Dissecting Transcription Factor-Target Interaction in Bovine Coronavirus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Genes Associated with Bovine Coronavirus Pathway
2.2. Prediction of Disease Associated Bovine Transcription Factors
2.3. Functional Analysis of Transcription Factors and Gene Targets
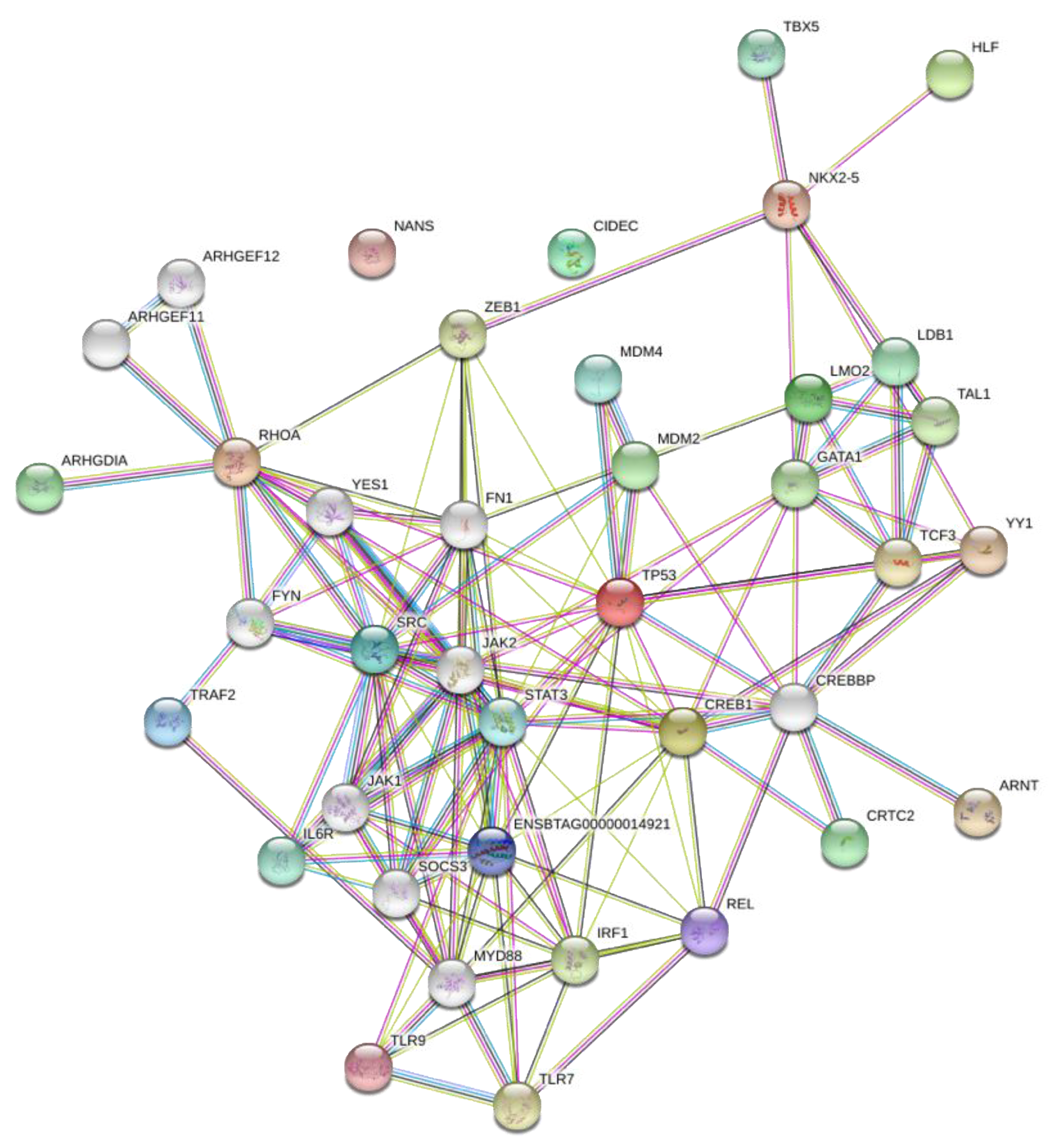
2.4. Network Analysis of Transcription Factors and Genes
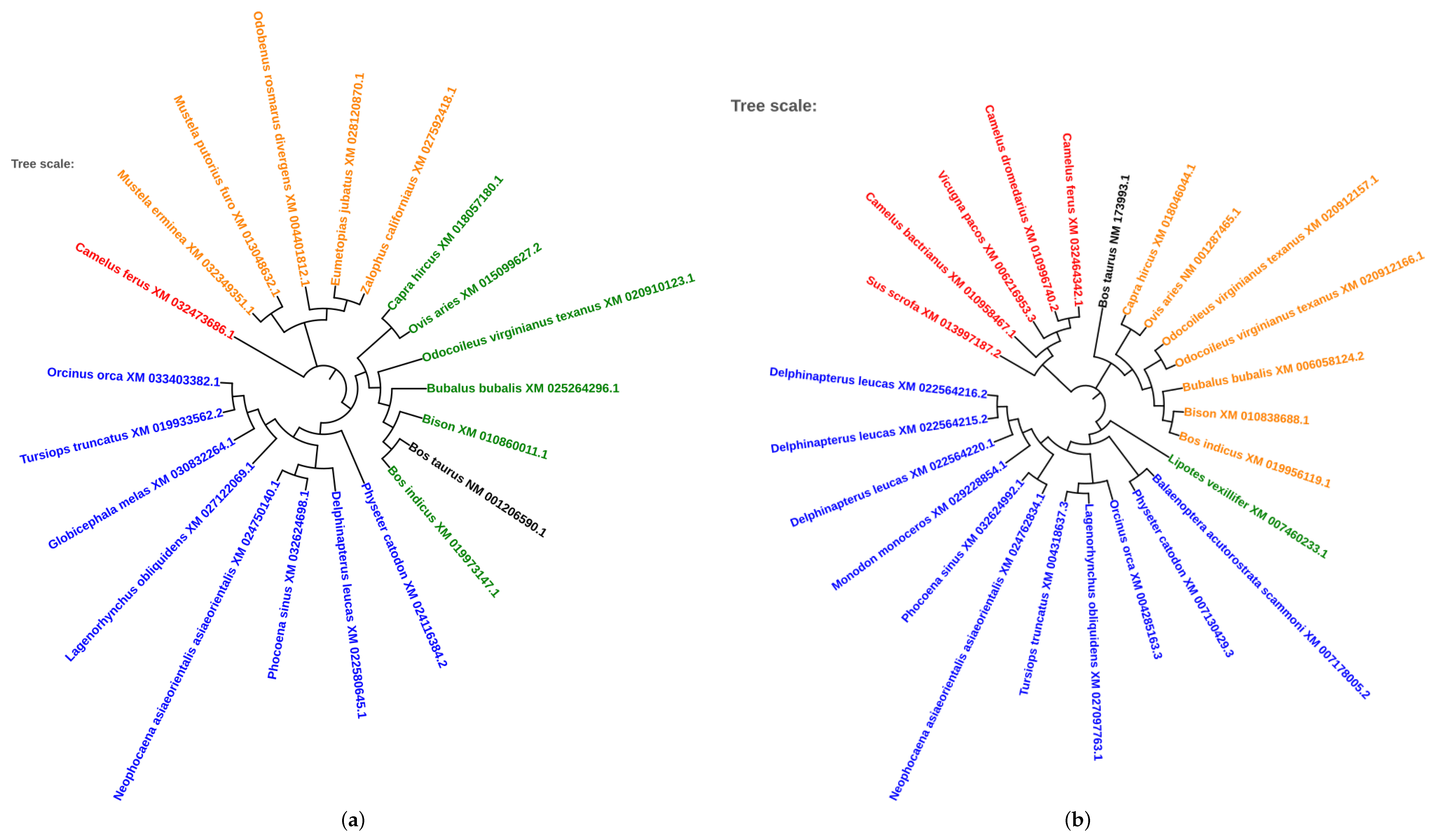
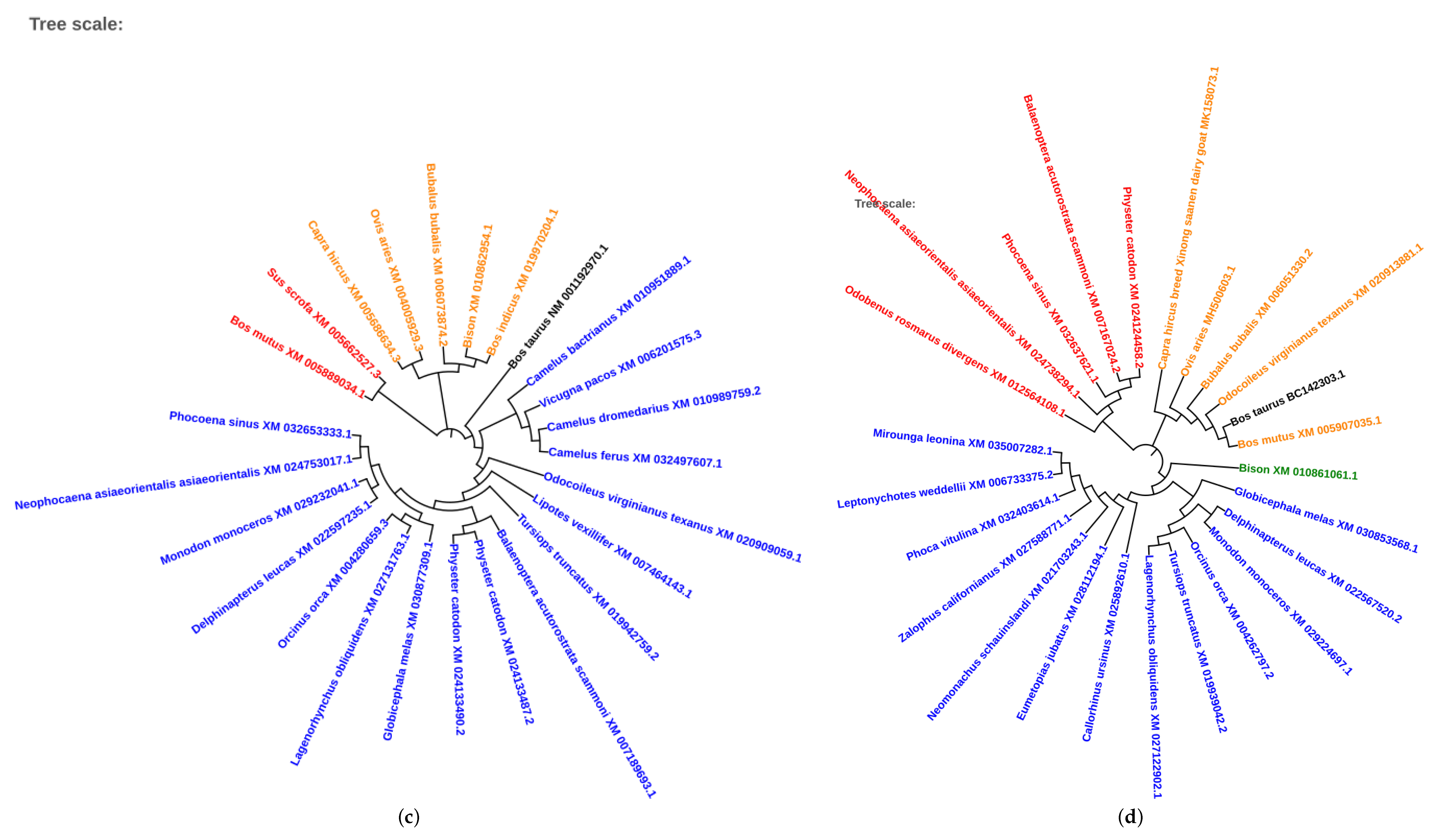
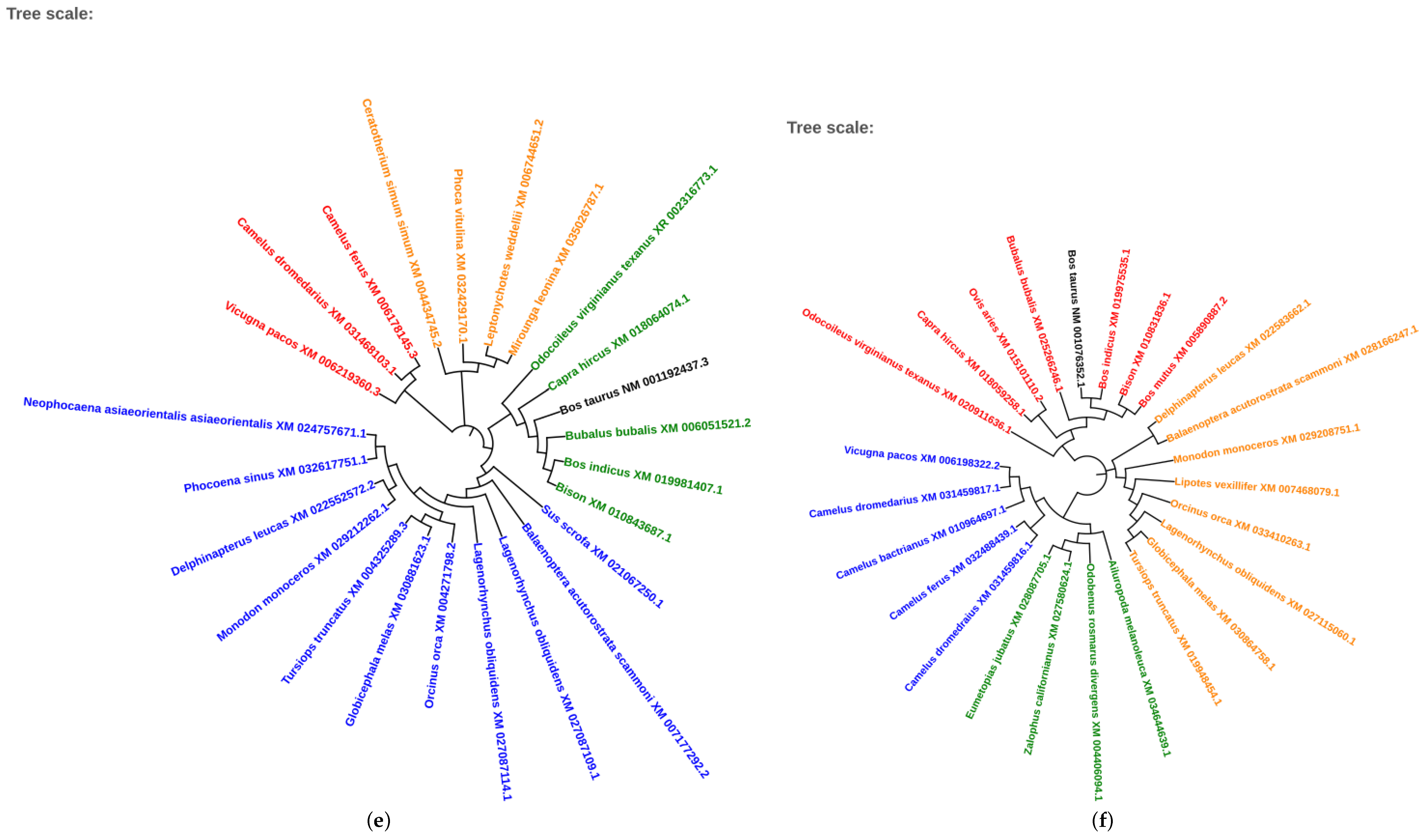
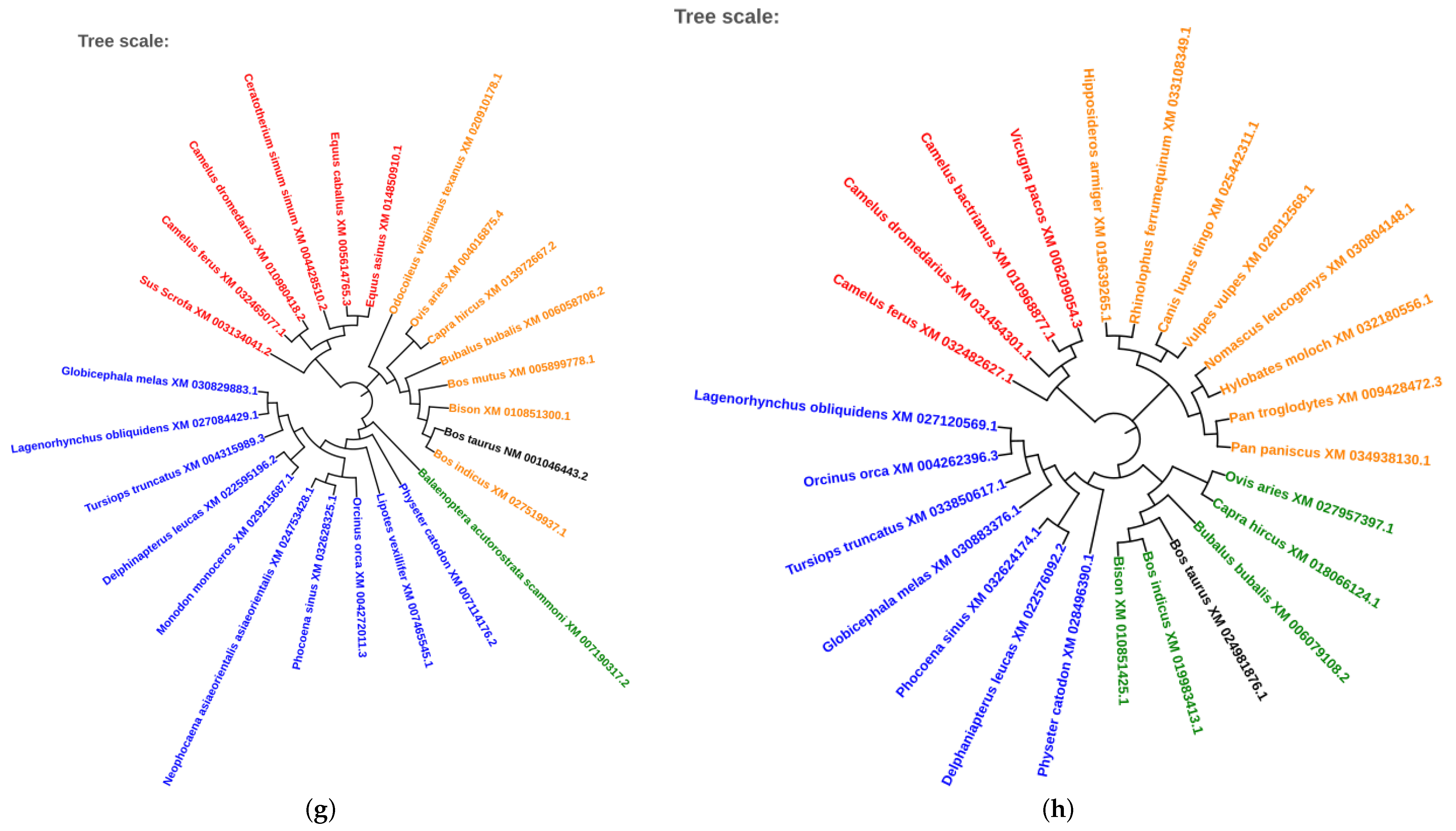
2.5. Phylogenetic Analysis of Conserved Transcription Factors
3. Results
3.1. Identification of Genes and TFs Associated with Bovine Coronavirus
3.2. Functional Analysis of Transcription Factors and Genes
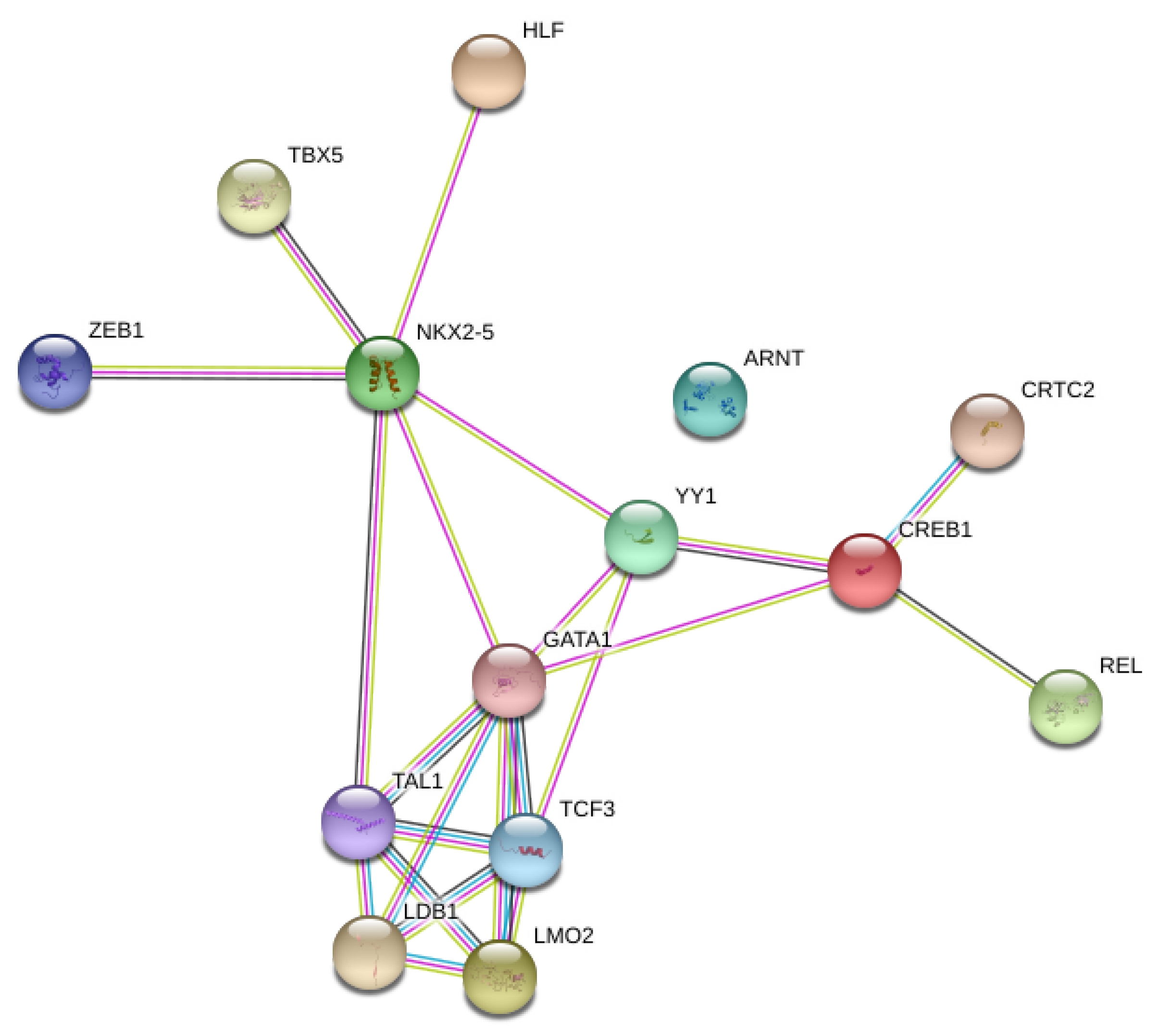
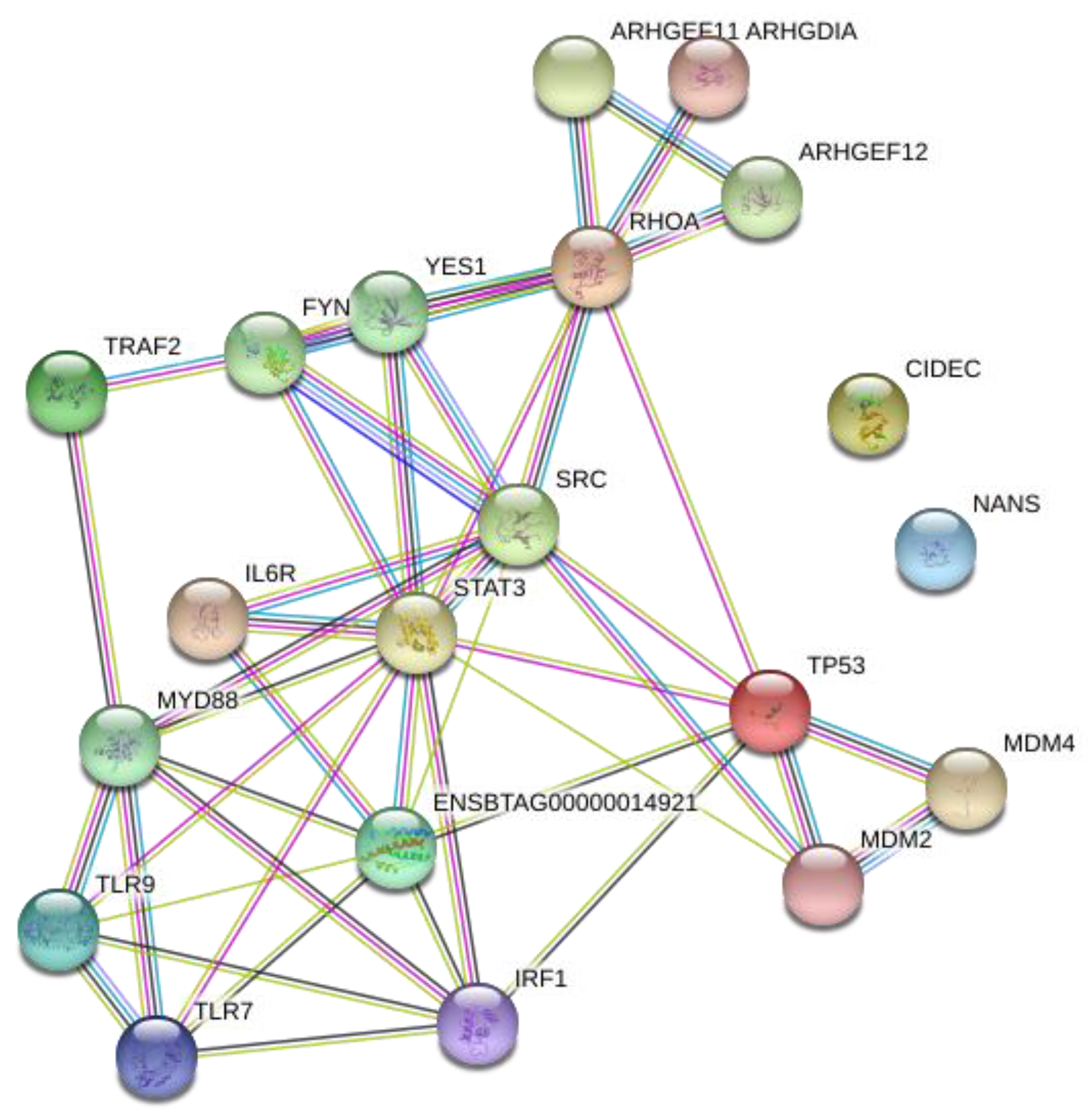
3.3. Network Analysis of Transcription Factors, Genes, and Genes and Transcription Factors
3.4. Evolutionary Conservation of Transcription Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shin, J.; Tark, D.; Le, V.P.; Choe, S.; Cha, R.M.; Park, G.-N.; Cho, I.-S.; Nga, B.T.T.; Lan, N.T.; An, D.-J. Genetic characterization of bovine coronavirus in Vietnam. Virus Genes 2019, 55, 415–420. [Google Scholar] [CrossRef]
- Ellis, J. What is the evidence that bovine coronavirus is a biologically significant respiratory pathogen in cattle? Can. Veter. J. 2019, 60, 147–152. [Google Scholar]
- Maclachlan, N.J.; Dubovi, E.J. (Eds.) Fenner’s Veterinary Virology; Academic Press: Salt Lake City, UT, USA, 2011. [Google Scholar]
- Bidokhti, M.R.M.; Tråvén, M.; Krishna, N.K.; Munir, M.; Belák, S.; Alenius, S.; Cortey, M. Evolutionary dynamics of bovine coronaviruses: Natural selection pattern of the spike gene implies adaptive evolution of the strains. J. Gen. Virol. 2013, 94, 2036–2049. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J. Bovine respiratory coronavirus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-I.; Yoon, K.-J. An overview of calf diarrhea—Infectious etiology, diagnosis, and intervention. J. Veter. Sci. 2014, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.; Giannitti, F.; Caffarena, R.D.; Casaux, M.L.; Schild, C.; Castells, D.; Riet-Correa, F.; Victoria, M.; Parreño, V.; Colina, R. Bovine coronavirus in Uruguay: Genetic diversity, risk factors and transboundary introductions from neighboring countries. Arch. Virol. 2019, 164, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Boileau, M.J.; Kapil, S. Bovine Coronavirus Associated Syndromes. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Oma, V.S.; Tråvén, M.; Alenius, S.; Myrmel, M.; Stokstad, M. Bovine coronavirus in naturally and experimentally exposed calves; viral shedding and the potential for transmission. Virol. J. 2016, 13, 100. [Google Scholar] [CrossRef]
- Burimuah, V.; Sylverken, A.; Owusu, M.; El-Duah, P.; Yeboah, R.; Lamptey, J.; Agbenyega, O.; Folitse, R.; Emikpe, B.; Tasiame, W.; et al. Molecular-based cross-Species evaluation of bovine coronavirus infection in cattle, sheep and goats in Ghana. BMC Vet. Res. 2020, preprint. [Google Scholar] [CrossRef]
- Geng, J.-J.; Gong, Z.-D.; Li, Q.-Y.; Shen, X.-Y.; Wei, S.-C. Specific Detection of Bovine Coronavirus N Protein with TaqMan Probe qRT-PCR. Acta Sci. Vet. 2019, 47. [Google Scholar] [CrossRef]
- Suzuki, T.; Otake, Y.; Uchimoto, S.; Hasebe, A.; Goto, Y. Genomic Characterization and Phylogenetic Classification of Bovine Coronaviruses through Whole Genome Sequence Analysis. Viruses 2020, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Moustaqil, M.; Gambin, Y.; Sierecki, E. Biophysical Techniques for Target Validation and Drug Discovery in Transcription-Targeted Therapy. Int. J. Mol. Sci. 2020, 21, 2301. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, K.; Pillay, S.; Bin Ahmad, N.R.; Bikádi, Z.; Hazai, E.; Yan, L.; Kolatkar, P.R.; Pervushin, K.; Jauch, R. Identification of a Polyoxometalate Inhibitor of the DNA Binding Activity of Sox2. ACS Chem. Biol. 2011, 6, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Souissi, I.; Ladam, P.; Cognet, J.; Le Coquil, S.; Varin-Blank, N.; Baran-Marszak, F.; Metelev, V.; Fagard, R. A STAT3-inhibitory hairpin decoy oligodeoxynucleotide discriminates between STAT1 and STAT3 and induces death in a human colon carcinoma cell line. Mol. Cancer 2012, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Dong, Z.; Chen, Y.; Wang, F.; Wang, C.J.; Peng, H.; He, Y.; Hangoc, G.; Pollok, K.; Sandusky, G.; et al. Small-molecule inhibitors targeting the DNA-binding domain of STAT3 suppress tumor growth, metastasis and STAT3 target gene expression in vivo. Oncogene 2016, 35, 783–792. [Google Scholar] [CrossRef]
- Morenikeji, O.B.; Hawkes, M.E.; Hudson, A.O.; Thomas, B.N. Computational Network Analysis Identifies Evolutionarily Conserved miRNA Gene Interactions Potentially Regulating Immune Response in Bovine Trypanosomosis. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Kontou, P.I.; Pavlopoulou, A.; Braliou, G.G.; Bogiatzi, S.; Dimou, N.L.; Bangalore, S.; Bagos, P.G. Identification of gene expression profiles in myocardial infarction: A systematic review and meta-analysis. BMC Med. Genom. 2018, 11, 109. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. The Gene Ontology Consortium Gene Ontology Consortium: Going forward. Nucleic Acids Res. 2015, 43, D1049–D1056. [Google Scholar] [CrossRef]
- Wang, G.; Wang, F.; Huang, Q.; Li, Y.; Liu, Y.; Wang, Y. Understanding Transcription Factor Regulation by Integrating Gene Expression and DNase I Hypersensitive Sites. BioMed. Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Farrel, A.; Guo, J.-T. An efficient algorithm for improving structure-based prediction of transcription factor binding sites. BMC Bioinform. 2017, 18, 342. [Google Scholar] [CrossRef]
- Hu, H.; Miao, Y.-R.; Jia, L.-H.; Yu, Q.-Y.; Zhang, Q.; Guo, A.-Y. AnimalTFDB 3.0: A comprehensive resource for annotation and prediction of animal transcription factors. Nucleic Acids Res. 2019, 47, D33–D38. [Google Scholar] [CrossRef] [PubMed]
- Aich, P.; Wilson, H.L.; Kaushik, R.S.; Potter, A.A.; Babiuk, L.A.; Griebel, P. Comparative analysis of innate immune responses following infection of newborn calves with bovine rotavirus and bovine coronavirus. J. Gen. Virol. 2007, 88, 2749–2761. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [CrossRef]
- Kel-Margoulis, O.V.; Kel, A.E.; Reuter, I.; Deineko, I.V.; Wingender, E. TRANSCompel: A database on composite regulatory elements in eukaryotic genes. Nucleic Acids Res. 2002, 30, 332–334. [Google Scholar] [CrossRef]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams (2007–2015). Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 21 July 2020).
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Kumar, S.; Tamura, K.; Nei, M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Briefings Bioinform. 2004, 5, 150–163. [Google Scholar] [CrossRef]
- Groen, A.F.; Steine, T.; Colleau, J.-J.; Pedersen, J.; Pribyl, J.; Reinsch, N. Economic values in dairy cattle breeding, with special reference to functional traits. Report of an EAAP-working group. Livest. Prod. Sci. 1997, 49, 1–21. [Google Scholar] [CrossRef]
- Macdonald, M.R.; Xia, J.; Smith, A.L.; Magor, K.E. The duck toll like receptor 7: Genomic organization, expression and function. Mol. Immunol. 2008, 45, 2055–2061. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, S.; Zhao, Q.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Liu, F.; Chen, X.; Cheng, A. Molecular cloning, tissue distribution, and immune function of goose TLR7. Immunol. Lett. 2015, 163, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hasan, U.; Bates, E.; Takeshita, F.; Biliato, A.; Accardi, R.; Bouvard, V.; Mansour, M.; Vincent, I.; Gissmann, L.; Iftner, T.; et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 2007, 178, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Bros, M.; Haas, K.; Moll, L.; Grabbe, S. Moll RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells 2019, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- López-Posadas, R.; Fastancz, P.; Martínez-Sánchez, L.D.C.; Panteleev-Ivlev, J.; Thonn, V.; Kisseleva, T.; Becker, L.S.; Schulz-Kuhnt, A.; Zundler, S.; Wirtz, S.; et al. Inhibiting PGGT1B Disrupts Function of RHOA, Resulting in T-cell Expression of Integrin α4β7 and Development of Colitis in Mice. Gastroenterology 2019, 157, 1293–1309. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. The Biology and Medical Implications of Interleukin-6. Cancer Immunol. Res. 2014, 2, 288–294. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Klement, J.D.; Payne, D.; Lu, C.; Redd, P.S.; Liu, K. Abstract 5744: Chronic inflammation activates IL6 signaling to upregulate DNMT1 and DNMT3b to promote colon tumorigenesis. Immunology 2018, 78, 5744. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Short, K.R.; Veeris, R.; Leijten, L.M.; van den Brand, J.M.; Jong, V.L.; Stittelaar, K.; Osterhaus, A.; Andeweg, A.; Van Riel, D. Proinflammatory Cytokine Responses in Extra-Respiratory Tissues During Severe Influenza. J. Infect. Dis. 2017, 216, 829–833. [Google Scholar] [CrossRef]
- Rangarajan, E.S.; Ruane, K.M.; Proteau, A.; Schrag, J.D.; Valladares, R.; Gonzalez, C.F.; Gilbert, M.; Yakunin, A.F.; Cygler, M. Structural and enzymatic characterization of NanS (YjhS), a 9-O-Acetyl N-acetylneuraminic acid esterase from Escherichia coli O157:H7. Protein Sci. 2011, 20, 1208–1219. [Google Scholar] [CrossRef]
- Van Karnebeek, C.D.M.; Bonafé, L.; Wen, X.-Y.; Tarailo-Graovac, M.; Balzano, S.; Royer-Bertrand, B.; Ashikov, A.; Garavelli, L.; Mammi, I.; Turolla, L.; et al. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat. Genet. 2016, 48, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Jaitin, D.A.; Weiner, A.; Yofe, I.; Lara-Astiaso, D.; Keren-Shaul, H.; David, E.; Salame, T.M.; Tanay, A.; Van Oudenaarden, A.; Amit, I. Dissecting Immune Circuits by Linking CRISPR-Pooled Screens with Single-Cell RNA-Seq. Cell 2016, 167, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiao, J.; Li, J.; Liu, J.; Wang, C.; Feng, C.; Feng, H. TRAF2 of black carp upregulates MAVS-mediated antiviral signaling during innate immune response. Fish Shellfish. Immunol. 2017, 71, 1–9. [Google Scholar] [CrossRef]
- Qu, F.; Xiang, Z.; Zhou, Y.; Qin, Y. A molluscan TNF receptor-associated factor 2 (TRAF2) was involved in host defense against immune challenges. Fish Shellfish. Immunol. 2017, 71, 105–115. [Google Scholar] [CrossRef]
- Ojurongbe, O.; Funwei, R.I.; Snyder, T.J.; Farid, I.; Aziz, N.; Li, Y.; Falade, C.O.; Thomas, B. Genetic variants of tumor necrosis factor-α -308G/A (rs1800629) but not Toll-interacting proteins or vitamin D receptor genes enhances susceptibility and severity of malaria infection. Immunogenetics 2018, 70, 135–140. [Google Scholar] [CrossRef]
- Pan, Y.-C.; Li, C.-F.; Ko, C.-Y.; Pan, M.-H.; Chen, P.-J.; Tseng, J.T.; Wu, W.-C.; Chang, W.-C.; Huang, A.-M.; Sterneck, E.; et al. CEBPD Reverses RB/E2F1-Mediated Gene Repression and Participates in HMDB-Induced Apoptosis of Cancer Cells. Clin. Cancer Res. 2010, 16, 5770–5780. [Google Scholar] [CrossRef]
- Sheshadri, N.; Sharan, S.; Sterneck, E. Abstract 4501: CEBPD is an early endoplasmic reticulum stress response gene implicated in breast cancer cell survival. Cancer Res. 2017, 77, 4501. [Google Scholar]
- Giacomelli, A.O.; Yang, X.; Lintner, R.E.; McFarland, J.M.; Duby, M.; Kim, J.; Howard, T.P.; Takeda, D.Y.; Ly, S.H.; Kim, E.; et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat. Genet. 2018, 50, 1381–1387. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, Z.; Shan, L.; Zeng, H.; Hua, Y.; Cai, Z. The importance of Src signaling in sarcoma. Oncol. Lett. 2015, 10, 17–22. [Google Scholar] [CrossRef]
- Nakashima, Y.; Yanez, D.A.; Touma, M.; Nakano, H.; Jaroszewicz, A.; Jordan, M.C.; Pellegrini, M.; Roos, K.P.; Nakano, A. Nkx2-5 Suppresses the Proliferation of Atrial Myocytes and Conduction SystemNovelty and Significance. Circ. Res. 2014, 114, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Kaplan, D.I.; Bell, K.M.; Koutsis, K.; Haynes, J.M.; Mills, R.J.; Phelan, D.G.; Qian, E.L.; Leitoguinho, A.R.; Arasaratnam, D.; et al. NKX2-5 regulates human cardiomyogenesis via a HEY2 dependent transcriptional network. Nat. Commun. 2018, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Kardassis, D.; Gafencu, A.V.; Zannis, V.I.; Dávalos, A. Regulation of HDL Genes: Transcriptional, Posttranscriptional, and Posttranslational. In High Density Lipoproteins; Spring: Cham, Switzerland, 2015; Volume 224, pp. 113–179. [Google Scholar] [CrossRef]
- Galdeano, C.; Gadd, M.S.; Soares, P.; Scaffidi, S.; Van Molle, I.; Birced, I.; Hewitt, S.; Dias, D.M.; Ciulli, A. Structure-Guided Design and Optimization of Small Molecules Targeting the Protein–Protein Interaction between the von Hippel–Lindau (VHL) E3 Ubiquitin Ligase and the Hypoxia Inducible Factor (HIF) Alpha Subunit with in Vitro Nanomolar Affinities. J. Med. Chem. 2014, 57, 8657–8663. [Google Scholar] [CrossRef] [PubMed]

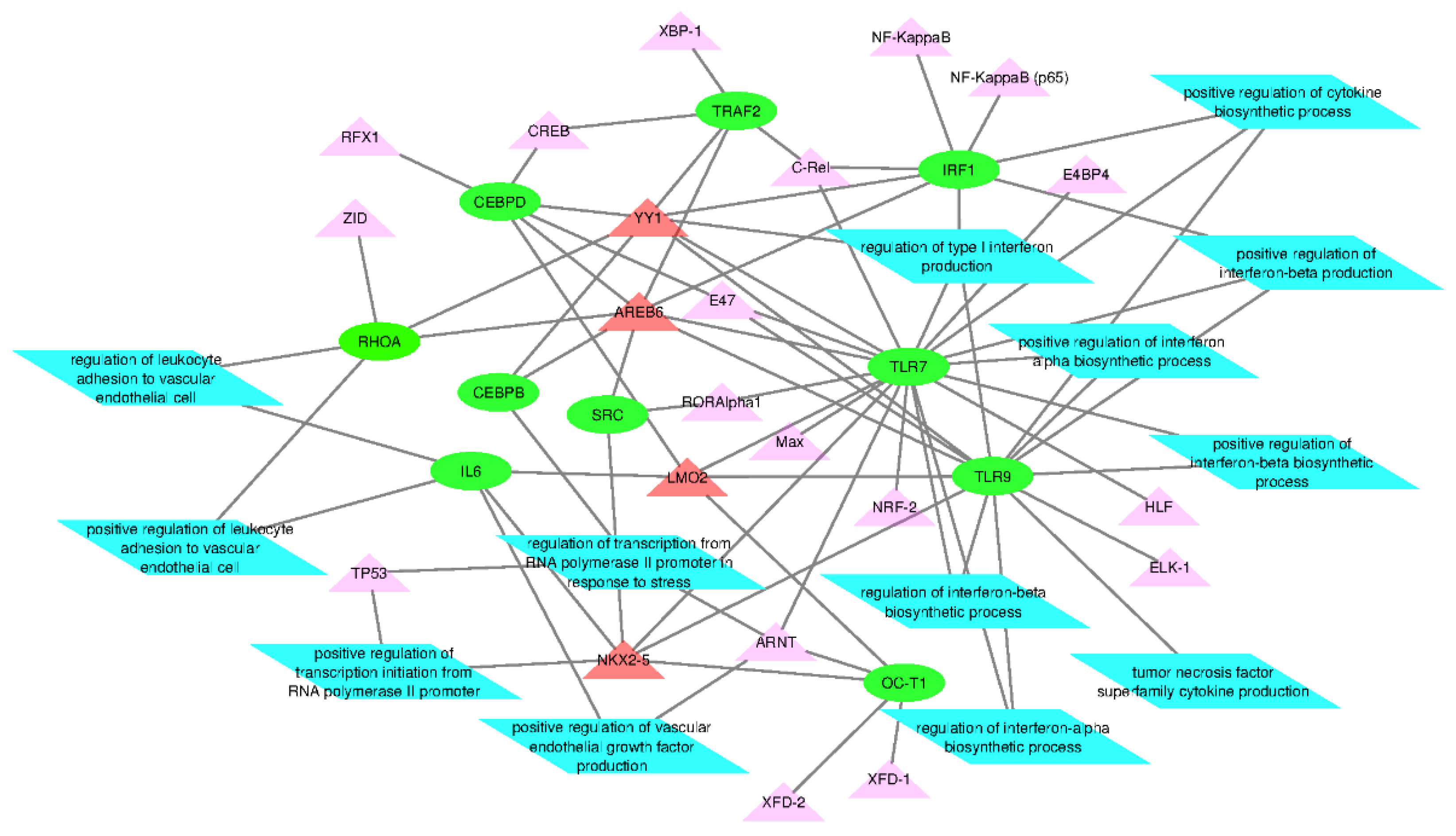
| SN | Genes | Number of GO Pathways | GO Pathways Identified Within |
|---|---|---|---|
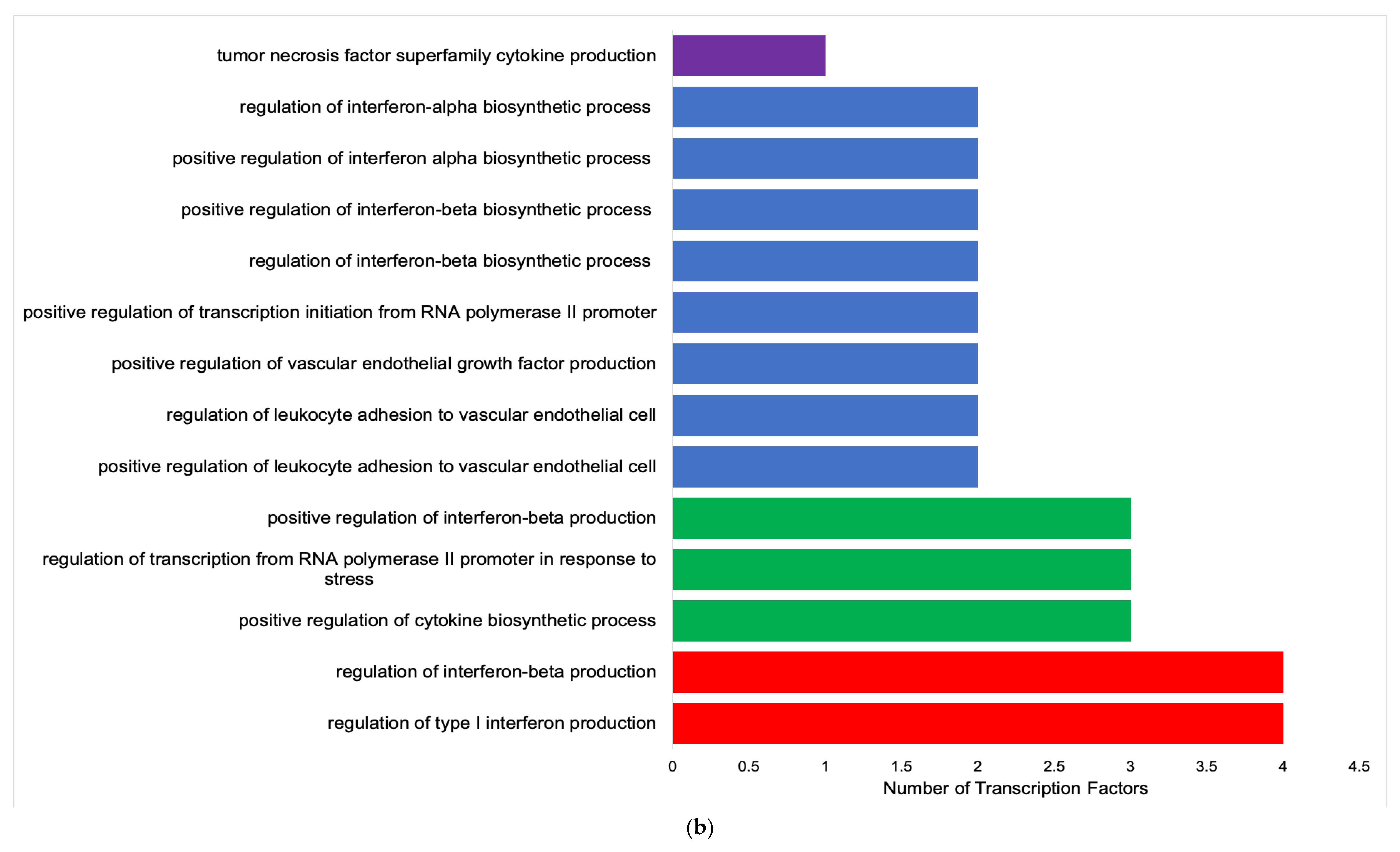
| 1 | TLR9 | 11 | positive regulation of interferon-α, β and γ biosynthetic process; regulation of interferon-α, β and γ biosynthetic process; positive regulation of toll-like receptor 9 signaling pathway; toll-like receptor 9 signaling pathway; positive regulation of interleukin-8 biosynthetic process; tumor necrosis factor production |
| 2 | TLR7 | 8 | positive regulation of interferon-α, β and γ biosynthetic process; regulation of interferon-α, β and γ biosynthetic process; positive regulation of interleukin-8 biosynthetic process; regulation of interferon-γ biosynthetic process |
| 3 | RHOA | 4 | apolipoprotein A-I mediated signaling pathway; stress fiber assembly; contractile actin filament bundle assembly; positive regulation of podosome assembly |
| 4 | CEBPD | 3 | epithelial cell proliferation involved in liver morphogenesis; liver regeneration; liver morphogenesis |
| 5 | SRC | 3 | stress fiber assembly; positive regulation of podosome assembly; contractile actin filament bundle assembly |
| 6 | IRF1 | 3 | Interferon-γ-mediated signaling pathway; contractile actin filament bundle assembly; positive regulation of interleukin-12 biosynthetic process |
| 7 | IL-6 | 2 | positive regulation of production of miRNAs involved in gene silencing by miRNA; regulation of interferon-γ biosynthetic process |
| 8 | TP53 | 2 | interferon-γ-mediated signaling pathway; positive regulation of production of miRNAs involved in gene silencing by miRNA |
| 9 | CEBPB | 2 | epithelial cell proliferation involved in liver morphogenesis; hepatocyte proliferation |
| 10 | TRAF2 | 1 | regulation of interferon-γ biosynthetic process |
| 11 | NANS | 0 |
| S/No | Genes | Number of Significantly Predicted TFs | Significantly Predicted Transcription Factors |
|---|---|---|---|
| 1 | TLR7 | 10 | HLF, YY1, AREB6, C-Rel, NKX2-5, E47, E4BP4, RORAlpha1, LMO2, ARNT |
| 2 | IRF1 | 6 | YY1, C-Rel, AREB6, NKX2-5, LMO2, ARNT |
| 3 | TLR9 | 5 | YY1, E47, AREB6, LMO2, NKX2-5 |
| 4 | CEBPD | 5 | YY1, E47, AREb6, CREB, LMO2 |
| 5 | TRAF2 | 4 | YY1 AREB6, C-Rel, CREB |
| 6 | TP53 | 4 | HLF, AREB6, RORAlpha1, E4BP4 |
| 7 | SRC | 3 | NKX2-5, RORAlpha1, AREB6 |
| 8 | IL-6 | 2 | LMO2, NKX2-5 |
| 9 | RHOA | 2 | YY1, AREB6 |
| 10 | CEBPB | 2 | YY1, AREB6 |
| 11 | NANS | 1 | AREB6 |
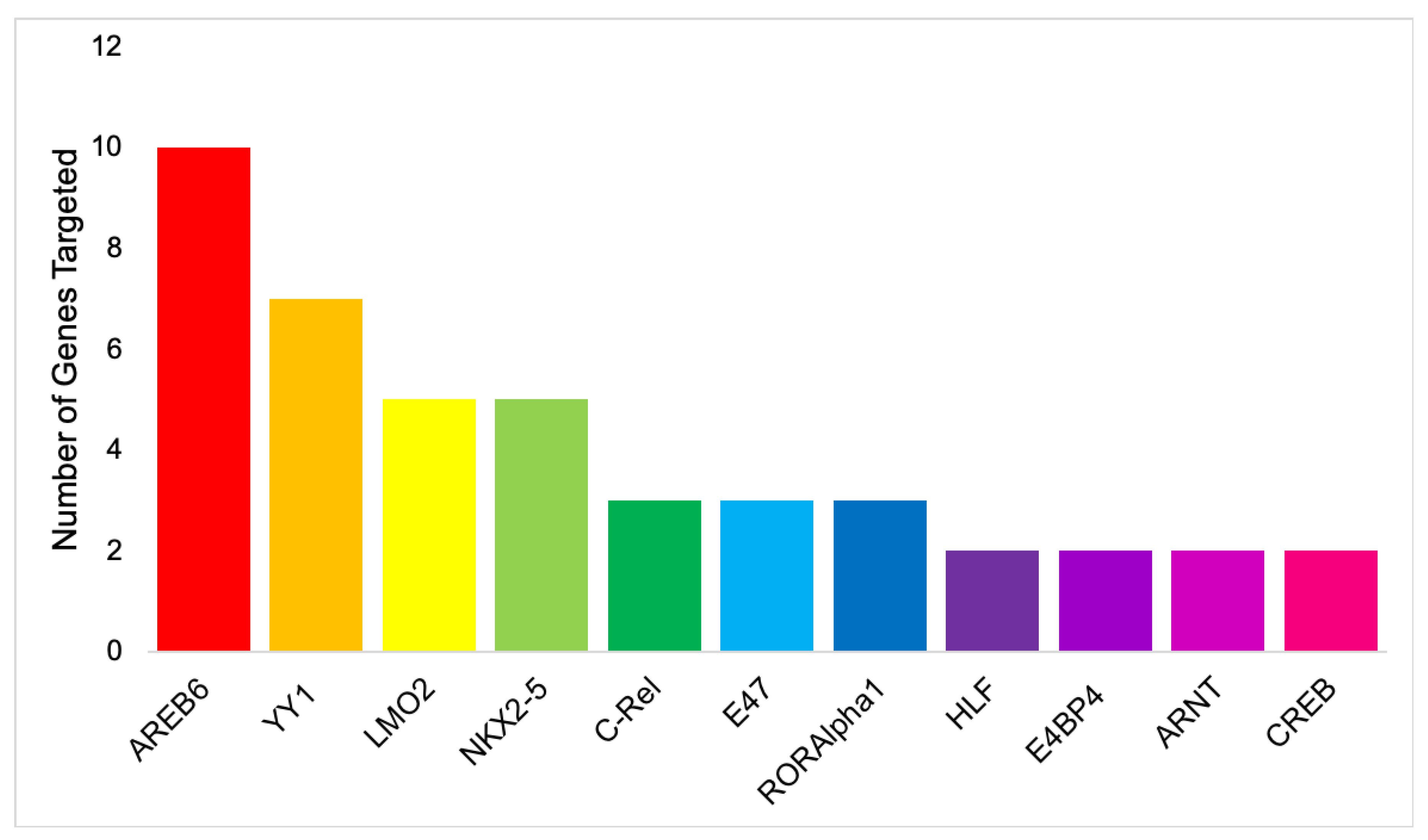
| S/No | Transcription Factor | Number of Gene Targets | Genes Targeted |
|---|---|---|---|
| 1 | AREB6 | 10 | TLR7, TLR9, IRF1, CEBPD, TRAF2, RHOA, TP53, CEBPB, SRC, NANS |
| 2 | YY1 | 7 | TLR7, TLR9, IRF1, CEBPD, TRAF2, RHOA, CEBPB |
| 3 | LMO2 | 5 | TRL7, TLR9, IRF1, IL-6, CEBPD, |
| 4 | NKX2-5 | 5 | IRF1, IL-6, TLR7, TLR9, SRC |
| 5 | C-Rel | 3 | TLR7, IRF1, TRAF2 |
| 6 | E47 | 3 | TLR7, TLR9, CEBPD |
| 7 | RORAlpha1 | 3 | TLR7, TP53, SRC |
| 8 | HLF | 2 | TLR7, TP53 |
| 9 | E4BP4 | 2 | TLR7, TP53 |
| 10 | ARNT | 2 | TLR7, IRF1 |
| 11 | CREB | 2 | CEBPD, TRAF2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morenikeji, O.B.; Strutton, E.; Wallace, M.; Bernard, K.; Yip, E.; Thomas, B.N. Dissecting Transcription Factor-Target Interaction in Bovine Coronavirus Infection. Microorganisms 2020, 8, 1323. https://doi.org/10.3390/microorganisms8091323
Morenikeji OB, Strutton E, Wallace M, Bernard K, Yip E, Thomas BN. Dissecting Transcription Factor-Target Interaction in Bovine Coronavirus Infection. Microorganisms. 2020; 8(9):1323. https://doi.org/10.3390/microorganisms8091323
Chicago/Turabian StyleMorenikeji, Olanrewaju B., Ellis Strutton, Madeleine Wallace, Kahleel Bernard, Elaine Yip, and Bolaji N. Thomas. 2020. "Dissecting Transcription Factor-Target Interaction in Bovine Coronavirus Infection" Microorganisms 8, no. 9: 1323. https://doi.org/10.3390/microorganisms8091323
APA StyleMorenikeji, O. B., Strutton, E., Wallace, M., Bernard, K., Yip, E., & Thomas, B. N. (2020). Dissecting Transcription Factor-Target Interaction in Bovine Coronavirus Infection. Microorganisms, 8(9), 1323. https://doi.org/10.3390/microorganisms8091323






