Probiotics: A Dietary Factor to Modulate the Gut Microbiome, Host Immune System, and Gut–Brain Interaction
Abstract
1. Introduction
2. Effect on Gut Microbiota
2.1. Neonates
2.2. Adults
2.3. Constipation
2.4. Obesity
3. Immune Control
3.1. URTIs
3.2. IBD
3.3. Cancer
4. Gut–Brain Interaction
4.1. Involvement of Gut Microbiota
4.2. Modulation of the Microbiota–Gut–Brain Axis by Probiotics
5. Possible Mechanisms of Physiological Effects
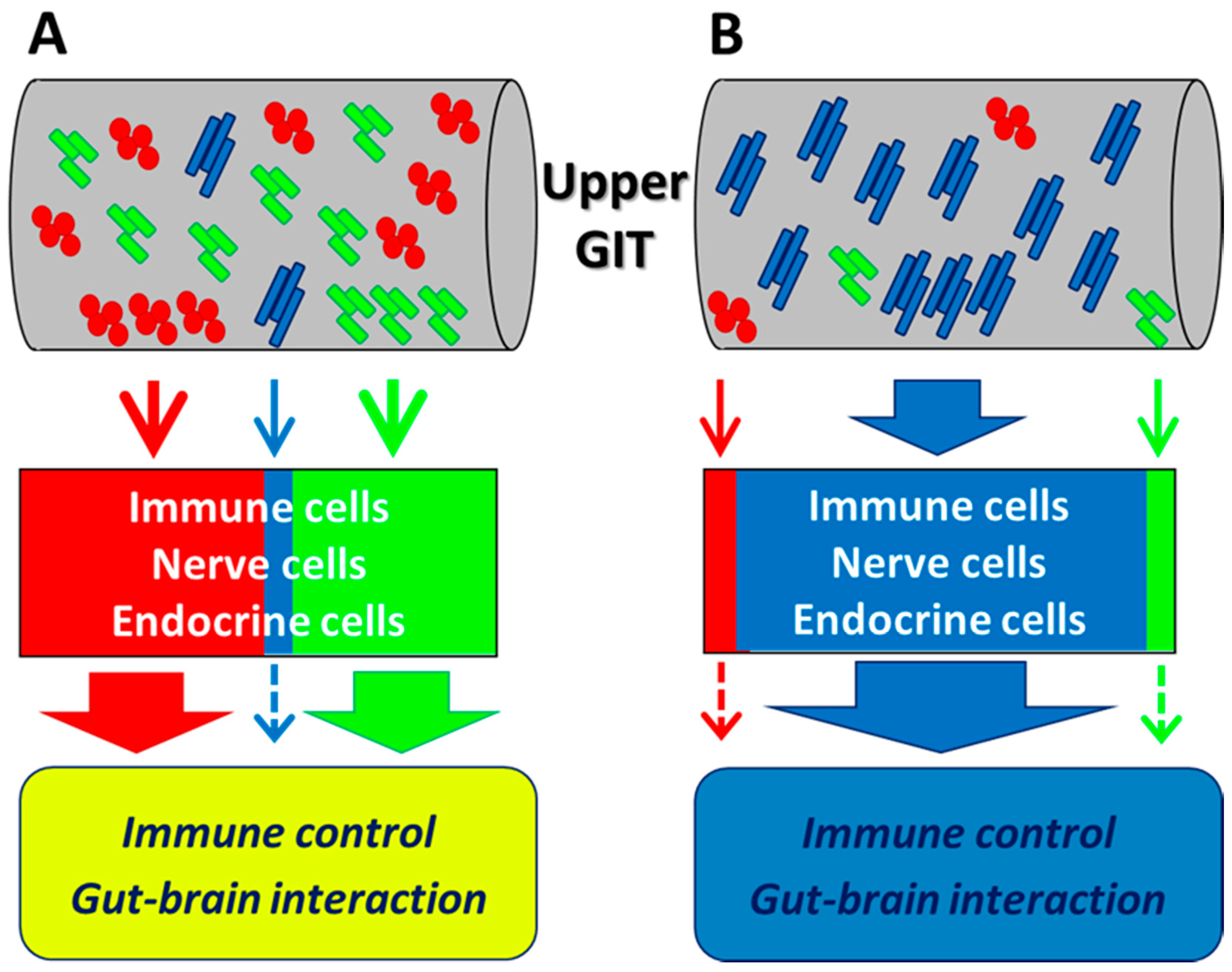
5.1. Dynamics in the Small Intestine
5.2. Immune Control
5.3. Modulation of Brain Function via the Gut–Brain Axis
6. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO (Food and Agricultural Organization of the United Nations and World Health Organization). Guidelines for the Evaluation of Probiotics in Food. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. London Ontario (CA); 30 April and 1 May 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 3 July 2020).
- Ashwell, M. Concepts of Functional Foods. Brussels (BE): International Life Sciences Institute (ILSI) Europe. Available online: https://ilsi.eu/publication/concepts-of-functional-foods/ (accessed on 3 July 2020).
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Takada, T.; Shimizu, K.; Kado, Y.; Kawakami, K.; Makino, I.; Yamaoka, Y.; Hirano, K.; Nishimura, A.; Kajimoto, O.; et al. The effects of a probiotic milk product containing Lactobacillus casei strain Shirota on the defecation frequency and the intestinal microflora of sub-optimal health state volunteers: A randomized placebo-controlled cross-over study. Biosci. Microflora 2006, 25, 39–48. [Google Scholar] [CrossRef]
- Ferrario, C.; Taverniti, V.; Milani, C.; Fiore, W.; Laureati, M.; De Noni, I.; Stuknyte, M.; Chouaia, B.; Riso, P.; Guglielmetti, S. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J. Nutr. 2014, 144, 1787–1796. [Google Scholar] [CrossRef]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef]
- Nagata, S.; Asahara, T.; Wang, C.; Suyama, Y.; Chonan, O.; Takano, K.; Daibou, M.; Takahashi, T.; Nomoto, K.; Yamashiro, Y. The effectiveness of Lactobacillus beverages in controlling infections among the residents of an aged care facility: A randomized placebo-controlled double-blind trial. Ann. Nutr. Metab. 2016, 68, 51–59. [Google Scholar] [CrossRef]
- Oshiro, T.; Nagata, S.; Wang, C.; Takahashi, T.; Tsuji, H.; Asahara, T.; Nomoto, K.; Takei, H.; Nittono, H.; Yamashiro, Y. Bifidobacterium supplementation of colostrum and breast milk enhances weight gain and metabolic responses associated with microbiota establishment in very-preterm infants. Biomed. Hub 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Takada, T.; Chinda, D.; Mikami, T.; Shimizu, K.; Oana, K.; Hayamizu, S.; Miyazawa, K.; Arai, T.; Katto, M.; Nagara, Y.; et al. Dynamic analysis of human small intestinal microbiota after an ingestion of fermented milk by small-intestinal fluid perfusion using an endoscopic retrograde bowel insertion technique. Gut Microbes 2020, 11, 1662–1676. [Google Scholar] [CrossRef]
- El Aila, N.A.; Tency, I.; Claeys, G.; Verstraelen, H.; Saerens, B.; Lopes dos Santos Santiago, G.; De Backer, E.; Cools, P.; Temmerman, M.; Verhelst, R.; et al. Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC Infect. Dis. 2009, 9, 167. [Google Scholar] [CrossRef]
- Makino, H.; Kushiro, A.; Ishikawa, E.; Kubota, H.; Gawad, A.; Sakai, T.; Oishi, K.; Martin, R.; Ben-Amor, K.; Knol, J.; et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS ONE 2013, 8, e78331. [Google Scholar] [CrossRef]
- Collins, M.D.; Gibson, G.R. Probiotics, prebiotics, and synbiotics: Approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999, 69, 1052s–1057s. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tian, L.; Luo, J.; Yu, J. Ongoing supplementation of probiotics to cesarean-born neonates during the first month of life may impact the gut microbial. Am. J. Perinatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Salminen, S.; Isolauri, E. Intestinal colonization, microbiota, and probiotics. J. Pediatr. 2006, 149, S115–S120. [Google Scholar] [CrossRef]
- Turroni, F.; Ribbera, A.; Foroni, E.; van Sinderen, D.; Ventura, M. Human gut microbiota and bifidobacteria: From composition to functionality. Antonie Van Leeuwenhoek 2008, 94, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Sakata, S.; Kalliomäki, M.; Isolauri, E.; Benno, Y.; Salminen, S. Effect of maternal consumption of Lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 166–170. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Boyle, R.J.; Kivivuori, S.; Oppedisano, F.; Smith, K.R.; Robins-Browne, R.; Salminen, S.J.; Tang, M.L.K. Prenatal probiotic administration can influence Bifidobacterium microbiota development in infants at high risk of allergy. J. Allergy Clin. Immunol. 2009, 123, 499–501. [Google Scholar] [CrossRef]
- Makino, H.; Kushiro, A.; Ishikawa, E.; Muylaert, D.; Kubota, H.; Sakai, T.; Oishi, K.; Martin, R.; Ben Amor, K.; Oozeer, R.; et al. Transmission of intestinal Bifidobacterium longum subsp. longum strains from mother to infant, determined by multilocus sequencing typing and amplified fragment length polymorphism. Appl. Environ. Microbiol. 2011, 77, 6788–6793. [Google Scholar] [CrossRef]
- Maldonado, J.; Cañabate, F.; Sempere, L.; Vela, F.; Sánchez, A.R.; Narbona, E.; López-Huertas, E.; Geerlings, A.; Valero, A.D.; Olivares, M.; et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 55–61. [Google Scholar] [CrossRef]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Kang, D.W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed]
- McHardy, I.H.; Li, X.; Tong, M.; Ruegger, P.; Jacobs, J.; Borneman, J.; Anton, P.; Braun, J. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome 2013, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Malinen, E.; Rinttilä, T.; Kajander, K.; Mättö, J.; Kassinen, A.; Krogius, L.; Saarela, M.; Korpela, R.; Palva, A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 2005, 100, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Biagi, E.; Heilig, H.G.H.J.; Kajander, K.; Kekkonen, R.A.; Tims, S.; De Vos, W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, P.P.; Yu, D.; Liao, G.C.; Wu, S.L.; Fang, A.P.; Chen, P.Y.; Wang, X.Y.; Luo, Y.; Long, J.A.; et al. Effects of probiotic supplementation on serum trimethylamine-N-oxide level and gut microbiota composition in young males: A double-blinded randomized controlled trial. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Khalif, I.L.; Quigley, E.M.M.; Konovitch, E.A.; Maximova, I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’Homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Kim, S.E.; Choi, S.C.; Park, K.S.; Park, M.I.; Shin, J.E.; Lee, T.H.; Jung, K.W.; Koo, H.S.; Myung, S.J. Change of fecal flora and effectiveness of the short-term VSL#3 probiotic treatment in patients with functional constipation. J. Neurogastroenterol. Motil. 2015, 21, 111–120. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Chen, J.; Chen, X.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 2016, 150, 367–379. [Google Scholar] [CrossRef]
- Ishizuka, A.; Tomizuka, K.; Aoki, R.; Nishijima, T.; Saito, Y.; Inoue, R.; Ushida, K.; Mawatari, T.; Ikeda, T. Effects of administration of Bifidobacterium animalis subsp. lactis GCL2505 on defecation frequency and bifidobacterial microbiota composition in humans. J. Biosci. Bioeng. 2012, 113, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Salminen, E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand. J. Gastroenterol. Suppl. 1997, 32, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Veiga, P.; Pons, N.; Agrawal, A.; Oozeer, R.; Guyonnet, D.; Brazeilles, R.; Faurie, J.M.; Van Hylckama Vlieg, J.E.T.; Houghton, L.A.; Whorwell, P.J.; et al. Changes of the human gut microbiome induced by a fermented milk product. Sci. Rep. 2014, 4, 6328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Z.; Han, M.; Guo, B.-H. Efficacy of pasteurised yoghurt in improving chronic constipation: A randomised, double-blind, placebo-controlled trial. Int. Dairy J. 2015, 40, 1–5. [Google Scholar] [CrossRef]
- Sakai, T.; Makino, H.; Ishikawa, E.; Oishi, K.; Kushiro, A. Fermented milk containing Lactobacillus casei strain Shirota reduces incidence of hard or lumpy stools in healthy population. Int. J. Food Sci. Nutr. 2011, 62, 423–430. [Google Scholar] [CrossRef]
- Riezzo, G.; Orlando, A.; D’Attoma, B.; Guerra, V.; Valerio, F.; Lavermicocca, P.; De Candia, S. Randomised clinical trial: Efficacy of Lactobacillus paracasei-enriched artichokes in the treatment of patients with functional constipation-a double-blind, controlled, crossover study. Aliment. Pharmacol. Ther. 2012, 35, 441–450. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Marsono, Y.; Illman, R.J.; Clarke, J.M.; Trimble, R.P.; Topping, D.L. Plasma lipids and large bowel volatile fatty acids in pigs fed on white rice, brown rice and rice bran. Br. J. Nutr. 1993, 70, 503–513. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gøbel, R.J.; Michaelsen, K.F.; Forssten, S.D.; Lahtinen, S.J.; Jakobsen, M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin. Nutr. 2013, 32, 935–940. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.-K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef]
- Currò, D.; Ianiro, G.; Pecere, S.; Bibbò, S.; Cammarota, G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2017, 174, 1426–1449. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 2017, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.A.-L. Prevention and treatment of influenza, influenza-like illness, and common cold by herbal, complementary, and natural therapies. J. Evid. Based Complement. Altern. Med. 2017, 22, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei group: History and health related applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic bacteria: A promising tool in cancer prevention and therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Hyarat, S.Y.; Subih, M.; Rayan, A.; Salami, I.; Harb, A. Health related quality of life among patients with multiple sclerosis: The role of psychosocial adjustment to illness. Arch. Psychiatr. Nurs. 2019, 33, 11–16. [Google Scholar] [CrossRef]
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A systematic review. J. Neuroinflammation 2019, 16, 231. [Google Scholar] [CrossRef]
- Makinodan, T.; James, S.J.; Inamizu, T.; Chang, M.P. Immunologic basis for susceptibility to infection in the aged. Gerontology 1984, 30, 279–289. [Google Scholar] [CrossRef]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fitness 1990, 30, 316–328. [Google Scholar]
- Cohen, S. Psychological stress and susceptibility to upper respiratory infections. Am. J. Respir. Crit. Care Med. 1995, 152, S53–S58. [Google Scholar] [CrossRef] [PubMed]
- Nokso-Koivisto, J.; Pitkäranta, A.; Blomqvist, S.; Jokinen, J.; Kleemola, M.; Takala, A.; Kilpi, T.; Hovi, T. Viral etiology of frequently recurring respiratory tract infections in children. Clin. Infect. Dis. 2002, 35, 540–546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biron, C.A.; Nguyen, K.B.; Pien, G.C.; Cousens, L.P.; Salazar-Mather, T.P. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu. Rev. Immunol. 1999, 17, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef]
- Merenstein, D.; Murphy, M.; Fokar, A.; Hernandez, R.K.; Park, H.; Nsouli, H.; Sanders, M.E.; Davis, B.A.; Niborski, V.; Tondu, F.; et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: The DRINK study A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur. J. Clin. Nutr. 2010, 64, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; Tauler, P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Fujita, R.; Iimuro, S.; Shinozaki, T.; Sakamaki, K.; Uemura, Y.; Takeuchi, A.; Matsuyama, Y.; Ohashi, Y. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: A multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am. J. Infect. Control. 2013, 41, 1231–1235. [Google Scholar] [CrossRef]
- Waki, N.; Matsumoto, M.; Fukui, Y.; Suganuma, H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: An open-label pilot study. Lett. Appl. Microbiol. 2014, 59, 565–571. [Google Scholar] [CrossRef]
- Strasser, B.; Geiger, D.; Schauer, M.; Gostner, J.M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Probiotic supplements beneficially affect tryptophan–kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: A randomized, double-blinded, placebo-controlled trial. Nutrients 2016, 8, 752. [Google Scholar] [CrossRef]
- Taipale, T.J.; Pienihäkkinen, K.; Isolauri, E.; Jokela, J.T.; Söderling, E.M. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatr. Res. 2016, 79, 65–69. [Google Scholar] [CrossRef]
- Pu, F.; Guo, Y.; Li, M.; Zhu, H.; Wang, S.; Shen, X.; He, M.; Huang, C.; He, F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin. Interv. Aging 2017, 12, 1223–1231. [Google Scholar] [CrossRef]
- Shida, K.; Sato, T.; Iizuka, R.; Hoshi, R.; Watanabe, O.; Igarashi, T.; Miyazaki, K.; Nanno, M.; Ishikawa, F. Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur. J. Nutr. 2017, 56, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus plantarum DR7 improved upper respiratory tract infections via enhancing immune and inflammatory parameters: A randomized, double-blind, placebo-controlled study. J. Dairy Sci. 2019, 102, 4783–4797. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Díaz-Ropero, M.P.; Sierra, S.; Lara-Villoslada, F.; Fonollá, J.; Navas, M.; Rodríguez, J.M.; Xaus, J. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition 2007, 23, 254–260. [Google Scholar] [CrossRef]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L.; Gopal, P.K. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobactedum lactis HN019. Am. J. Clin. Nutr. 2001, 74, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes. J. Clin. Immunol. 2001, 21, 264–271. [Google Scholar] [CrossRef]
- Nagao, F.; Nakayama, M.; Muto, T.; Okumura, K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci. Biotechnol. Biochem. 2000, 64, 2706–2708. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Suzuki, T.; Shimada, S.I.; Shida, K.; Nanno, M.; Okumura, K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin. Exp. Immunol. 2006, 146, 109–115. [Google Scholar] [CrossRef]
- Reale, M.; Boscolo, P.; Bellante, V.; Tarantelli, C.; Di Nicola, M.; Forcella, L.; Li, Q.; Morimoto, K.; Muraro, R. Daily intake of Lactobacillus casei Shirota increases natural killer cell activity in smokers. Br. J. Nutr. 2012, 108, 308–314. [Google Scholar] [CrossRef]
- Zocco, M.A.; Dal Verme, L.Z.; Cremonini, F.; Piscaglia, A.C.; Nista, E.C.; Candelli, M.; Novi, M.; Rigante, D.; Cazzato, I.A.; Ojetti, V.; et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 1567–1574. [Google Scholar] [CrossRef]
- Mitsuyama, K.; Matsumoto, S.; Yamasaki, H.; Masuda, J.; Kuwaki, K.; Takedatsu, H.; Magaoka, M.; Andoh, A.; Tsuruta, O.; Sata, M. Beneficial effects of Lactobacillus casei in ulcerative colitis: A pilot study. J. Clin. Biochem. Nutr. 2008, 43, 78–81. [Google Scholar]
- Tursi, A.; Brandimarte, G.; Papa, A.; Giglio, A.; Elisei, W.; Giorgetti, G.M.; Forti, G.; Morini, S.; Hassan, C.; Pistoia, M.A.; et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 2010, 105, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Gerardi, V.; Mangiola, F.; Lopetuso, L.R.; Pizzoferrato, M.; Petito, V.; Papa, A.; Stojanovic, J.; Poscia, A.; Cammarota, G.; et al. Role and mechanisms of action of Escherichia coli Nissle 1917 in the maintenance of remission in ulcerative colitis patients: An update. World J. Gastroenterol. 2016, 22, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef]
- Braat, H.; Van Den Brande, J.; Van Tol, E.; Hommes, D.; Peppelenbosch, M.; Van Deventer, S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am. J. Clin. Nutr. 2004, 80, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Plamondon, S.; Kamm, M.A.; Hart, A.L.; Al-Hassi, H.O.; Guenther, T.; Stagg, A.J.; Knight, S.C. Immunosuppressive effects via human intestinal dendritic cells of probiotic bacteria and steroids in the treatment of acute ulcerative colitis. Inflamm. Bowel Dis. 2010, 16, 1286–1298. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef]
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-de-Mesquita, B.; Skeie, G.; Olsen, A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; et al. Consumption of dairy products and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS ONE 2013, 8, e72715. [Google Scholar] [CrossRef]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef]
- Aso, Y.; Akaza, H.; Kotake, T.; Tsukamoto, T.; Imai, K.; Naito, S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. Eur. Urol. 1995, 27, 104–109. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakai, S.; Tsukamoto, T.; Masumori, N.; Akaza, H.; Miyanaga, N.; Kitamura, T.; Kawabe, K.; Kotake, T.; Kuroda, M.; et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol. Int. 2002, 68, 273–280. [Google Scholar] [CrossRef]
- Ishikawa, H.; Akedo, I.; Otani, T.; Suzuki, T.; Nakamura, T.; Takeyama, I.; Ishiguro, S.; Miyaoka, E.; Sobue, T.; Kakizoe, T. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int. J. Cancer 2005, 116, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Koga, H.; Yamaguchi, A.; Fujimoto, N.; Hasui, Y.; Kuramoto, H.; Iguchi, A.; Kinukawa, N. Prevention of recurrence with epirubicin and Lactobacillus casei after transurethral resection of bladder cancer. J. Urol. 2008, 179, 485–490. [Google Scholar] [CrossRef]
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic beverage with soy isoflavone consumption for breast cancer prevention: A case-control study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 2014, 817, 115–133. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Badawy, A.A.B. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 2017, 112, 248–263. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Teratani, T.; Mikami, Y.; Nakamoto, N.; Suzuki, T.; Harada, Y.; Okabayashi, K.; Hagihara, Y.; Taniki, N.; Kohno, K.; Sibata, S.; et al. The liver–brain–gut neural arc maintains the Treg cell niche in the gut. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.F.; Trotman, H.D.; Pearce, B.D.; Addington, J.; Cadenhead, K.S.; Cornblatt, B.A.; Heinssen, R.; Mathalon, D.H.; Perkins, D.O.; Seidman, L.J.; et al. Cortisol levels and risk for psychosis: Initial findings from the North American Prodrome Longitudinal Study. Biol. Psychiatry 2013, 74, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Agostini, S.; Goubern, M.; Tondereau, V.; Salvador-Cartier, C.; Bezirard, V.; Lévèque, M.; Keränen, H.; Theodorou, V.; Bourdu-Naturel, S.; Goupil-Feuillerat, N.; et al. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol. Motil. 2012, 24, 376-e172. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology 2017, 153, 448–459. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401. [Google Scholar] [CrossRef]
- Marcos, A.; Wärnberg, J.; Nova, E.; Gómez, S.; Alvarez, A.; Alvarez, R.; Mateos, J.A.; Cobo, J.M. The effect of milk fermented by yogurt cultures plus Lactobacillus casei DN-114001 on the immune response of subjects under academic examination stress. Eur. J. Nutr. 2004, 43, 381–389. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef]
- Diop, L.; Guillou, S.; Durand, H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: A double-blind, placebo-controlled, randomized trial. Nutr. Res. 2008, 28, 1–5. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut–brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Sugawara, T.; Aoki, Y.; Fujiwara, S.; Rokutan, K. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods 2017, 36, 112–121. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Itoh, N.; Yamamoto, S.; Higo-Yamamoto, S.; Nakakita, Y.; Kaneda, H.; Shigyo, T.; Oishi, K. Dietary heat-killed Lactobacillus brevis SBC8803 promotes voluntary wheel-running and affects sleep rhythms in mice. Life Sci. 2014, 111, 47–52. [Google Scholar] [CrossRef]
- Nakakita, Y.; Tsuchimoto, N.; Takata, Y.; Nakamura, T. Effect of dietary heat-killed Lactobacillus brevis SBC8803 (SBL88TM) on sleep: A non-randomised, double blind, placebo-controlled, and crossover pilot study. Benef. Microbes 2016, 7, 501–509. [Google Scholar] [CrossRef]
- Yamamura, S.; Morishima, H.; Kumano-Go, T.; Suganuma, N.; Matsumoto, H.; Adachi, H.; Sigedo, Y.; Mikami, A.; Kai, T.; Masuyama, A.; et al. The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. Eur. J. Clin. Nutr. 2009, 63, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Gondo, Y.; Kikuchi-Hayakawa, H.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Kuwano, Y.; Miyazaki, K.; et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: A double-blind, randomised, placebo-controlled trial. Benef. Microbes 2017, 8, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Yuki, N.; Watanabe, K.; Mike, A.; Tagami, Y.; Tanaka, R.; Ohwaki, M.; Morotomi, M. Survival of a probiotic, Lactobacillus casei strain Shirota, in the gastrointestinal tract: Selective isolation from faeces and identification using monoclonal antibodies. Int. J. Food Microbiol. 1999, 48, 51–57. [Google Scholar] [CrossRef]
- Kado, Y.; Yuki, N.; Kushiro, A.; Watanabe, K.; Morotomi, M. Survival of a probiotic, Bifidobacterium breve strain Yakult, in the human gastrointestinal tract: Selective isolation from feces and identification using randomly amplified polymorphic DNA polymerase chain reaction technique. J. Intest. Microbiol. 2001, 15, 9–14. [Google Scholar] [CrossRef]
- Pochart, P.; Marteau, P.; Bouhnik, Y.; Goderel, I.; Bourlioux, P.; Ramboud, J.C. Survival of bifidobacteria ingested via fermented milk during their passage through the human small intestine: An in vivo study using intestinal perfusion. Am. J. Clin. Nutr. 1992, 55, 78–80. [Google Scholar] [CrossRef]
- Oozeer, R.; Leplingard, A.; Mater, D.D.G.; Mogenet, A.; Michelin, R.; Seksek, I.; Marteau, P.; Doré, J.; Bresson, J.L.; Corthier, G. Survival of Lactobacillus casei in the human digestive tract after consumption of fermented milk. Appl. Environ. Microbiol. 2006, 72, 5615–5617. [Google Scholar] [CrossRef]
- Vesa, T.; Pochart, P.; Marteau, P. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment. Pharmacol. Ther. 2000, 14, 823–828. [Google Scholar] [CrossRef]
- Johansson, M.L.; Molin, G.; Jeppsson, B.; Nobaek, S.; Ahrné, S.; Bengmark, S. Administration of different Lactobacillus strains in fermented oatmeal soup: In vivo colonization of human intestinal mucosa and effect on the indigenous flora. Appl. Environ. Microbiol. 1993, 59, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Murosaki, S.; Fujiki, T.; Yamamoto, Y.; Yoshikai, Y.; Yamashita, M. Lipoteichoic acids on Lactobacillus plantarum cell surfaces correlate with induction of interleukin-12p40 production. Microbiol. Immunol. 2010, 54, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Ikari, N.; Kouchi, T.; Kowatari, Y.; Kubota, Y.; Shimojo, N.; Tsuji, N.M. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci. Rep. 2018, 8, 5065. [Google Scholar] [CrossRef] [PubMed]
- Shida, K.; Kiyoshima-Shibata, J.; Nagaoka, M.; Watanabe, K.; Nanno, M. Induction of interleukin-12 by Lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J. Dairy Sci. 2006, 89, 3306–3317. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hara, T.; Hori, T.; Mitsuyama, K.; Nagaoka, M.; Tomiyasu, N.; Suzuki, A.; Sata, M. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin. Exp. Immunol. 2005, 140, 417–426. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hara, T.; Nagaoka, M.; Mike, A.; Mitsuyama, K.; Sako, T.; Yamamoto, M.; Kado, S.; Takada, T. A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology 2009, 128, e170–e180. [Google Scholar] [CrossRef]
- Gao, K.; Wang, C.; Liu, L.; Dou, X.; Liu, J.; Yuan, L.; Zhang, W.; Wang, H. Immunomodulation and signaling mechanism of Lactobacillus rhamnosus GG and its components on porcine intestinal epithelial cells stimulated by lipopolysaccharide. J. Microbiol. Immunol. Infect. 2017, 50, 700–713. [Google Scholar] [CrossRef]
- Morikawa, M.; Tsujibe, S.; Kiyoshima-Shibata, J.; Watanabe, Y.; Kato-Nagaoka, N.; Shida, K.; Matsumoto, S. Microbiota of the small intestine is selectively engulfed by phagocytes of the lamina propria and peyer’s patches. PLoS ONE 2016, 11, e0163607. [Google Scholar] [CrossRef]
- Shima, T.; Fukushima, K.; Setoyama, H.; Imaoka, A.; Matsumoto, S.; Hara, T.; Suda, K.; Umesaki, Y. Differential effects of two probiotic strains with different bacteriological properties on intestinal gene expression, with special reference to indigenous bacteria. FEMS Immunol. Med. Microbiol. 2008, 52, 69–77. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Hirano, T.; Murakami, M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity 2020, 52, 731–733. [Google Scholar] [CrossRef]
- Burgueno, J.F.; Reich, A.; Hazime, H.; Quintero, M.A.; Fernandez, I.; Fritsch, J.; Santander, A.M.; Brito, N.; Damas, O.M.; Deshpande, A.; et al. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm. Bowel Dis. 2020, 26, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Lai, J.B.; Du, Y.L.; Xu, Y.; Ruan, L.M.; Hu, S.H. Current understanding of gut microbiota in mood disorders: An update of human studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T.; et al. Fermented milk containing Lactobacillus casei strain Shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health benefits of Lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: A randomized, double-blind, placebo-controlled study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef]
- Sawada, D.; Kuwano, Y.; Tanaka, H.; Hara, S.; Uchiyama, Y.; Sugawara, T.; Fujiwara, S.; Rokutan, K.; Nishida, K. Daily intake of Lactobacillus gasseri CP2305 relieves fatigue and stress-related symptoms in male university Ekiden runners: A double-blind, randomized, and placebo-controlled clinical trial. J. Funct. Foods 2019, 57, 465–476. [Google Scholar] [CrossRef]
- de Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; Gobbetti, M. Autism spectrum disorders and intestinal microbiota. Gut Microbes 2015, 6, 207–213. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Probiotics and psychobiotics: The role of microbial neurochemicals. Probiotics Antimicrob. Proteins 2019, 11, 1071–1085. [Google Scholar] [CrossRef]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef]
- Kim, H.J.; Leeds, P.; Chuang, D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: A prospective open-label trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.A.; Ossenkopp, K.-P.; Kavaliers, M.; Macfabe, D.F. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS ONE 2014, 9, e87072. [Google Scholar] [CrossRef]
- Abdelli, L.S.; Samsam, A.; Naser, S.A. Propionic acid induces gliosis and neuro-inflammation through modulation of PTEN/AKT pathway in autism spectrum disorder. Sci. Rep. 2019, 9, 8824. [Google Scholar] [CrossRef] [PubMed]
- Nakaita, Y.; Kaneda, H.; Shigyo, T. Heat-killed Lactobacillus brevis SBC8803 induces serotonin release from intestinal cells. Food Nutr. Sci. 2013, 4, 767–771. [Google Scholar] [CrossRef][Green Version]
- Opp, M.R. Corticotropin-releasing hormone involvement in stressor-induced alterations in sleep and in the regulation of waking. Adv. Neuroimmunol. 1995, 5, 127–143. [Google Scholar] [CrossRef]
- Hori, H.; Teraishi, T.; Sasayama, D.; Ozeki, Y.; Matsuo, J.; Kawamoto, Y.; Kinoshita, Y.; Hattori, K.; Higuchi, T.; Kunugi, H. Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/CRH test in a non-clinical population. J. Psychiatr. Res. 2011, 45, 1257–1263. [Google Scholar] [CrossRef]
- Tanida, M.; Takada, M.; Kato-Kataoka, A.; Kawai, M.; Miyazaki, K.; Shibamoto, T. Intragastric injection of Lactobacillus casei strain Shirota suppressed spleen sympathetic activation by central corticotrophin-releasing factor or peripheral 2-deoxy-d-glucose in anesthetized rats. Neurosci. Lett. 2016, 619, 114–120. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- The International Dairy Federation (IDF) Inventory of Microbial Food Cultures with Safety Demonstration in Fermented Food Products. Available online: https://store.fil-idf.org/product/bulletin-idf-n-495-2018-inventory-microbial-food-cultures-safety-demonstration-fermented-food-products/ (accessed on 19 August 2020).
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423. [Google Scholar] [CrossRef] [PubMed]

| References | Probiotics (CFUs/Day) | Target Subjects | Result |
|---|---|---|---|
| [56] | Lactobacillus casei DN-114001 (2.0 × 1010) | Children (3–6 years) (n = 638) | Incidence of common infectious diseases decreased by ingestion of probiotic drink. |
| [55] | Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1 (2.5 × 1010) | Elderly (n = 142) | Risk of catching a cold decreased by intake of yogurt. |
| [57] | Lactobacillus casei Shirota (1.3 × 1010) | Athletes (n = 84) | Incidence of URTI was reduced by intake of probiotic drink. |
| [58] | Lactobacillus casei Shirota (4.0 × 1010) | Elderly (n = 154) | Probiotic treatment reduced the duration of acute URTIs. |
| [59] | Lactobacillus brevis KB290 (6.0 × 109) | Schoolchildren (6–12 years) (n = 1089) | Incidence of influenza was reduced by intake of probiotic drink. |
| [61] | Bifidobacterium animalis subsp. lactis BB-12 (1.0 × 1010) | Infants (1 month) (n = 109) | Incidence of URTI was reduced by probiotic treatment. |
| [60] | Sachet: Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Enterococcus faecium W54, Lactobacillus acidophilus W22, Lactobacillus brevis W63 and Lactococcus lactis W58 (1.0 × 1010) | Athletes (n = 33) | Incidence of URTI was reduced by probiotic treatment. |
| [63] | Lactobacillus casei Shirota (1.0 × 1011) | Stress-burdened office workers (n = 96) | Incidence of URTI was reduced by probiotic drink. |
| [62] | Lactobacillus paracasei N1115 (1.0 × 1010) | Healthy elderly over 45 years (n = 205) | The incidence of acute URTI was reduced by probiotic treatment. |
| [64] | Lactobacillus plantarum DR7 (1.0 × 109) | Adults (n = 109) | Probiotic treatment reduced the duration of nasal symptoms and the frequency of URTI. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hori, T.; Matsuda, K.; Oishi, K. Probiotics: A Dietary Factor to Modulate the Gut Microbiome, Host Immune System, and Gut–Brain Interaction. Microorganisms 2020, 8, 1401. https://doi.org/10.3390/microorganisms8091401
Hori T, Matsuda K, Oishi K. Probiotics: A Dietary Factor to Modulate the Gut Microbiome, Host Immune System, and Gut–Brain Interaction. Microorganisms. 2020; 8(9):1401. https://doi.org/10.3390/microorganisms8091401
Chicago/Turabian StyleHori, Tetsuji, Kazunori Matsuda, and Kenji Oishi. 2020. "Probiotics: A Dietary Factor to Modulate the Gut Microbiome, Host Immune System, and Gut–Brain Interaction" Microorganisms 8, no. 9: 1401. https://doi.org/10.3390/microorganisms8091401
APA StyleHori, T., Matsuda, K., & Oishi, K. (2020). Probiotics: A Dietary Factor to Modulate the Gut Microbiome, Host Immune System, and Gut–Brain Interaction. Microorganisms, 8(9), 1401. https://doi.org/10.3390/microorganisms8091401





