG, BR, BY, CA, CH, CL, CZ, DE, EE, FI, FR, HR, IE, IT, LT, MD, PL, PT, PY, RS, RU, SI, SK, UA, UY | ||||
| Myclobutanil | AR, AT, BE, BR, BG, CA, CH, CL, CR, CZ, DE, EC, ES, FR, GR, HR, IL, IN, IT, JP, MA, MD, MX, PE, PT, RO, RS, SI, SK, TN, TR, UA, ZA | ||||
| Penconazole | AR, AT, BE, BG, BY, CH, CL, CZ, DE, EE, ES, FR, GR, HR, IL, IN, IT, LT, LV, MA, MD, NL, PE, PK, PL, PT, RS, RU, SI, SK, TN, TR, UA, UZ, ZA | ||||
| Prochloraz | AT, BE, BG, BY, CH, CL, CZ, DE, EE, ES, FR, GR, HR, IE, IT, LT, LV, MD, MX, NL, PE, PL, TR, RU, SK, TR, UA, ZA | ||||
| Propiconazole | BG, BO, BR, BY, CA, CH, CL, CO, IN, JP, MX, PE, PK, RS, RU, TN, TR, UA, UY, UZ, ZA | ||||
| Prothioconazole | AT, BE, BG, BR, BY, CA, CH, CL, CZ, DE, DK, EE, ES, FI, FR, GR, HR, HU, IE, IT, LT, LV, MD, NL, PL, PT, RS, RU, SI, SK, TN, TR, UA, UY, ZA | ||||
| Simeconazole | JP | ||||
| Tebuconazole | AR, AT, BE, BG, BR, BY, CA, CH, CL, CZ, DE, DK, EC, EE, ES, FI, FR, GR, HR, HU, IE, IL, IN, IT, JP, LT, LV, MA, MD, MX, NL, PE, PL, PT, PY, RO, RU, RS, SI, SK, TN, TR, UA, UY, UZ, ZA | ||||
| Tetraconazole | AT, BE, BG, BR, CA, CL, CZ, DE, ES, FR, GR, HR, IL, IT, MA, MD, PE, PL, PT, RU, SI, SK, TR, UA, ZA | ||||
| Triadimefon | AR, BY, CL, CR, IN, KE, MX, RU, TZ, UA, UZ, ZA | ||||
| Triadimenol | AR, BR, BG, CH, CL, CZ, DE, EC, EE, FR, GR, HR, IL, IT, LT, LV, MA, MD, NZ, PE, PL, RO, RS, RU, SI, SK, TN, TR, UA, ZA | ||||
| Triflumizole | BE, BR, CL, JP, MA, MD, MX, NL, PE | ||||
| Triforine | CL, JP, MX, PK, UZ | ||||
| Triticonazole | BR, BY, CL, MD, RU, UA, ZA | ||||
| 3- UNKNOWN MODE OF ACTION | |||||
| Unknown | Unknown | Phenyl-acetamide | Cyflufenamid | AT, BR, BG, CH, CL, CZ, DE, EE, ES, FR, GR, HR, IN, IE, IL, IT, JP, LT, LV, MA, NL, PE, PL, PT, RS, RU, SI, SK, TN, TR, UA, ZA | U06 |
| Thiazolidine | Flutianil | JP | U13 | ||
| Group Name | Active Ingredient | Year of Registration 1 | Resistant Pathogen | Date First Observed | Reference |
|---|---|---|---|---|---|
| Aryl-phenyl ketones | Metrafenone | 2006 | B. graminis f. sp. tritici | 2009 | [27] |
| E. necator | 2010 | [32] | |||
| Pyriofenone | 2014 | P. xanthii | 2017 | [33] | |
| Aza-naphthalenes | Quinoxyfen | 1996 | E. necator | 2002 | [42] |
| B. graminis f. sp. tritici | 2003 | [38] | |||
| P. xanthii | 2004 | [49] | |||
| Hydroxy-(2-amino) pyrimidines | Dimethirimol | 1968 | P. xanthii | 1970 | [55] |
| Ethirimol | 1968 | B. graminis f. sp. hordei | 1971 | [57] | |
| Bupirimate | 1975 | P. xanthii | 1986 | [60] | |
| G. cichoracearum | 1990 | [62] | |||
| Methyl-Benzimidazole Carbamates (MBC fungicides) | Benomyl | 1968 | P. xanthii | 1967 | [66] |
| S. pannosa | 1974 | [75] | |||
| E. necator | 1977 | [76] | |||
| G. cichoracearum | 2001 | [70] | |||
| Thiophanate-methyl | 1971 | P. xanthii | 2002 | [64] | |
| E. necator | 2005 | [46] | |||
| G. cichoracearum | 2005 | [73] | |||
| Phosphorothiolates | Pyrazophos | 1970 | P. xanthii | 1975 | [81] |
| Quinone outside Inhibitors (QoI-fungicides) | Azoxystrobin | 1992 | P. xanthii | 1998 | [86] |
| E. necator | 1999 | [89] | |||
| G. cichoracearum | 2010 | [73] | |||
| L. taurica | 2015 | [99] | |||
| Kresoxim-methyl | 1996 | P. xanthii | 1998 | [86] | |
| B. graminis f. sp. tritici | 1998 | [87] | |||
| B. graminis f. sp. hordei | 2003 | [88] | |||
| Famoxadone | 1998 | B. graminis f. sp. tritici | 2000 | [83] | |
| P. xanthii | |||||
| Trifloxystrobin | 1999 | B. graminis f. sp. tritici | 1998 | [87] | |
| E. necator | 2002 | [90] | |||
| P. leuchotricha | 2002 | [98] | |||
| P. xanthii | 2002 | [84] | |||
| B. graminis f. sp. hordei | 2003 | [88] | |||
| E. betae | 2011 | [96] | |||
| Pyraclostrobin | 2000 | E. betae | 2011 | [96] | |
| Fenamidone | 2001 | B. graminis f. sp. tritici | 2000 | [83] | |
| P. xanthii | |||||
| Amines (“morpholines”) | Tridemorph | 1969 | B. graminis f. sp. hordei | 1976 | [58] |
| P. xanthii | 1988 | [61] | |||
| Fenpropimorph | 1983 | B. graminis f. sp. hordei | 1979 | [109] | |
| Fenpropidin | 1986 | B. graminis f. sp. hordei | 1979 | [109] | |
| Spiroxamine | 1997 | E. necator | 2002 | [77] | |
| DeMethylation Inhibitors (DMI-fungicides) | Triforine | 1970 | P. xanthii | 1982 | [128] |
| Fenarimol | 1975 | P. xanthii | 1984 | [129] | |
| E. necator | 1985 | [138] | |||
| G. cichoracearum | 2005 | [73] | |||
| Triadimefon | 1976 | B. graminis f. sp. hordei | 1979 | [110] | |
| B.graminis f. sp. tritici | 1981 | [112] | |||
| E. necator | 1985 | [138] | |||
| P. xanthii | 1988 | [61] | |||
| Imazalil | 1977 | P. xanthii | 1982 | [128] | |
| Prochloraz | 1977 | B.graminis f. sp. tritici | 1981 | [112] | |
| B. graminis f. sp. hordei | |||||
| Triadimenol | 1978 | B. graminis f. sp. hordei | 1979 | [110] | |
| B.graminis f. sp. tritici | 1981 | [112] | |||
| E. necator | 1990 | [139] | |||
| P. xanthii | 2002 | [133] | |||
| Diclobutrazol | 1979 | B.graminis f. sp. tritici | 1981 | [112] | |
| B. graminis f. sp. hordei | |||||
| Propioconazole | 1980 | B.graminis f. sp. tritici | 1981 | [112] | |
| B. graminis f. sp. hordei | |||||
| P. xanthii | 1991 | [67] | |||
| Nuarimol | 1980 | B.graminis f. sp. tritici | 1981 | [112] | |
| B. graminis f. sp. hordei | |||||
| Flutriafol | 1981 | B. graminis f. sp. hordei | 1992 | [116] | |
| Penconazole | 1983 | P. xanthii | 1988 | [61] | |
| E. necator | 1996 | [141] | |||
| P. aphanis | 2009 | [148] | |||
| Flusiazole | 1984 | E. necator | 1996 | [141] | |
| Cyproconazole | 1986 | B. graminis f. sp. hordei | 1991 | [119] | |
| Hexaconazole | 1986 | E. necator | 2009 | [146] | |
| Myclobutanil | 1986 | E. necator | 1985 | [138] | |
| P. xanthii | 1991 | [67] | |||
| P. aphanis | 2009 | [148] | |||
| Tebuconazole | 1986 | B. graminis f. sp. hordei | 1992 | [116] | |
| E. necator | 2004 | [90] | |||
| Triflumizole | 1986 | E. necator | 2004 | [90] | |
| Difenoconazole | 1988 | P. xanthii | 2008 | [132] | |
| Epoxiconazole | 1993 | B. graminis f. sp. hordei | 1991 | [119] | |
| Succinate DeHydrogenase Inhibitors (SDHI-fungicides) | Boscalid | 2002 | P. xanthii | 2005 | [134] |
| E. necator | 2014 | [164] | |||
| Penthiopyrad | 2010 | P. xanthii | 2017 | [161] | |
| Isopyrazam | 20172017 | ||||
| Pyraziflumid | |||||
| Isofetamid | 2018 | 2018 | |||
| Phenyl-acetamide | Cyflufenamid | 2002 | P. xanthii | 2012 | [167] |
| Cyano-methylenethiazolidines | Flutianil | 2008 | P. xanthii | 2017 | [33] |
| Group Name | Active Ingredient | Resistant Pathogen | Target Protein | Amino Acid Change | Phenotype | References | |
|---|---|---|---|---|---|---|---|
| Methyl-Benzimidazole Carbamates (MBC fungicides) | Thiophanate-methyl | P. xanthii | β-tubulin | E198A | Highly resistant | [74,80] | |
| Quinone outside Inhibitors (QoI-fungicides) | Azoxystrobin Kresoxim-methyl Pyraclostrobin Tryfloxystrobin | B. graminis f. sp. hordei | Cytb | G143A | Resistant | [88] | |
| B. graminis f. sp. tritici | [83,101,102,103,104,105] | ||||||
| E. betae | [96,97] | ||||||
| E. necator | [46,91,92,93,94,95] | ||||||
| L. taurica1 | [99] | ||||||
| P. leucotricha1 | [98] | ||||||
| P. xanthii1 | [82,83,106] | ||||||
| DeMethylation Inhibitors (DMI-fungicides) | Propiconazole | B. graminis f. sp. tritici | Cyp51 | Y136F, K147Q | Resistant | [150] | |
| Triadimenol | |||||||
| Epoxiconazole | B. graminis f. sp. hordei | Cyp51 | Y136F, S509T | Resistant | [153] | ||
| Tebuconazole | Y136F, S509T | Resistant | [151,152,153] | ||||
| Triadimenol | Y136F, K147Q | Highly-low resistant | [149,150] | ||||
| Fenarimol | E. necator | Cyp51 | Y136F, A1119C 2 | Resistant, highly resistant | [22] | ||
| Myclobutanil | Y136F, A1119C 2 | Highly resistant | [21,22,145] | ||||
| Tebuconazole | Y136F, A1119C 2 | Resistant, highly resistant | [22] | ||||
| Triadimenol | Y136F | Highly resistant | [154] | ||||
| Succinate DeHydrogenase Inhibitors (SDHI-fungicides) | Boscalid | E. necator | SdhB | H242R/Y | Resistant | [165] | |
| Fluopyram Fluxapyroxad | E. necator | SdhB | H242R/Y | Moderately resistant | [31] | ||
| SdhC | G169D | Highly resistant | |||||
| Penthiopyrad Isopyrazam Pyraziflumid | P. xanthii | SdhC | G151R | Highly resistant | [161] | ||
| G172D | |||||||
| SdhD | H137R | ||||||
| S121P | Moderately resistant | ||||||
| Isofetamid | SdhC | A86V | Highly resistant |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide Resistance in Powdery Mildew Fungi. Microorganisms 2020, 8, 1431. https://doi.org/10.3390/microorganisms8091431
Vielba-Fernández A, Polonio Á, Ruiz-Jiménez L, de Vicente A, Pérez-García A, Fernández-Ortuño D. Fungicide Resistance in Powdery Mildew Fungi. Microorganisms. 2020; 8(9):1431. https://doi.org/10.3390/microorganisms8091431
Chicago/Turabian StyleVielba-Fernández, Alejandra, Álvaro Polonio, Laura Ruiz-Jiménez, Antonio de Vicente, Alejandro Pérez-García, and Dolores Fernández-Ortuño. 2020. "Fungicide Resistance in Powdery Mildew Fungi" Microorganisms 8, no. 9: 1431. https://doi.org/10.3390/microorganisms8091431
APA StyleVielba-Fernández, A., Polonio, Á., Ruiz-Jiménez, L., de Vicente, A., Pérez-García, A., & Fernández-Ortuño, D. (2020). Fungicide Resistance in Powdery Mildew Fungi. Microorganisms, 8(9), 1431. https://doi.org/10.3390/microorganisms8091431





