Genome-Assisted Characterization of Lactobacillus fermentum, Weissella cibaria, and Weissella confusa Strains Isolated from Sorghum as Starters for Sourdough Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Phenotypic Characterization of LAB Isolated from Sorghum Flour
2.2. Safety of the Selected Isolates
2.3. Whole-Genome Sequencing and Bioinformatic Analyses
2.4. Dough Preparation
2.5. Technological Characterization of the Dough
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phenotypic Characterization of LAB Isolated from Sorghum Flour
3.2. Safety of the Selected Isolates
3.3. General Genomic Features of Candidate Starter Cultures
3.3.1. Carbohydrate Metabolism
3.3.2. Protein and Amino Acid Metabolism
3.3.3. Bioactive Compounds
3.4. Technological Properties of the Dough
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Hansen, A.; Schieberle, P. Generation of aroma compounds during sourdough fermentation: Applied and fundamental aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Katina, K.; Arendt, E.; Liukkonen, K.H.; Autio, K.; Flander, L.; Poutanen, K. Potential of sourdough for healthier cereal products. Trends Food Sci. Technol. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Fekri, A.; Torbati, M.; Yari Khosrowshahi, A.; Bagherpour Shamloo, H.; Azadmard-Damirchi, S. Functional effects of phytate-degrading, probiotic lactic acid bacteria and yeast strains isolated from Iranian traditional sourdough on the technological and nutritional properties of whole wheat bread. Food Chem. 2020. [Google Scholar] [CrossRef]
- Karaman, K.; Sagdic, O.; Durak, M.Z. Use of phytase active yeasts and lactic acid bacteria isolated from sourdough in the production of whole wheat bread. LWT-Food Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Pepe, O.; Ventorino, V.; Cavella, S.; Fagnano, M.; Brugno, R. Prebiotic content of bread prepared with flour from immature wheat grain and selected dextran-producing Lactic Acid Bacteria. Appl. Environ. Microbiol. 2013. [Google Scholar] [CrossRef]
- Arendt, E.K.; Moroni, A.; Zannini, E. Medical nutrition therapy: Use of sourdough lactic acid bacteria as a cell factory for delivering functional biomolecules and food ingredients in gluten free bread. Microb. Cell Fact. 2011. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Dal Bello, F. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Yang, Y.; Yi, H.; Zhang, L.; He, G. The influence of different lactic acid bacteria on sourdough flavor and a deep insight into sourdough fermentation through RNA sequencing. Food Chem. 2020, 1, 125529. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide producing lactic acid bacteria: Their techno-functional role and potential application in gluten-free bread products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Pétel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Denkova, R.; Ilieva, S.; Denkova, Z.; Georgieva, L.; Yordanova, M.; Nikolova, D.; Evstatieva, Y. Production of wheat bread without preservatives using sourdough starters. Biotechnol. Biotechnol. Equip. 2014. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.; Fois, S.; Falchi, G.; Campus, M.; Roggio, T.; Catzeddu, P. Effect of liquid sourdough technology on the pre-biotic, texture, and sensory properties of a crispy flatbread. Food Sci. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Giannone, V.; Giarnetti, M.; Spina, A.; Todaro, A.; Pecorino, B.; Summo, C.; Caponio, F.; Paradiso, V.M.; Pasqualone, A. Physico-chemical properties and sensory profile of durum wheat Dittaino PDO (Protected Designation of Origin) bread and quality of re-milled semolina used for its production. Food Chem. 2018. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; Di Cagno, R.; Gobbetti, M. Use of sourdough fermented wheat germ for enhancing the nutritional, texture and sensory characteristics of the white bread. Eur. Food Res. Technol. 2010. [Google Scholar] [CrossRef]
- Corsetti, A. Technology of sourdough fermentation and sourdough applications. In Handbook on Sourdough Biotechnology; Springer: Boston, MA, USA, 2013; ISBN 9781461454250. [Google Scholar]
- Denkova, R.; Ilieva, S.; Denkova, Z.; Georgieva, L.; Krastanov, A. Examination of the technological properties of newly isolated strains of the genus Lactobacillus and possibilities for their application in the composition of starters. Biotechnol. Biotechnol. Equip. 2014. [Google Scholar] [CrossRef]
- Milanovic, V.; Osimani, A.; Garofalo, C.; Belleggia, L.; Maoloni, A.; Cardinali, F.; Mozzon, M.; Foligni, R.; Aquilanti, L.; Clementi, F. Selection of cereal-sourced lactic acid bacteria as candidate starters for the baking industry. PLoS ONE 2020, 15, e0236190. [Google Scholar] [CrossRef]
- Ventimiglia, G.; Alfonzo, A.; Galluzzo, P.; Corona, O.; Francesca, N.; Caracappa, S.; Moschetti, G.; Settanni, L. Codominance of Lactobacillus plantarum and obligate heterofermentative lactic acid bacteria during sourdough fermentation. Food Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Carbó, R.; Gordún, E.; Fernández, A.; Ginovart, M. Elaboration of a spontaneous gluten-free sourdough with a mixture of amaranth, buckwheat, and quinoa flours analyzing microbial load, acidity, and pH. Food Sci. Technol. Int. 2019, 26, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Di Cagno, R.; Edema, M.O.; Nionelli, L.; Gobbetti, M. Exploitation of Acha (Digitaria exiliis) and Iburu (Digitaria iburua) flours: Chemical characterization and their use for sourdough fermentation. Food Microbiol. 2010, 27, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Moroni, A.V.; Iametti, S.; Bonomi, F.; Arendt, E.K.; Dal Bello, F. Solubility of proteins from non-gluten cereals: A comparative study on combinations of solubilising agents. Food Chem. 2010, 121, 1225–1230. [Google Scholar] [CrossRef]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Corona, O.; Alfonzo, A.; Ventimiglia, G.; Nasca, A.; Francesca, N.; Martorana, A.; Moschetti, G.; Settanni, L. Industrial application of selected lactic acid bacteria isolated from local semolinas for typical sourdough bread production. Food Microbiol. 2016, 59, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Gaggiano, M.; Di Cagno, R.; De Angelis, M.; Arnault, P.; Tossut, P.; Fox, P.F.; Gobbetti, M. Defined multi-species semi-liquid ready-to-use sourdough starter. Food Microbiol. 2007, 24, 15–24. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-forming weissella strains as starter cultures for sorghum and wheat sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Galle, S.; Gänzle, M.G.; Waters, D.M.; Arendt, E.K. Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef]
- Ricciardi, A.; Parente, E.; Zotta, T. Modelling the growth of Weissella cibaria as a function of fermentation conditions. J. Appl. Microbiol. 2009, 107, 1528–1535. [Google Scholar] [CrossRef]
- Di Cagno, R.; Rizzello, C.G.; De Angelis, M.; Cassone, A.; Giuliani, G.; Benedusi, A.; Limitone, A.; Surico, R.F.; Gobbetti, M. Use of selected sourdough strains of Lactobacillus for removing gluten and enhancing the nutritional properties of gluten-free bread. J. Food Prot. 2008, 71, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Trani, A.; Knaapila, A.; Hietala, S.; Coda, R.; Katina, K.; Maina, N.H. The effect of in situ produced dextran on flavour and texture perception of wholegrain sorghum bread. Food Hydrocoll. 2020, 106, 105913. [Google Scholar] [CrossRef]
- Katina, K.; Maina, N.H.; Juvonen, R.; Flander, L.; Johansson, L.; Virkki, L.; Tenkanen, M.; Laitila, A. In situ production and analysis of Weissella confusa dextran in wheat sourdough. Food Microbiol. 2009, 26, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Mustafa, M.M. Isolation, characterization and identification of lactic acid bacteria from fermented sorghum dough used in Sudanese Kisra preparation. Pakistan J. Nutr. 2009, 8, 1814–1818. [Google Scholar] [CrossRef][Green Version]
- Sterr, Y.; Weiss, A.; Schmidt, H. Evaluation of lactic acid bacteria for sourdough fermentation of amaranth. Int. J. Food Microbiol. 2009, 136, 75–82. [Google Scholar] [CrossRef]
- AACCI. Approved Methods of Analysis; American Association of Cereal Chemists: Saint Paul, MI, USA, 2001. [Google Scholar]
- Sekwati-Monang, B.; Gänzle, M.G. Microbiological and chemical characterisation of ting, a sorghum-based sourdough product from Botswana. Int. J. Food Microbiol. 2011, 150, 115–121. [Google Scholar] [CrossRef]
- Versalovic, J.; Schneider, M.; De Bruijn, F.J.; Lupski, J.R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 1994, 5, 25–40. [Google Scholar]
- Di Cello, F.; Fani, R. A molecular strategy for the study of natural bacterial communities by PCR-based techniques. Minerva Biotecnol. 1996, 8, 126–134. [Google Scholar]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Alfonzo, A.; Ventimiglia, G.; Corona, O.; Di Gerlando, R.; Gaglio, R.; Francesca, N.; Moschetti, G.; Settanni, L. Diversity and technological potential of lactic acid bacteria of wheat flours. Food Microbiol. 2013, 36, 343–354. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.; Vera Pingitore, E.; Rollan, G.; Martos, G.; Saavedra, L.; Fontana, C.; Hebert, E.M.; Vignolo, G. Biodiversity and technological potential of lactic acid bacteria isolated from spontaneously fermented amaranth sourdough. Lett. Appl. Microbiol. 2016, 63, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bounaix, M.S.; Gabriel, V.; Morel, S.; Robert, H.; Rabier, P.; Remaud-Siméon, M.; Gabriel, B.; Fontagné-Faucher, C. Biodiversity of exopolysaccharides produced from sucrose by sourdough lactic acid bacteria. J. Agric. Food Chem. 2009, 57, 10889–10897. [Google Scholar] [CrossRef] [PubMed]
- Nachi, I.; Fhoula, I.; Smida, I.; Ouzari, H.I.; Hassouna, M. Microbiological analysis and assessment of biotechnological potential of lactic acid bacteria isolated from Tunisian flours. Ann. Microbiol. 2019. [Google Scholar] [CrossRef]
- ISO 10932:2010. Milk and Milk Products—Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria (LAB); ISO: Geneva, Switzerland, 2010. [Google Scholar]
- European Food Safety Authority Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 1–10. [CrossRef]
- Perreten, V.; Vorlet-Fawer, L.; Slickers, P.; Ehricht, R.; Kuhnert, P.; Frey, J. Microarray-based detection of 90 antibiotic resistance genes of gram-positive bacteria. J. Clin. Microbiol. 2005, 43, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; Fluit, A.C.; Gondolf, M.; Beyrau, R.; Lindenlauf, E.; Verhoef, J.; Heinz, H.P.; Jones, M.E. The prevalence of aminoglycoside resistance and corresponding resistance genes in clinical isolates of staphylococci from 19 European hospitals. J. Antimicrob. Chemother. 1999, 43, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bover-Cid, S.; Holzapfel, W.H. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol. 1999, 53, 33–41. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- de Jong, A.; van Hijum, S.A.F.T.; Bijlsma, J.J.E.; Kok, J.; Kuipers, O.P. BAGEL: A web-based bacteriocin genome mining tool. Nucleic Acids Res. 2006. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; Garciá-Fernández, A.; Larsen, M.V.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In Silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014. [Google Scholar] [CrossRef]
- Picozzi, C.; Mariotti, M.; Cappa, C.; Tedesco, B.; Vigentini, I.; Foschino, R.; Lucisano, M. Development of a Type I gluten-free sourdough. Lett. Appl. Microbiol. 2016, 62, 119–125. [Google Scholar] [CrossRef]
- Petrofsky, K.E.; Hoseney, R.C. Rheological properties of dough made with starch and gluten from several cereal sources. Cereal Chem. 1995, 72, 53–58. [Google Scholar]
- Jin, H.; Jeong, Y.; Yoo, S.H.; Johnston, T.V.; Ku, S.; Ji, G.E. Isolation and characterization of high exopolysaccharide - producing Weissella confusa VP30 from young children’s feces. Microb. Cell Factories 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Falasconi, I.; Molinari, P.; Treu, L.; Basile, A.; Vezzi, A.; Campanaro, S.; Morelli, L. Genomic comparison of Lactobacillus helveticus strains highlights probiotic potential. Front. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Little, D.J.; Li, G.; Ing, C.; DiFrancesco, B.R.; Bamford, N.C.; Robinson, H.; Nitz, M.; Pomès, R.; Howell, P.L. Modification and periplasmic translocation of the biofilm exopolysaccharide poly-β-1,6-N-acetyl-D-glucosamine. Proc. Natl. Acad. Sci. USA 2014. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Vermeulen, N.; Vogel, R.F. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 2007. [Google Scholar] [CrossRef]
- Omoba, O.S.; Isah, L.R. Influence of sourdough fermentation on amino acids composition, phenolic profile, and antioxidant properties of sorghum biscuits. Prev. Nutr. Food Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, F.; Mandrioli, R.; Potente, G.; Taneyo Saa, D.L.; Gianotti, A. Changes in carotenoids, phenolic acids and antioxidant capacity in bread wheat doughs fermented with different lactic acid bacteria strains. Food Chem. 2019. [Google Scholar] [CrossRef]
- Mukhametzyanova, A.D.; Akhmetova, A.I.; Sharipova, M.R. Microorganisms as phytase producers. Microbiology (Russian Fed.) 2012, 81, 267–275. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Chen, N.; Shelef, L.A. Relationship between water activity, salts of lactic acid, and growth of Listeria monocytogenes in a meat model system. J. Food Prot. 1992, 55, 574–578. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K. Rheological, textural and nutritional properties of gluten-free sourdough made with functionally important lactic acid bacteria and yeast from Nigerian sorghum. LWT 2020, 120, 108875. [Google Scholar] [CrossRef]
- Schober, T.J.; Bean, S.R.; Boyle, D.L. Gluten-free sorghum bread improved by sourdough fermentation: Biochemical, rheological, and microstructural background. J. Agric. Food Chem. 2007, 55, 5137–5146. [Google Scholar] [CrossRef] [PubMed]
- Weipert, D. The benefits of basic rheometry in studying dough rheology. Cereal Chem. 1990, 67, 311–317. [Google Scholar]
- Ahmed, A.R.; Mohammed, I.; Senge, B. Oscillation Measurements and Creep Test of Bread Prepared from Wheat-Lupin Flours and Wheat Flour-Lupin Fibre Dough’s Blends. Annu. Transcations Nord. Rheol. Soc. 2012, 20, 145–152. [Google Scholar]
- Lamacchia, C.; Chillo, S.; Lamparelli, S.; Suriano, N.; La Notte, E.; Del Nobile, M.A. Amaranth, quinoa and oat doughs: Mechanical and rheological behaviour, polymeric protein size distribution and extractability. J. Food Eng. 2010, 96, 97–106. [Google Scholar] [CrossRef]
- Monthe, O.C.; Grosmaire, L.; Nguimbou, R.M.; Dahdouh, L.; Ricci, J.; Tran, T.; Ndjouenkeu, R. Rheological and textural properties of gluten-free doughs and breads based on fermented cassava, sweet potato and sorghum mixed flours. LWT 2019, 101, 575–582. [Google Scholar] [CrossRef]
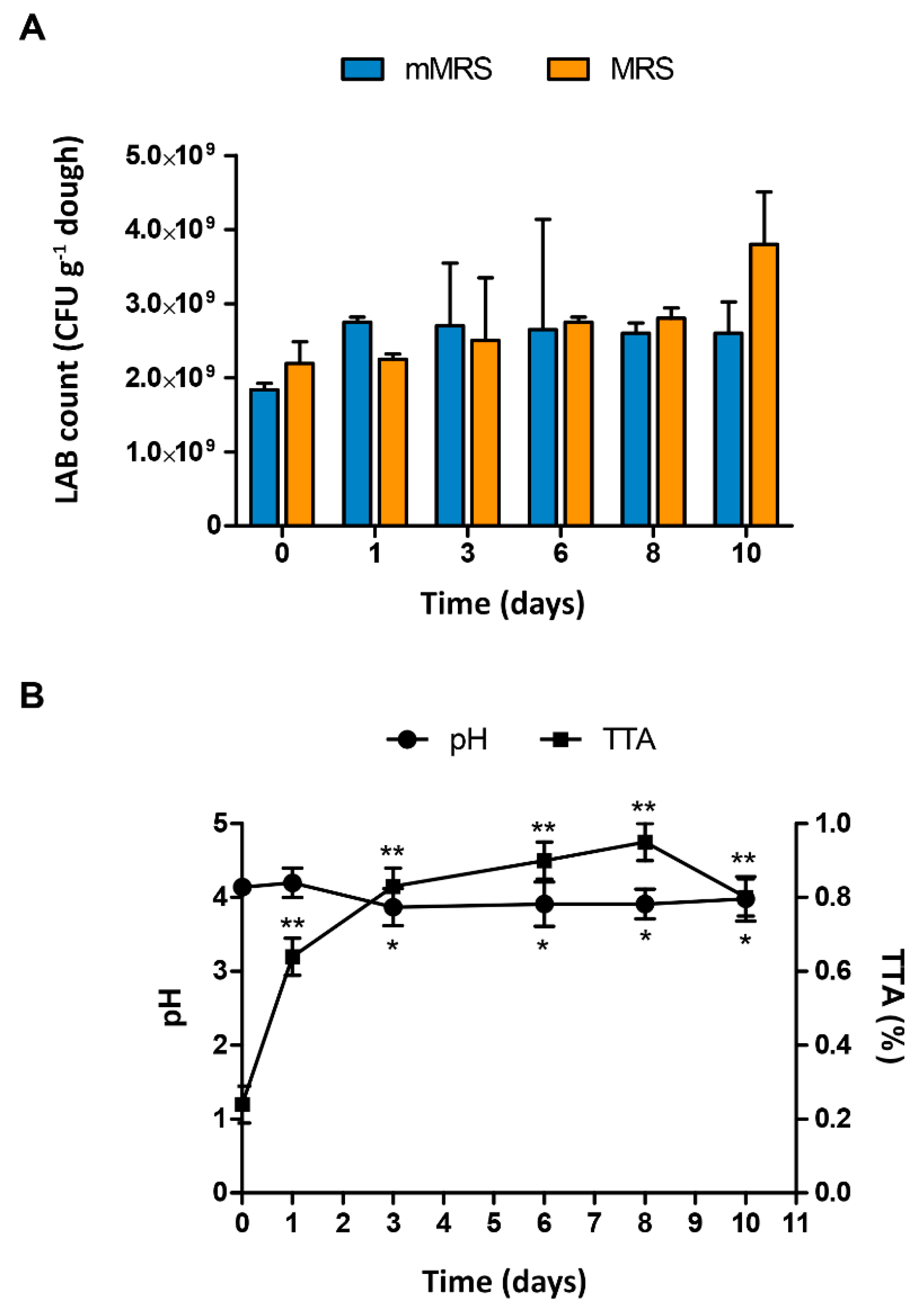
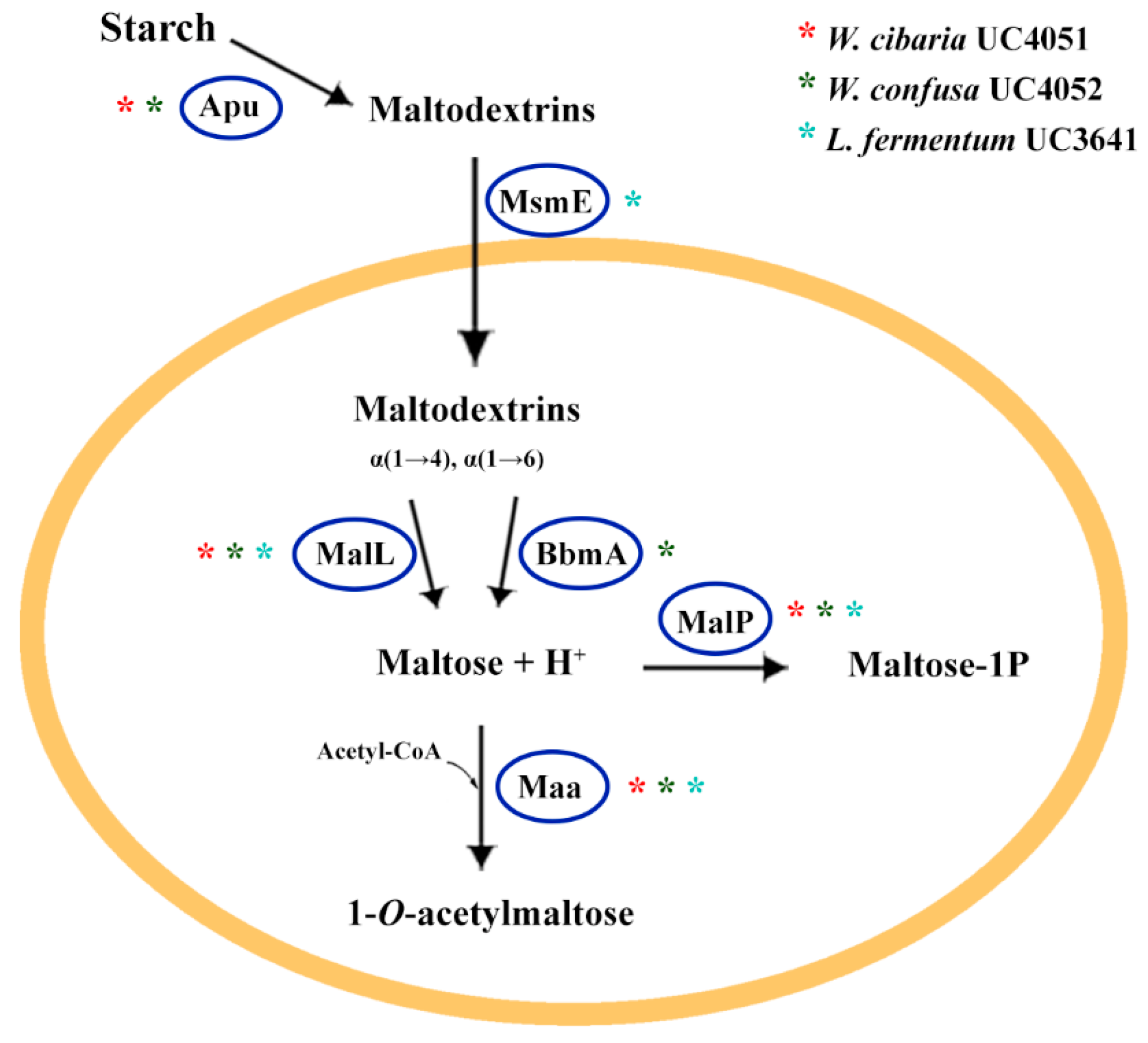
| Microorganism | Acidification (Final pH) | CO2 Production 1 | Amylolytic Activity | EPS Production 2 |
|---|---|---|---|---|
| W. paramesenteroides W1 | 3.99 ± 0.01 | + | − | − |
| W. cibaria W2 (UC4051) | 3.84 ± 0.04 | + | + | + |
| W. confusa W3 | 3.67 ± 0.03 | +++ | − | + |
| W. confusa W4 | 3.66 ± 0.01 | + | − | + |
| W. cibaria W5 | 3.98 ± 0.21 | + | − | + |
| W. paramesenteroides W6 | 3.92 ± 0.13 | + | − | − |
| W. confusa W7 | 4.08 ± 0.03 | ++ | − | + |
| W. confusa W8 (UC4052) | 3.80 ± 0.06 | + | − | ++ |
| W. cibaria W9 | 3.73 ± 0.04 | ++ | − | + |
| W. confusa W10 | 3.72 ± 0.14 | + | − | + |
| P. pentasaceus P1 | 3.64 ± 0.00 | − | − | − |
| P. pentasaceus P2 | 3.32 ± 0.04 | − | − | − |
| P. pentasaceus P3 | 3.31 ± 0.02 | − | − | − |
| P. pentasaceus P4 | 3.35 ± 0.28 | − | − | − |
| P. pentasaceus P5 | 3.77 ± 0.08 | − | − | − |
| P. pentasaceus P6 | 3.51 ± 0.11 | − | − | − |
| P. acidilactici P7 | 3.71 ± 0.06 | − | − | − |
| L. fermentum L1 | 4.03 ± 0.13 | + | − | − |
| L. fermentum L2 (UC3641) | 3.75 ± 0.12 | +++ | + | − |
| Lc. taiwanensis L3 | 3.81 ± 0.21 | − | − | − |
| Parameter | Yeast | LAB | Yeast + LAB |
|---|---|---|---|
| 50% Sorghum 1 | |||
| pH | 5.528 ± 0.024 a | 4.202 ± 0.004 b | 4.178 ± 0.008 c |
| aw | 0.9743 ± 0.0008 a | 0.9701 ± 0.0053 b | 0.9629 ± 0.0008 c |
| Weight (g) | 113.05 ± 0.61 a | 113.98 ± 0.42 b | 114.79 ± 0.42 c |
| Height (H) (cm) | 3.43 ± 0.17 a | 3.10 ± 0.17 b | 3.45 ± 0.21 a |
| Diameter (D) (cm) | 9.13 ± 0.21 a | 9.25 ± 0.29 a | 9.00 ± 0.00 a |
| Spread factor (D/H) | 2.66 ± 0.15 a | 2.98 ± 0.19 b | 2.61 ± 0.16 a |
| LAB Log (CFU/g) | 7.10 ± 0.04 a | 9.16 ± 0.06 b | 9.25 ± 0.07 b |
| Yeast Log (CFU/g) | 7.59 ± 0.01 a | 4.62 ± 0.12 b | 5.77 ± 0.10 c |
| 100 % Sorghum 2 | |||
| pH | 5.406 ± 0.011 a | 4.298 ± 0.025 b | 4.356 ± 0.040 c |
| aw | 0.9752 ± 0.0016 ab | 0.9713 ± 0.0043 b | 0.9792 ± 0.0038 a |
| Weight (g) | 114.67 ± 0.61 a | 113.08 ± 0.86 b | 110.55 ± 0.23 c |
| Height (H) (cm) | 2.75 ± 0.17 a | 2.75 ± 0.13 a | 2.80 ± 0.18 a |
| Diameter (D) (cm) | 6.88 ± 0.05 a | 7.13 ± 0.10 b | 7.15 ± 0.06 b |
| Spread factor (D/H) | 2.50 ± 0.16 a | 2.59 ± 0.13 a | 2.55 ± 0.17 a |
| LAB Log (CFU/g) | 7.62 ± 0.01 a | 9.31 ± 0.11 b | 9.42 ± 0.08 b |
| Yeast Log (CFU/g) | 7.37 ± 0.10 a | 5.49 ± 0.15 b | 6.13 ± 0.02 c |
| T0 1 | T24 | T24 | |
|---|---|---|---|
| Parameter | 1% Sugar | 10% Sugar | |
| 50% Sorghum 2 | |||
| pH | 5.876 ± 0.006 a | 4.166 ± 0.006 b | 4.356 ± 0.006 c |
| aw | 0.9795 ± 0.0107 a | 0.9717 ± 0.0018 b | 0.9676 ± 0.0023 b |
| Weight (g) | 120.23 ± 0.13 a | 112.53 ± 0.24 b | 112.48 ± 0.31 b |
| Height (H) (cm) | 2.87 ± 0.16 a | 3.28 ± 0.22 b | 2.89 ± 0.24 b |
| Diameter (D) (cm) | 6.90 ± 0.07 a | 9.15 ± 0.58 b | 9.70 ± 0.26 b |
| Spread factor (D/H) | 2.40 ± 0.14 a | 2.79 ± 0.26 b | 3.43 ± 0.29 c |
| LAB Log (CFU/g) | 7.16 ± 0.04 a | 9.46 ± 0.06 b | 9.16 ± 0.02 c |
| Yeast Log (CFU/g) | 6.19 ± 0.06 a | 6.59 ± 0.10 a | 6.50 ± 0.28 a |
| 100% Sorghum | |||
| pH | 5.930 ± 0.054 a | 4.546 ± 0.011 b | 4.684 ± 0.018 c |
| aw | 0.9760 ± 0.0098 a | 0.9704 ± 0.0020 b | 0.9626 ± 0.0010 c |
| Weight (g) | 120.40 ± 0.19 a | 113.91 ± 0.30 b | 114.12 ± 0.30 b |
| Height (H) (cm) | 2.75 ± 0.07 a | 2.90 ± 0.00 b | 2.98 ± 0.05 b |
| Diameter (D) (cm) | 6.66 ± 0.03 a | 6.50 ± 0.10 a | 6.65 ± 0.13 a |
| Spread factor (D/H) | 2.43 ± 0.06 a | 2.24 ± 0.10 b | 2.23 ± 0.06 b |
| LAB Log (CFU/g) | 7.78 ± 0.01 a | 9.39 ± 0.02 b | 9.17 ± 0.07 b |
| Yeast Log (CFU/g) | 6.15 ± 0.02 a | 5.71 ± 0.16 b | 5.61 ± 0.19 b |
| t0 | t24 | |||||
|---|---|---|---|---|---|---|
| Dough | n (−) | G0′ (Pa) | R2 | n (−) | G0′ (Pa) | R2 |
| 100% Sorghum + Yeast | 0.118 | 158,800 | 0.992 | 0.103 | 166,809 | 0.996 |
| 100% Sorghum + LAB | 0.107 | 178,813 | 0.996 | 0.114 | 157,217 | 0.999 |
| 100% Sorghum + Yeast + LAB | 0.113 | 149,142 | 0.994 | 0.120 | 54,790 | 0.994 |
| 50% Sorghum + Yeast | 0.160 | 17,128 | 0.997 | 0.283 | 5,857 | 0.987 |
| 50% Sorghum + LAB | 0.158 | 28,474 | 0.989 | 0.187 | 17,689 | 0.997 |
| 50% Sorghum + Yeast + LAB | 0.175 | 21,535 | 0.993 | 0.241 | 12,405 | 0.984 |
| 100% Sorghum + 10% Sucrose + Yeast + LAB | 0.100 | 119,729 | 0.992 | 0.126 | 104,761 | 0.999 |
| 100% Sorghum + 1% Sucrose + Yeast + LAB | 0.113 | 149,142 | 0.994 | 0.120 | 54,790 | 0.994 |
| 50% Sorghum + 10% Sucrose + Yeast + LAB | 0.160 | 12515 | 0.994 | 0.316 | 4688 | 0.981 |
| 50% Sorghum + 1% Sucrose + Yeast + LAB | 0.176 | 24949 | 0.993 | 0.251 | 8054 | 0.981 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falasconi, I.; Fontana, A.; Patrone, V.; Rebecchi, A.; Duserm Garrido, G.; Principato, L.; Callegari, M.L.; Spigno, G.; Morelli, L. Genome-Assisted Characterization of Lactobacillus fermentum, Weissella cibaria, and Weissella confusa Strains Isolated from Sorghum as Starters for Sourdough Fermentation. Microorganisms 2020, 8, 1388. https://doi.org/10.3390/microorganisms8091388
Falasconi I, Fontana A, Patrone V, Rebecchi A, Duserm Garrido G, Principato L, Callegari ML, Spigno G, Morelli L. Genome-Assisted Characterization of Lactobacillus fermentum, Weissella cibaria, and Weissella confusa Strains Isolated from Sorghum as Starters for Sourdough Fermentation. Microorganisms. 2020; 8(9):1388. https://doi.org/10.3390/microorganisms8091388
Chicago/Turabian StyleFalasconi, Irene, Alessandra Fontana, Vania Patrone, Annalisa Rebecchi, Guillermo Duserm Garrido, Laura Principato, Maria Luisa Callegari, Giorgia Spigno, and Lorenzo Morelli. 2020. "Genome-Assisted Characterization of Lactobacillus fermentum, Weissella cibaria, and Weissella confusa Strains Isolated from Sorghum as Starters for Sourdough Fermentation" Microorganisms 8, no. 9: 1388. https://doi.org/10.3390/microorganisms8091388
APA StyleFalasconi, I., Fontana, A., Patrone, V., Rebecchi, A., Duserm Garrido, G., Principato, L., Callegari, M. L., Spigno, G., & Morelli, L. (2020). Genome-Assisted Characterization of Lactobacillus fermentum, Weissella cibaria, and Weissella confusa Strains Isolated from Sorghum as Starters for Sourdough Fermentation. Microorganisms, 8(9), 1388. https://doi.org/10.3390/microorganisms8091388








