Integrated qPCR and Staining Methods for Detection and Quantification of Enterocytozoon hepatopenaei in Shrimp Litopenaeus vannamei
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Treatment and Dna Extraction
2.2. Synthesis of Primers and Conventional PCR Amplification
2.3. Construction of the Standard Sample
2.4. Optimization of the Reaction System
2.5. Generation of the Standard Curve
2.6. Specificity Analysis
2.7. Sensitivity Analysis
2.8. Repeatability Analysis
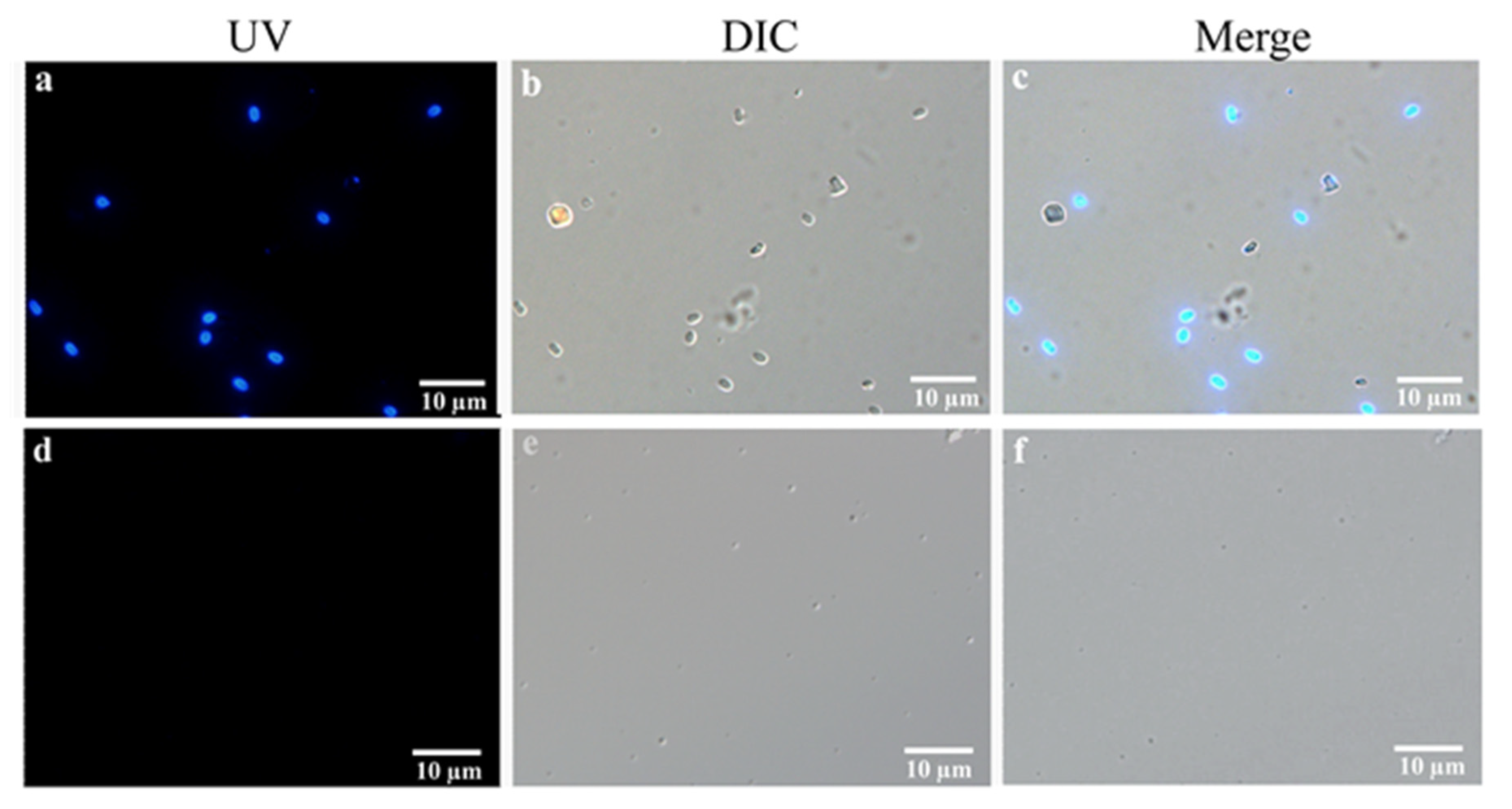
2.9. Microscopy Analysis
3. Results
3.1. qPCR Standard Curve
3.2. Specificity Analysis
3.3. Sensitivity Analysis
3.4. Repeatability Analysis
3.5. Integrated PTP2-qPCR and Microscopy Analysis EHP in Field-Shrimp
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barandun, J.; Hunziker, M.; Vossbrinck, C.R.; Klinge, S. Evolutionary compaction and adaptation visualized by the structure of the dormant microsporidian ribosome. Nat. Microbiol. 2019, 4, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Franchet, A.; Niehus, S.; Caravello, G.; Ferrandon, D. Phosphatidic acid as a limiting host metabolite for the proliferation of the microsporidium Tubulinosema ratisbonensis in Drosophila flies. Nat. Microbiol. 2019, 4, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Duzlu, O.; Yildirim, A.; Onder, Z.; Ciloglu, A.; Yetismis, G.; Inci, A. Prevalence and genotyping of microsporidian parasites in dogs in Turkey: Zoonotic concerns. J. Eukaryot. Microbiol. 2019, 66, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Weiss, L.M. Microsporidia: Obligate intracellular pathogens within the fungal kingdom. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Long, M.; Tan, Y.; Yu, B.; Pan, G.; Zhou, Z. Expression of Nosema bombycis polar tube protein 1 in lepidopteran Sf9 cells and its effect on microsporidian proliferation. J. Invertebr. Pathol. 2020, 172, 107350. [Google Scholar] [CrossRef]
- Chayaburakul, K.; Nash, G.; Pratanpipat, P.; Sriurairatana, S.; Withyachumnarnkul, B. Multiple pathogens found in growth-retarded black tiger shrimp Penaeus monodon cultivated in Thailand. Dis. Aquat. Organ. 2004, 60, 89–96. [Google Scholar] [CrossRef]
- Texier, C.; Vidau, C.; Viguès, B.; El Alaoui, H.; Delbac, F. Microsporidia: A model for minimal parasite-host interactions. Curr. Opin. Microbiol. 2010, 13, 443–449. [Google Scholar] [CrossRef]
- Santhoshkumar, S.; Sivakumar, S.; Vimal, S.; Abdul Majeed, S.; Taju, G.; Haribabu, P.; Uma, A.; Sahul Hameed, A.S. Biochemical changes and tissue distribution of Enterocytozoon hepatopenaei (EHP) in naturally and experimentally EHP-infected whiteleg shrimp, Litopenaeus vannamei (Boone, 1931), in India. J. Fish Dis. 2017, 40, 529–539. [Google Scholar] [CrossRef]
- Tang, K.; Han, J.E.; Aranguren, L.F.; WhiteNoble, B.; Schmidt, M.M.; Piamsomboon, P.; Risdiana, E.; Hanggono, B. Dense populations of the microsporidian Enterocytozoon hepatopenaei (EHP) in feces of Penaeus vannamei exhibiting white feces syndrome and pathways of their transmission to healthy shrimp. J. Invertebr. Pathol. 2016, 140, 1–7. [Google Scholar] [CrossRef]
- Tangprasittipap, A.; Srisala, J.; Chouwdee, S.; Somboon, M.; Chuchird, N.; Limsuwan, C.; Srisuvan, T.; Flegel, T.W.; Sritunyalucksana, K. The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome in whiteleg shrimp Penaeus (Litopenaeus) vannamei. BMC Vet. Res. 2013, 9, 139. [Google Scholar] [CrossRef]
- Rajendran, K.V.; Shivam, S.; Praveena, P.E.; Rajan, J.J.S.; Kumar, T.S.; Avunje, S.; Jagadeesan, V.; Babu, S.V.A.N.V.P.; Pande, A.; Krishnan, A.N.; et al. Emergence of Enterocytozoon hepatopenaei (EHP) in farmed Penaeus (Litopenaeus) vannamei in India. Aquaculture 2016, 454, 272–280. [Google Scholar] [CrossRef]
- Aranguren, L.F.; Han, J.E.; Tang, K.F.J. Enterocytozoon hepatopenaei (EHP) is a risk factor for acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN) in the Pacific white shrimp Penaeus vannamei. Aquaculture 2017, 471, 37–42. [Google Scholar] [CrossRef]
- Tang, K.; Aranguren, L.F.; Piamsomboon, P.; Han, J.E.; Maskaykina, I.Y.; Schmidt, M.M. Detection of the microsporidian Enterocytozoon hepatopenaei (EHP) and Taura syndrome virus in Penaeus vannamei cultured in Venezuela. Aquaculture 2017, 480, 17–21. [Google Scholar] [CrossRef]
- Weiss, L.M. Microsporidia: Emerging pathogenic protists. Acta Trop. 2001, 78, 89–102. [Google Scholar] [CrossRef]
- Salachan, P.V.; Jaroenlak, P.; Thitamadee, S.; Itsathitphaisarn, O.; Sritunyalucksana, K. Laboratory cohabitation challenge model for shrimp hepatopancreatic microsporidiosis (HPM) caused by Enterocytozoon hepatopenaei (EHP). BMC Vet. Res. 2017, 13, 9. [Google Scholar] [CrossRef]
- Vu-Khac, H.; Thanh, T.N.T.; Thu, G.N.T.; Le, C.H.; Nguyen, V.D. Vertical transmission and early diagnosis of the microsporidian Enterocytozoon hepatonaei in whiteleg shrimp Penaeus vannamei. J. Pure Appl. Microbiol. 2018, 12, 1125–1131. [Google Scholar] [CrossRef]
- Aldama-Cano, D.J.; Sanguanrut, P.; Munkongwongsiri, N.; Ibarra-Gámez, J.C.; Itsathitphaisarn, O.; Vanichviriyakit, R.; Flegel, T.W.; Sritunyalucksana, K.; Thitamadee, S. Bioassay for spore polar tube extrusion of shrimp Enterocytozoon hepatopenaei (EHP). Aquaculture 2018, 490, 156–161. [Google Scholar] [CrossRef]
- Zhao, R.; Gao, W.; Qiu, L.; Chen, X.; Dong, X.; Li, C.; Huang, J. A staining method for detection of Enterocytozoon hepatopenaei (EHP) spores with calcofluor white. J. Invertebr. Pathol. 2020, 172, 107347. [Google Scholar] [CrossRef]
- Suebsing, R.; Prombun, P.; Srisala, J.; Kiatpathomchai, W. Loop-mediated isothermal amplification combined with colorimetric nanogold for detection of the microsporidian Enterocytozoon hepatopenaei in penaeid shrimp. J. Appl. Microbiol. 2013, 114, 1254–1263. [Google Scholar] [CrossRef]
- Ning, M.; Wei, P.; Shen, H.; Wan, X.; Jin, M.; Li, X.; Shi, H.; Qiao, Y.; Jiang, G.; Gu, W.; et al. Proteomic and metabolomic responses in hepatopancreas of whiteleg shrimp Litopenaeus vannamei infected by microsporidian Enterocytozoon hepatopenaei. Fish Shellfish. Immunol. 2019, 87, 534–545. [Google Scholar] [CrossRef]
- Cai, S.; Kong, F.; Xu, S.; Yao, C. Real-time loop-mediated isothermal amplification for rapid detection of Enterocytozoon hepatopenaei. PeerJ 2018, 6, e5993. [Google Scholar] [CrossRef] [PubMed]
- Jaroenlak, P.; Sanguanrut, P.; Williams, B.A.; Stentiford, G.D.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. A nested PCR assay to avoid false positive detection of the microsporidian Enterocytozoon hepatopenaei (EHP) in environmental samples in shrimp farms. PLoS ONE 2016, 11, e0166320. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Weiss, L.M. The microsporidian polar tube: A highly specialised invasion organelle. Int. J. Parasitol. 2005, 35, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Delbac, F.; Peuvel, I.; Metenier, G.; Peyretaillade, E.; Vivares, C.P. Microsporidian invasion apparatus: Identification of a novel polar tube protein and evidence for clustering of ptp1 and ptp2 genes in three Encephalitozoon species. Infect. Immun. 2001, 69, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Peuvel, I.; Peyret, P.; Méténier, G.; Vivarès, C.P.; Delbac, F. The microsporidian polar tube: Evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi. Mol. Biochem. Parasitol. 2002, 122, 69–80. [Google Scholar] [CrossRef]
- Brosson, D.; Kuhn, L.; Delbac, F.; Garin, J.; Vivarès, C.P.; Texier, C. Proteomic analysis of the eukaryotic parasite Encephalitozoon cuniculi (microsporidia): A reference map for proteins expressed in late sporogonial stages. Proteomics 2006, 6, 3625–3635. [Google Scholar] [CrossRef]
- Polonais, V.; Prensier, G.; Méténier, G.; Vivarès, C.P.; Delbac, F. Microsporidian polar tube proteins: Highly divergent but closely linked genes encode PTP1 and PTP2 in members of the evolutionarily distant Antonospora and Encephalitozoon groups. Fungal Genet. Biol. 2005, 42, 791–803. [Google Scholar] [CrossRef]
- Cornman, R.S.; Chen, Y.P.; Schatz, M.C.; Street, C.; Zhao, Y.; Desany, B.; Egholm, M.; Hutchison, S.; Pettis, J.S.; Lipkin, W.I.; et al. Genomic analyses of the microsporidian Nosema ceranae, an emergent pathogen of honey bees. PLoS Pathog. 2009, 5, e1000466. [Google Scholar] [CrossRef]
- Pan, G.; Xu, J.; Li, T.; Xia, Q.; Liu, S.; Zhang, G.; Li, S.; Li, C.; Liu, H.; Yang, L.; et al. Comparative genomics of parasitic silkworm microsporidia reveal an association between genome expansion and host adaptation. BMC Genom. 2013, 14, 186. [Google Scholar] [CrossRef]
- Kanitchinda, S.; Srisala, J.; Suebsing, R.; Prachumwat, A.; Chaijarasphong, T. CRISPR-Cas fluorescent cleavage assay coupled with recombinase polymerase mmplification for sensitive and specific detection of Enterocytozoon hepatopenaei. Biotechnol. Rep. 2020, 27, e00485. [Google Scholar] [CrossRef]
- Rasconi, S.; Jobard, M.; Jouve, L.; Sime-Ngando, T. Use of calcofluor white for detection, identification, and quantification of phytoplanktonic fungal parasites. Appl. Environ. Microbiol. 2009, 75, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Polonais, V.; Sugi, T.; Yakubu, R.; Takvorian, P.M.; Cali, A.; Maier, K.; Long, M.; Levy, M.; Tanowitz, H.B.; et al. The role of microsporidian polar tube protein 4 (PTP4) in host cell infection. PLoS Pathog. 2017, 13, e1006341. [Google Scholar] [CrossRef] [PubMed]
- Tourtip, S.; Wongtripop, S.; Stentiford, G.D.; Batemanc, K.S.; Sriurairatanad, S.; Chavadeja, J.; Sritunyalucksanad, K.; Withyachumnarnkulad, B. Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae), a parasite of the black tiger shrimp Penaeus monodon (Decapoda: Penaeidae): Fine structure and phylogenetic relationships. J. Invertebr. Pathol. 2009, 102, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, L.; Sheng, A.; Wan, X.; Cheng, D.; Huang, J. Quantitative detection method of Enterocytozoon hepatopenaei using TaqMan probe real-time PCR. J. Invertebr. Pathol. 2018, 151, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Makesh, M. Visual loop-mediated isothermal amplification (LAMP) for the rapid diagnosis of Enterocytozoon hepatopenaei (EHP) infection. Parasitol. Res. 2018, 117, 1485–1493. [Google Scholar] [CrossRef]
- Tang, K.F.; Pantoja, C.R.; Redman, R.M.; Han, J.E.; Tran, L.H.; Lightner, D.V. Development of in situ hybridization and PCR assays for the detection of Enterocytozoon hepatopenaei (EHP), a microsporidian parasite infecting penaeid shrimp. J. Invertebr. Pathol. 2015, 130, 37–41. [Google Scholar] [CrossRef]
- Snow, J.W.; Ceylan Koydemir, H.; Karinca, D.K.; Liang, K.; Tseng, D.; Ozcan, A. Rapid imaging, detection, and quantification of Nosema ceranae spores in honey bees using mobile phone-based fluorescence microscopy. Lab. Chip. 2019, 19, 789–797. [Google Scholar] [CrossRef]




| Primer | Sequence (5′-3′) | PCR Length |
|---|---|---|
| EHP-PTP2-F (qPCR) | GCAGCACTCAAGGAATGGC | 238 bp |
| EHP-PTP2-R (qPCR) | TTTCGTTAGGCTTACCCTGTGA | |
| EHP-PTP2-F | ATGAGTCTTTATAATGCACTG | 855 bp |
| EHP-PTP2-R | TTATTCGTTGGATGTTAATG | |
| EHP-SSU-F | GATGGCTCCCACGTCCAAGG | 913 bp |
| EHP-SSU-R | GAACAGGGACACATTCACAA | |
| EHP-Tubulin-F | ATGAGAGAAATTATTCATGTACAGG | 1317 bp |
| EHP-Tubulin-R | TTAATAACCTCCTTCTTCAATAAC |
| Reaction System | |
|---|---|
| 2×Hieff® qPCR SYBR Green Master Mix | 5.0 μL |
| EHP-PTP2-F | 0.2 μM |
| EHP-PTP2-R | 0.2 μM |
| Template DNA | 1.0 μL |
| ddH2O | Add to 10 μL |
| Sample No. | People | Mean Cq Value ± S | Cq SD | CV/% |
|---|---|---|---|---|
| 1 | 3 | 29.64 ± 0.30 | 0.2154 | 0.7266 |
| 2 | 3 | 21.59 ± 0.05 | 0.0370 | 0.1713 |
| 3 | 3 | 22.41 ± 0.08 | 0.0580 | 0.2589 |
| 4 | 3 | 15.37 ± 0.05 | 0.0412 | 0.2681 |
| 5 | 3 | 7.97 ± 0.07 | 0.0500 | 0.6274 |
| Samples | EHP # Copies/mg | Spore Number * /(field × mg) |
|---|---|---|
| 1 | 1.20 × 106 | 45.23 |
| 2 | 1.08 × 106 | 39.66 |
| 3 | 6.27 × 105 | 16.14 |
| 4 | 4.51 × 105 | 14.78 |
| 5 | 2.36 × 105 | 13.88 |
| 6 | 1.14 × 105 | 7.16 |
| 7 | 2.63 × 104 | 5.91 |
| 8 | 2.22 × 104 | 3.19 |
| 9 | 1.29 × 104 | 2.28 |
| 10 | 9.95 × 103 | 1.56 |
| 11 | 4.34 × 103 | 0.69 |
| 12 | 8.15 × 102 | 0.00 |
| 13 | 7.41 × 102 | 0.00 |
| 14 | 6.84 × 101 | 0.23 |
| 15 | 2.58 × 101 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Lv, Q.; He, Y.; Gu, R.; Zhou, B.; Chen, J.; Fan, X.; Pan, G.; Long, M.; Zhou, Z. Integrated qPCR and Staining Methods for Detection and Quantification of Enterocytozoon hepatopenaei in Shrimp Litopenaeus vannamei. Microorganisms 2020, 8, 1366. https://doi.org/10.3390/microorganisms8091366
Wang L, Lv Q, He Y, Gu R, Zhou B, Chen J, Fan X, Pan G, Long M, Zhou Z. Integrated qPCR and Staining Methods for Detection and Quantification of Enterocytozoon hepatopenaei in Shrimp Litopenaeus vannamei. Microorganisms. 2020; 8(9):1366. https://doi.org/10.3390/microorganisms8091366
Chicago/Turabian StyleWang, Lijun, Qing Lv, Yantong He, Ruocheng Gu, Bingqian Zhou, Jie Chen, Xiaodong Fan, Guoqing Pan, Mengxian Long, and Zeyang Zhou. 2020. "Integrated qPCR and Staining Methods for Detection and Quantification of Enterocytozoon hepatopenaei in Shrimp Litopenaeus vannamei" Microorganisms 8, no. 9: 1366. https://doi.org/10.3390/microorganisms8091366
APA StyleWang, L., Lv, Q., He, Y., Gu, R., Zhou, B., Chen, J., Fan, X., Pan, G., Long, M., & Zhou, Z. (2020). Integrated qPCR and Staining Methods for Detection and Quantification of Enterocytozoon hepatopenaei in Shrimp Litopenaeus vannamei. Microorganisms, 8(9), 1366. https://doi.org/10.3390/microorganisms8091366






