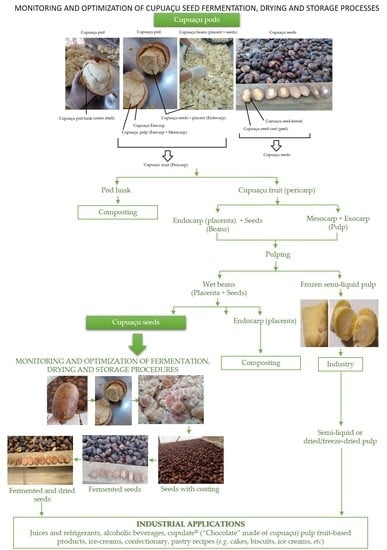
Monitoring and Optimization of Cupuaçu Seed Fermentation, Drying and Storage Processes
Abstract
1. Introduction
2. Materials and Methods
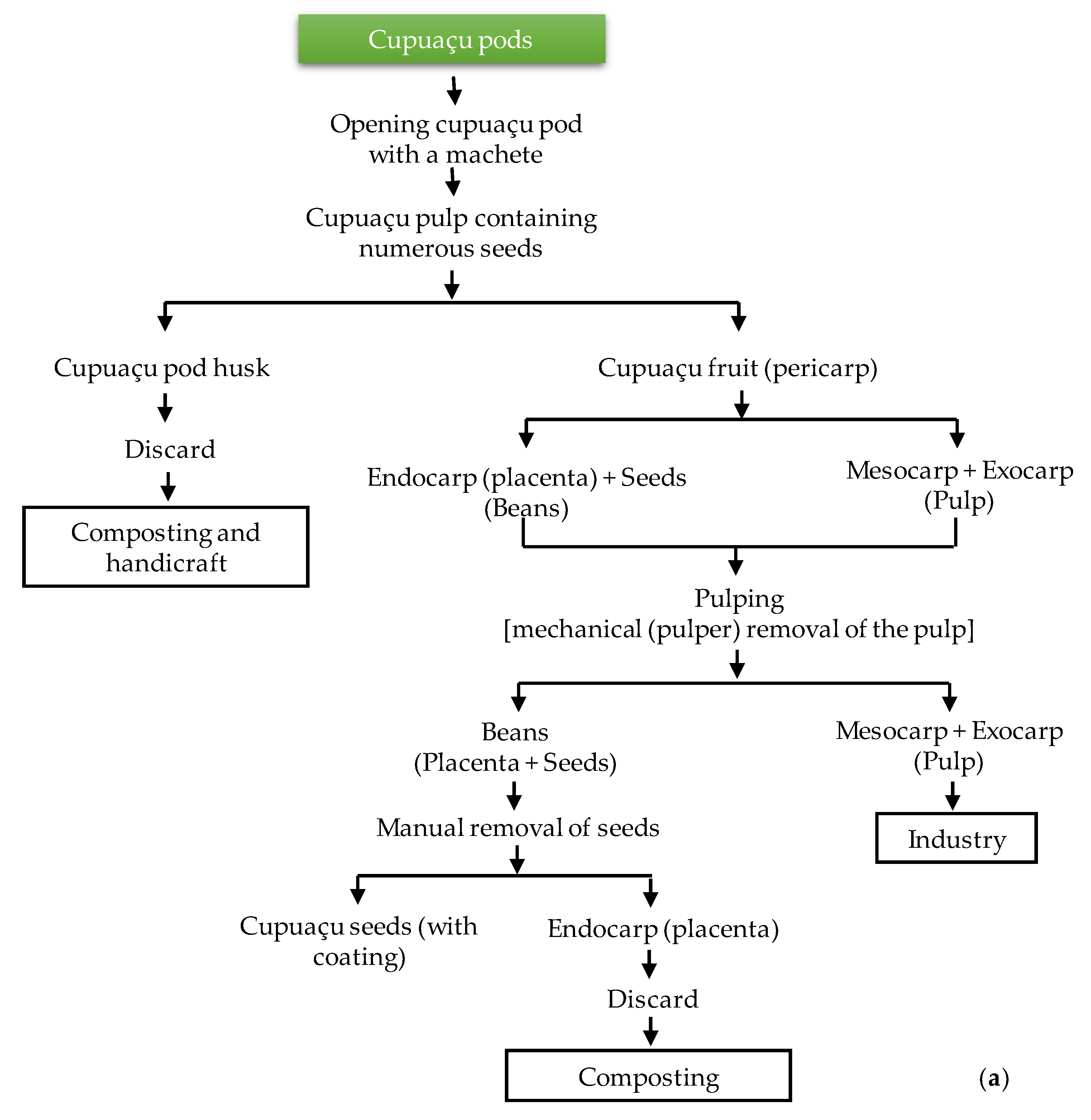
2.1. Sampling and Cupuaçu Seed Fermentation and Drying
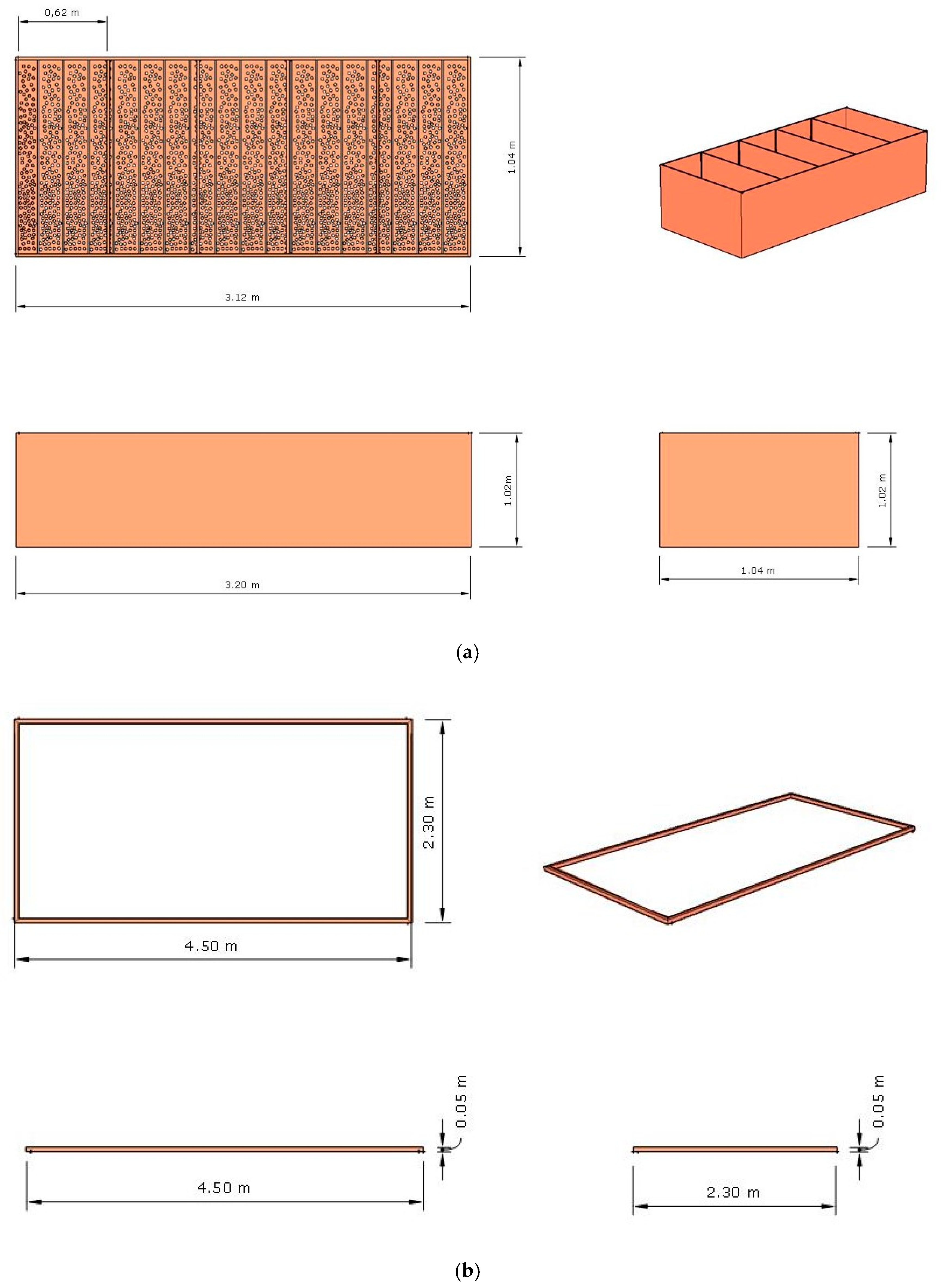
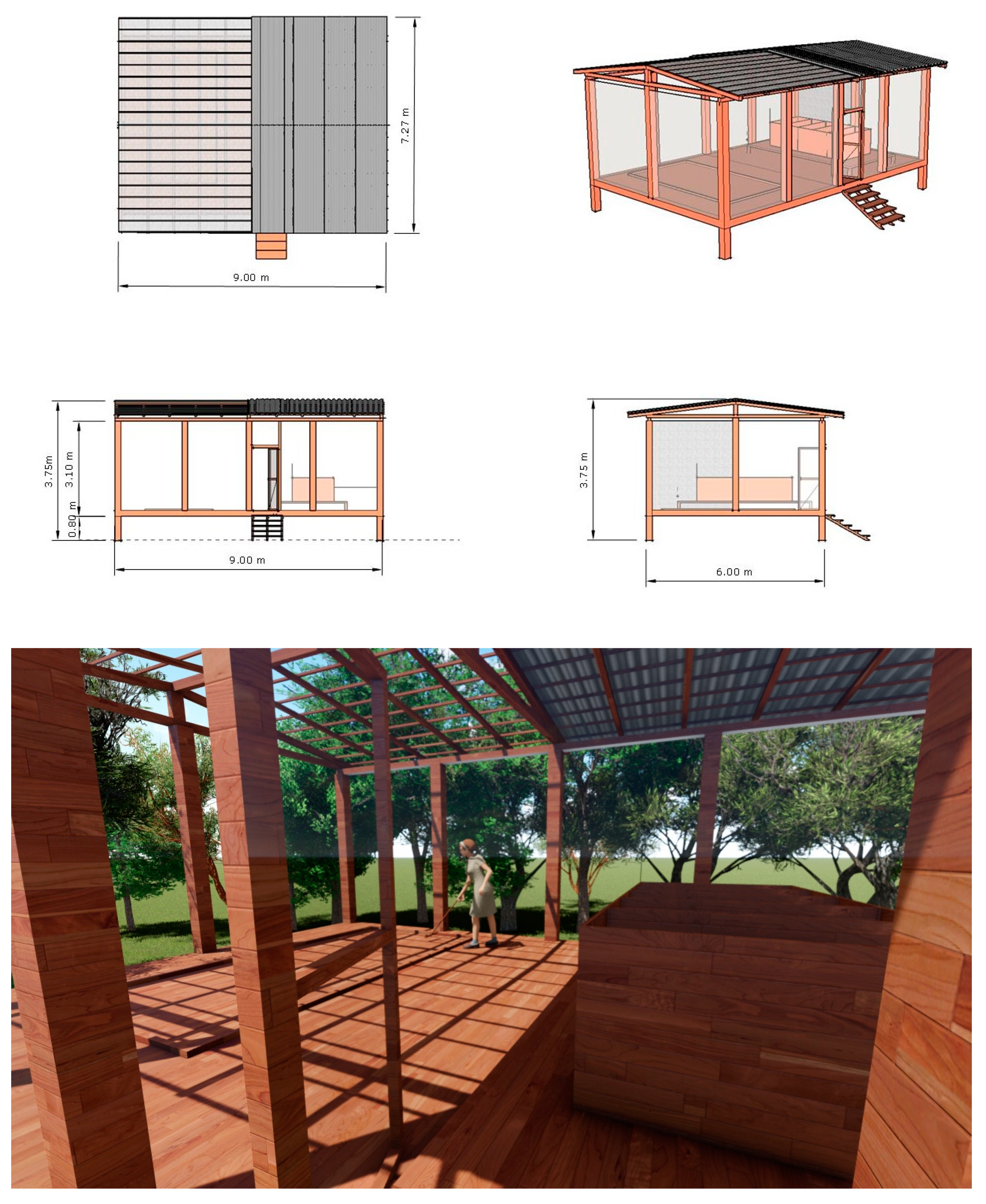
2.2. Project Design of the Fermenter, Solar Drier Terrace, and Greenhouse
2.3. Sampling and Physicochemical Analysis during Storage
2.4. Sampling and Microbiological Analysis during Storage
2.5. Statistical Analysis
3. Results and Discussion
3.1. Cupuaçu Seed Fermentation
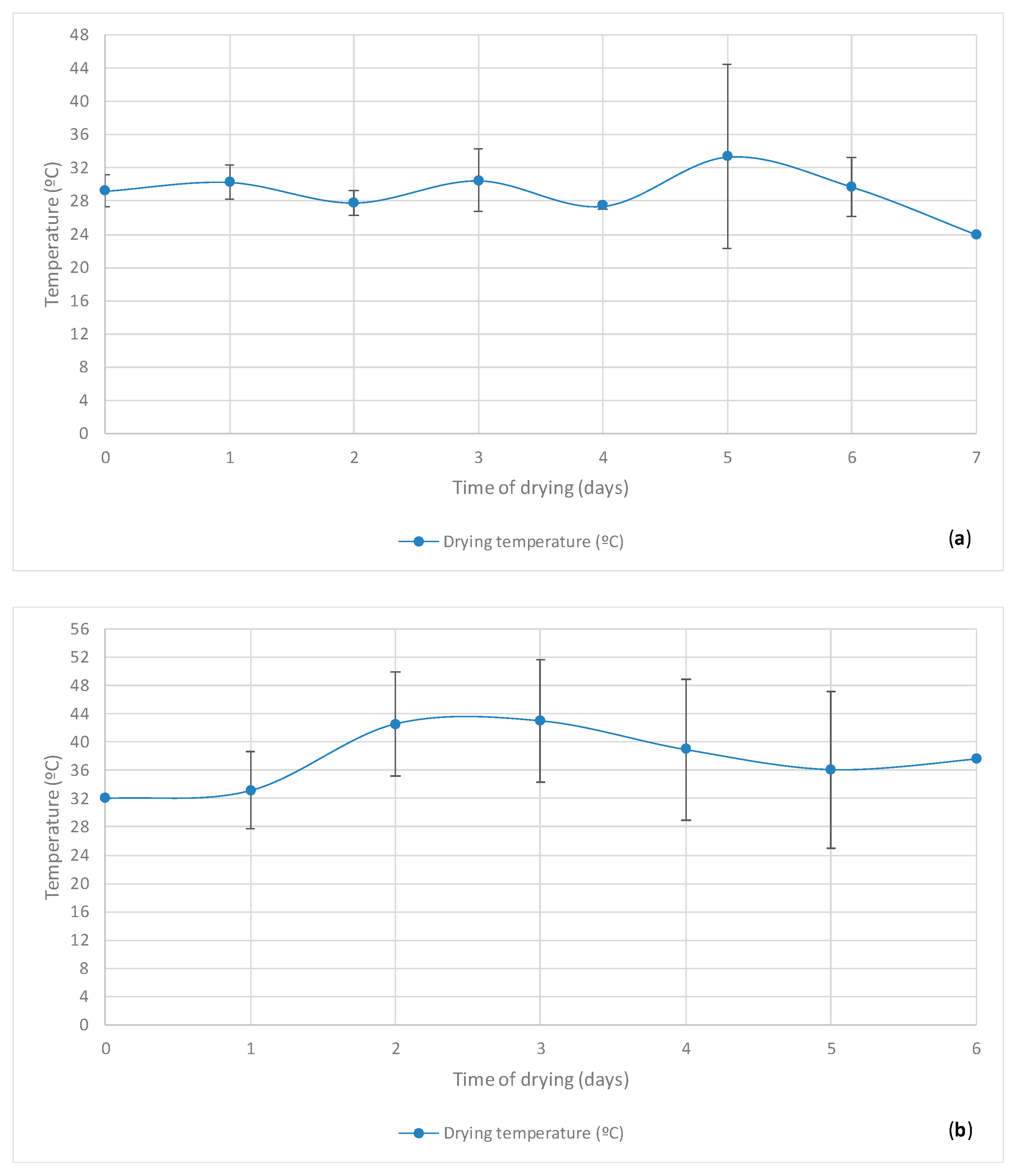
3.2. Cupuaçu Seed Drying
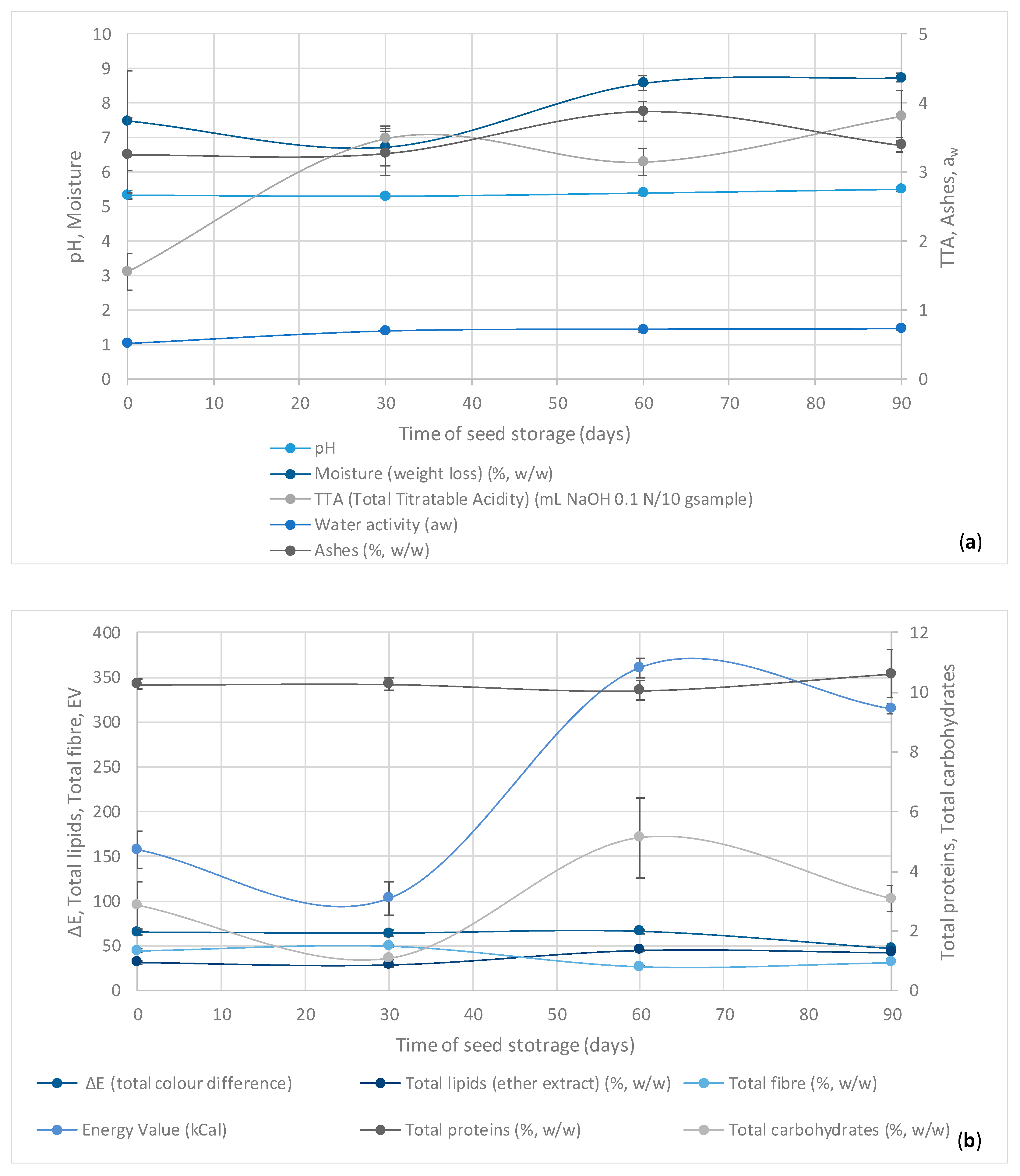
3.3. Physicochemical Analysis during Storage
3.4. Microbiological Analysis during Storage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Instituto Brasileiro de Geografia e Estatística [Brazilian Institute of Geography and Statistics] (IBGE). Agricultural Census. Number of Agricultural Establishments, Quantity Produced and Area Harvested, by Products from Temporary Crops. 2017. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/21814-2017-censo-agropecuario.html?=&t=resultados (accessed on 30 July 2020).
- Andrade, R.C.; Negreiros, J.R.S.; Araujo Neto, S.E.; Cavalcante, M.J.B.; Alécio, M.R.; Santos, R.S. Diagnóstico da Potencialidade da Fruticultura no Acre—Rio Branco, AC [Diagnosis of the Potential of Fruit Growing in Acre—Rio Branco, AC]; Embrapa Acre: Rio Branco, Brazil, 2011; Volume 125, Documents/Embrapa Acre; ISSN 01049046. 27p. [Google Scholar]
- Nazaré, R.F.R.; Barbosa, W.C.; Viégas, R.M.F. Processamento das Sementes de Cupuaçu Para a Obtenção de Cupulate® [Processing of Cupuaçu Seeds to Obtain Cupulate®]; Embrapa CPATU: Belém, Brazil, 1990. [Google Scholar]
- Gondim, T.; Thomazini, M.; Cavalcante, M.; Souza, J. Aspectos da Produção de Cupuaçu [Aspects of Cupuaçu Production]; Embrapa Acre: Rio Branco, AC, Brazil, 2001. [Google Scholar]
- Ramos, S.N.M. Influência dos Microrganismos Envolvidos no Processo de Fermentação de Cupuaçu (Theobroma grandiflorum Schum) na formação do sabor [Influence of Microorganisms Involved in the Cupuaçu Fermentation Process (Theobroma Grandiflorum Schum) on Flavor Formation]. Tese de Doutorado [Ph.D. Thesis], Universidade Estadual de Campinas [Campinas State University], Campinas, Brazil, 2015. [Google Scholar]
- Cohen, K.O.; Jackix, M.N.H. Estudo do liquor de cupuaçu [Study of cupuaçu liquor]. Ciência e Tecnologia de Alimentos [Food Science and Technology] 2005, 25, 182–190. [Google Scholar] [CrossRef]
- Cohen, K.O.; Jackix, M.N.H. Obtenção e caracterização física, química e físico-química de liquor de cupuaçu e de cacau [Obtaining and physical, chemical and physical-chemical characterization of cupuaçu and cocoa liquor]. Braz. J. Food Technol. 2004, 7, 57–67. [Google Scholar]
- Souza, J.M.L.; Cartaxo, C.B.C.; Andrade Neto, R.; Moura, S.I.A.; Maciel, V.T.; Furtado, C.M. Otimização dos Processos de Fermentação e Secagem de Sementes de Cupuaçu [Optimization of Cupuaçu Seed Fermentation and Drying Processes]. In Proceedings of the 25th Brazilian Congress of Food Science and Technology, Gramado, Brazil, 3–6 November 2016; UFRGS: Gramado, Brazil, 2016. [Google Scholar]
- Lopes, A.S.; García, N.H.P.; Vasconcelos, M.A.M. Avaliação das condições de torração após a fermentação de amêndoas de cupuaçu (Theobroma grandiflorum Schum) e cacau (Theobroma cacao L.) [Evaluation of roasting conditions after the fermentation of cupuaçu (Theobroma grandiflorum Schum) and cocoa (Theobroma cacao L.) almonds]. Braz. J. Food Technol. 2003, 6, 309–316. [Google Scholar]
- Carvalho, A.V.; García, N.H.P.; Wada, J.K.A. Caracterização físico-química e curvas de solubilidade proteica de sementes, amêndoas fermentadas e torradas de cupuaçu (Theobroma grandiflorum Schum). [Physicochemical characterization and protein solubility curves of seeds, fermented almonds and cupuaçu toast (Theobroma grandiflorum Schum)]. Braz. J. Food Technol. 2005, 8, 127–134. [Google Scholar]
- Carvalho, A.V.; García, N.H.P.; Farfán, J.A. Proteínas da semente de cupuaçu e alterações devidas à fermentação e à torração [Physico-chemical characterization and cupuaçu seed proteins and changes due to fermentation and roasting]. Ciência e Tecnologia de Alimentos [Food Science and Technology] 2008, 28, 986–993. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Mayrhofer, S.; Domig, K.; Starkute1, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; et al. Inhibition of pathogenic and opportunistic bacteria and molds by lactic acid bacteria isolated from spontaneous baking sourdough. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Küley, E.; Özyurt, G.; Özogul, I.; Boga, M.; Akyol, I.; Rocha, J.M.; Özogul, F. The Role of Selected Lactic Acid Bacteria on Organic Acid Accumulation during Wet and Spray-Dried Fish-based Silages. Contributions to the Winning Combination of Microbial Food Safety and Environmental Sustainability. Microorganisms 2020, 8, 172. [Google Scholar] [CrossRef]
- Novotni, D.; Gänzle, M.; Rocha, J.M. Chapter 5. Composition and activity of microbiota in sourdough and their effect on bread quality and safety. In Trends in Wheat and Bread Making; Galanaksis, C.M., Ed.; Elsevier-Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Georgescu, L.A.; Vasile, M.A.; Rocha, J.M.; Bahrim, G.-E. Selection of wild lactic acid bacteria strains as promoters of postbiotics in gluten-free sourdoughs. Microorganism 2020, 8, 643. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. On the microbiological profile of traditional Portuguese sourdough. J. Food Prot. 1999, 62, 1416–1429. Available online: http://www.scopus.com/inward/record.url?eid=2-s2.0-0345201643&partnerID=MN8TOARS (accessed on 27 August 2020).
- Rocha, J.M.; Malcata, F.X. Microbiological profile of maize and rye flours, and sourdough used for the manufacture of traditional Portuguese bread. Food Microbiol. 2012, 31, 72–88. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. Microbial ecology dynamics in Portuguese broa sourdough. J. Food Qual. 2016, 39, 634–648. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. Behavior of the complex micro-ecology in maize and rye flour and mother-dough for broa throughout storage. J. Food Qual. 2016, 39, 218–233. [Google Scholar] [CrossRef]
- Zokaityte, E.; Cernauskas, D.; Klupsaite, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Gruzauskas, R.; Juodeikiene, G.; Rocha, J.M.; et al. Bioconversion of milk permeate with selected lactic acid bacteria strains and apple by-products into beverages with antimicrobial properties and enriched with galactooligosaccharides. Microorganisms 2020, 8, 1182. [Google Scholar] [CrossRef] [PubMed]
- Schwan, R.; Wheals, A. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Camu, N.; De Winter, T.; Verbrugghe, K.; Cleenwerck, I.; Vandamme, P.; Takrama, J.S.; Vancanneyt, M.; Vuyst, L. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl. Environ. Microbiol. 2007, 73, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.; Zhao, J.; Fleet, G. Yeasts are essential for cocoa bean fermentation. Internat. J. Food Microb. 2014, 174, 72–87. [Google Scholar] [CrossRef]
- Souza, J.M.L.; Magalhaes, A.J.P.; Saraiva, L.S.; Araujo, A.P.S.; Cartaxo, C.B.C.; Maciel, V.T. Caracterização da microbiota fúngica em amêndoas de cupuaçu fermentadas secas [Characterization of the fungal microbiota in dried fermented cupuaçu seeds]. In Proceedings of the 26th Scientific Initiation Seminar at UFAC, Rio Branco, Brazil, 19–20 October 2017; Edufac: Rio Branco, Brazil,, 2017; Volume 1. [Google Scholar]
- Souza, J.M.L.; Cartaxo, C.B.C.; Álvares, V.S.; Ribeiro, S.A.L.; Cruz, S.C. Microbiota fúngica de amêndoas fermentadas de cupuaçu [Fungal microbiota of cupuaçu fermented almonds]. In Embrapa-Acre Seminar of Scientific Initiation and Graduation, 1; 2018, Rio Branco, AC. Pesquisa e Inovação Para a Agropecuária no Acre: Anais [Research and Innovation for Agriculture in Acre: Annals]; Embrapa Acre: Rio Branco, Brazil, 2019; pp. 83–86. [Google Scholar]
- Souza, J.M.L.; Lima, M.S.; Nascimento, F.C.C.; Álvares, V.S.; Yomura, R.B.; Nascimento, M.M. Characterization of depuiculated cupuaçu seeds and partially degreased pie. In Proceedings of the 2nd Embrapa Acre Scientific Initiation and Graduate Seminar, Rio Branco, Brazil, 10–11 September 2019; Álvares, V.S., Estanislau, F.M., Eds.; Embrapa Acre: Rio Branco, Brazil, 2019. [Google Scholar]
- Souza, J.M.L.; Lima, M.S.; Nascimento, F.C.C.; Álvares, V.S.; Yomura, R.B.T.; Nascimento, M.M. Qualidade de amêndoas fermentadas e secas de cupuaçu em função da despeliculação e do armazenamento [Quality of fermented and dried cupuaçu almonds as a function of depeliculation and storage]. In Proceedings of the Anais do II Seminário da Embrapa Acre de Iniciação Científica e pós-Graduação [Proceedings of the II Embrapa Acre Seminar of Scientific Initiation and Postgraduate Studies], Rio Branco, Embrapa Acre, Brazil, 10–11 September 2019. [Google Scholar]
- Álvares, V.S.; Souza, J.M.L.; Macedo, P.E.F.; Vasconcelos, M.A.M. Fungi associated with fermented and stored cupuaçu seeds. In Proceedings of the 2nd Embrapa Acre Scientific Initiation and Graduate Seminar, Rio Branco, Brazil, 10–11 September 2019; Álvares, V.S., Estanislau, F.M., Eds.; Embrapa Acre: Rio Branco, Brazil, 2019. [Google Scholar]
- AOAC. Official Method of Analysis: Association of Analytical Chemists, 16th ed.; AOAC: Arlington, TX, USA, 1995; Volume 2, p. 559. [Google Scholar]
- AOAC. Official methods of analysis 2001.11 Protein (Crude) in Animal Feed, Forage (Plant Tissue), Grain and Oilseeds. In Official Methods of Analysis of AOAC International (OMA); AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Official Method of Analysis: Association of Analytical Chemists. In Official Methods of Analysis of the AOAC International, 19th ed.; AOAC: Washington, DC, USA, 2012; p. 599. [Google Scholar]
- Zenebon, O.; Pascuet, N.S.; Tiglea, P. (Coordinators) Métodos Físico-Químicos Para Análise de Alimentos [Physico-Chemical Methods for Food Analysis], 4th ed.; State Department of Health, Disease Control Coordination, Institute Adolfo Lutz© (SES-CCD-IAL): São Paulo, Brazil, 2008; p. 1018. Available online: http://www.ial.sp.gov.br/resources/editorinplace/ial/2016_3_19/analisedealimentosial_2008.pdf?attach=true (accessed on 24 July 2020).
- Lorite, G.S.; Rocha, J.M.; Miilumäki, N.; Saavalainen, P.; Selkälä, T.; Morales-Cid, G.; Gonçalves, M.P.; Pongrácz, E.; Rocha, C.M.R.; Toth, G. Evaluation of physiochemical/microbial properties and life cycle assessment (LCA) of PLA-based nanocomposite active packaging. LWT Food Sci. Technol. 2016, 75, 305–315. [Google Scholar] [CrossRef]
- Vanderzant, C.; Splittstoesser, D.F. Compendium of Methods for the Microbiological Examination of Food, 3rd ed.; American Public Health Association: Washington, DC, USA, 1992; p. 1219. [Google Scholar]
- ICMSF. Comissão Internacional Para Especificações Microbiológicas dos Alimentos (ICMSF) [International Commission for Microbiological Specifications of Food (ICMSF)]; Varela: São Paulo, Brazil, 1997; p. 377. [Google Scholar]
- Downes, F.P.; Ito, H. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; American Public Health Association (APHA): Washington, DC, USA, 2001; p. 676. [Google Scholar]
- Barbosa, J.C.; Júnior, W.M. AgroEstat—Sistema para análises estatísticas de ensaios agronómicos [AgroEstat—system for statistical analysis of agronomic trials]; Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista (FCAV/UNESP) [Faculty of Agricultural and Veterinary Sciences, Paulista State University]: Jaboticabal, São Paulo, Brazil, 2015; p. 369. [Google Scholar]
- Hawkins, D.M.; Weisberg, S. Combining the box-cox power and generalised log transformations to accommodate nonpositive responses in linear and mixed-effects linear models. S. Afr. Stat. J. 2017, 51, 317–328. [Google Scholar]
- Royston, P. Remark AS R94: A Remark on Algorithm AS 181: The W-test for Normality. J. R. Stat. Soc. Ser. C (Appl. Stat.) 1995, 44, 547–551. [Google Scholar] [CrossRef]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The Impact of Levene’s Test of Equality of Variances on Statistical Theory and Practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef]
- Granados, A.P.F. Efeito da Irradiação Gama Sobre a Inibição de Fungos e Salmonella spp. e Sobre as Características Físico-Químicas do Cacau [Effect of Gamma Irradiation on Inhibition of Fungi and Salmonella spp. and on the Physical-Chemical Characteristics of Cocoa]. Master’s Thesis, Universidade Estadual de Campinas (UNICAMP) [Campinas State University (UNICAMP)], Campinas, Brazil, 2016. [Google Scholar]
- Vasconcelos, M.A.M. Transformações Físicas e Químicas Durante a Fermentação de Amêndoas do Cupuaçu (Theobroma grandiflorum Schum) [Physical and Chemical Transformations During Fermentation of Cupuaçu Seeds (Theobroma grandiflorum Schum)]. Master’s Thesis, Universidade Estadual de Campinas (UNICAMP) [Campinas State University (UNICAMP)], Campinas-SP, Brazil, 1999. [Google Scholar]
- 4Mattietto, R.A. Estudo comparativo das transformações estruturais e físico-químicas durante o processo fermentativo de amêndoas de cacau e cupuaçu (Theobroma cacau L.) e cupuaçu (Theobroma grandiflorum Schum) [Comparative Study of Structural and Physicochemical Transformations during the Fermentation Process of Cocoa (Theobroma cacau L.) and Cupuaçu (Theobroma grandiflorum Schum) Seeds]. Master’s Thesis, Universidade Estadual de Campinas (UNICAMP) [Campinas State University (UNICAMP)], Campinas, Brazil, 2001. [Google Scholar]
- Ramos, S.N.M.; Danzl, W.; Ziegleder, G.; Efraim, P. Formation of volatile compounds during cupuassu fermentation: Influence of pulp concentration. Food Res. Int. 2016, 87, 161–167. [Google Scholar] [CrossRef]
- Ramos, S.; Salazar, M.; Nascimento, L.; Carazzolle, M.; Pereira, G.; Delforno, T.; Nascimento, M.; Aleluia, T.; Celeghinia, R.; Efraim, P. Influence of pulp on the microbial diversity during cupuassu fermentation. Intern. J. Food Microb. 2020, 318, 108465. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.P. Enzimas Produzidas Durante os Diferentes Estágios de Fermentação das Sementes de Cupuaçu (Theobroma grandiflorum (Willdenow ex Sprengel) Schumann) [Enzymes Produced during the Different Fermentation Stages of Cupuaçu Seeds (Theobroma grandiflorum (Willdenow ex Sprengel) Schumann)]. Master’s Thesis, Universidade Federal do Amazonas (UFAM) [Amazonas Federal University (UFAM)], Manaus-AM, Brazil, 2006. [Google Scholar]
- Ozturk, G.; Young, G.M. Food Evolution: The Impact of Society and Science on the Fermentation of Cocoa Beans. Compr. Rev. Food Sci. Food Saf. 2017, 16, 431–455. [Google Scholar] [CrossRef]
- INMET (Instituto Nacional de Meteorologia) [National Institute of Meteorology]. Banco de Dados Meteorológicos do INMET [INMET Meteorological Database]. 2020. Available online: https://bdmep.inmet.gov.br/# (accessed on 20 July 2020).
- Pereira, J.D.S.; Álvares, V.S.; Souza, J.M.L.; Maciel, V.T. Storage of fermented and depulicated seeds of cupuaçu. In Proceedings of the 1st Embrapa Acre Seminar of Scientific Initiation and Graduation, Rio Branco, Brazil, 18–19 September 2018; Álvares, V.S., Estanislau, F.M., Eds.; Embrapa Acre: Rio Branco, Brazil, 2019; pp. 45–49. [Google Scholar]
- Pereira, J.D.S.; Álvares, V.S.; Souza, J.M.L.; Maciel, V.T. Armazenamento de amêndoas fermentadas e despeliculadas de cupuaçu [Storage of fermented and depulicated seeds of cupuaçu]. In Embrapa-Acre seminar of Scientific Initiation and Graduation 1 (Embrapa Acre. Eventos Técnicos & Científicos, 1), Proceedings of the Pesquisa e inovação para a Agropecuária no Acre: Anais [Research and innovation for Agriculture in Acre: Annals], Rio Branco, Brazil, 2018; Embrapa Acre: Rio Branco, AC, Brazil, 2019; pp. 83–86. [Google Scholar]
- Banboye, F.D.; Ngwabie, M.N.; Eneighe, S.A.; NDE, D.B. Assessment of greenhouse technologies on the drying behavior of cocoa beans. Food Sci. Nutr. 2020, 8, 2748–2757. [Google Scholar] [CrossRef]
- Oliveira, M. Temperatura na secagem e condições de armazenamento sobre propriedades da soja para consumo e produção de biodiesel [Drying temperature and storage conditions on soy properties for consumption and production of biodiesel]. Master’s Thesis, Universidade Federal de Pelotas, Pelotas, Brazil, 2008; p. 70. [Google Scholar]
- Santos, E.S.H.; Álvares, V.S.; Souza, J.M.L.; Vasconcelos, M.A.M. Qualidade de amêndoas fermentadas e secas de cupuaçu em função da despeliculação e do armazenamento [Quality of fermented and dried cupuaçu seeds as a function of depeliculation and storage]. In Proceedings of the II Seminário da Embrapa Acre de Iniciação Científica e pós-graduação [Embrapa Acre II Scientific Initiation and Postgraduate Seminar], Rio Branco, Embrapa Acre, Rio Branco, Brazil, 10–11 September 2019. [Google Scholar]
- Santos, E.S.H.; Álvares, V.S.; Souza, J.M.L.; Vasconcelos, M.A.M.; Cartaxo, C.B.C. Quality of fermented and dried seeds of cupuaçu due to depeliculation and storage. In Proceedings of the II Seminário da Embrapa Acre de Iniciação Científica e Pós-graduação Embrapa Acre Scientific Initiation and Graduate Seminar 2. Annals/[II Embrapa Acre II Scientific Initiation and Postgraduate Seminar], Rio Branco, AC, Embrapa Acre, Rio Branco, Brazil, 10–11 September 2019. [Google Scholar]
- Brigante, G.P. Deterioração de sementes de girassol durante o armazenamento [Deterioration of Sunflower Seeds during Storage]. Tese de doutoramento [Ph.D. Thesis], Universidade Federal de Lavras [Federal University of Lavras], Lavras, Brazil, 2013; p. 206. [Google Scholar]
- Oliveira, M.A.; Lorini, I.; Mandarino, J.M.G.; Benassi, V.T.; FRANÇA-NETO, J.B.; Henning, A.A.; Krzyzanowski, F.C.; Henning, F.A.; Hirakuri, M.H.; Leite, R.S.; et al. Determinação do índice de acidez titulável dos grãos de soja colhidos na safra 2014/15 [Determination of the Titratable Acidity Index of Soybeans Harvested in the 2014/15 harvest]. Dissertação de Mestrado [Master’s Thesis], Universidade Federal de Pelotas [Federal University of Pelotas], Pelotas, Brazil, 2016. [Google Scholar]
- Concex. Concex Resolution n° 160, of June 28, 1988. Brazil. Especificações da padronização de de sementes de cacau (Theobroma cacao L.) visando a sua classificação e fiscalização na exportação [Specifications for the standardization of cocoa beans (Theobroma cacao L.) aiming at their classification and inspection in exportation] 1988. Diário Oficial da União, Brasília, 29 September; Section I. p. 8.
- Efraim, P.; Pezoa-García, N.H.; Jardim, D.C.P.; Nishikawa, A.; Haddad, R.; Eberlin, M.N. Influência da fermentação e secagem de amêndoas de cacau no teor de compostos fenólicos e na aceitação sensorial [Influence of fermentation and drying of cocoa beans on the content of phenolic compounds and on sensory acceptance]. Ciênc. Tecnol. Aliment. 2010, 30 (Suppl. 1), 142–150. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-20612010000500022&lng=en&nrm=iso. (accessed on 29 July 2019). [CrossRef]
- Reed, C.; Doyungan, S.; Ioerger, B.; Getchell, A. Response of storage molds to different initial moisture contents of maize (corn) stored at 25ºC, and effect on respiration rate and nutrient composition. J. Stored Prod. Res. 2007, 43, 443–458. [Google Scholar] [CrossRef]
- CNPPA. Resolution—CNNPA No. 12, 1978. Special Technical Rules. Official Gazette, 24 July 1978. [Google Scholar]
- Ministry of Health; National Health Surveillance Agency (NHSA). RDC Resolution N° 12, of January 2, 2001. Approves the technical regulation on microbiological standards for food. Official Gazette of the Federative Republic of Brazil, 2 January 2001. [Google Scholar]
- Nascimento, A.R.; Mouchrek Filho, J.E.; Mouchrek Filho, V.E.; Martins, A.G.A.L.; Bayma, A.B.; Gomes, S.V.; Marinho, S.C.; Carvalho, P.A.B.; Garcias Junior, A.V. Incidência de Escherichia coli e Salmonella em alface (Lactuca sativa) [Incidence of Escherichia coli and Salmonella on lettuce (Lactuca sativa)]. Revista Higiene Alimentar [Food Hyg. Mag.] 2005, 19, 223–225. [Google Scholar]
- Okura, M.H.; Mariano, A.M.S.E.; Teixeira, A.N.S. Eficiência de sanitizantes no tratamento “minimamente processado” de alface cultivada em meio hidropônico [Efficiency of sanitizers in the “minimally processed” treatment of lettuce grown in a hydroponic médium]. Revista Higiene Alimentar [Food Hyg. Mag.] 2006, 20, 105–113. [Google Scholar]
- Franco, B.D.G.M.; Landgraf, M.T.D. Microbiologia dos alimentos [Food Microbiology]; Atheneu: São Paulo, Brazil, 2008; p. 182. [Google Scholar]
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, D.C. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- de Vries, H.; Mikolajczak, M.; Salmon, J.-M.; Abecassis, J.; Chaunier, L.; Guessasma, S.; Lourdin, D.; Belhabib, S.; Leroy, E.; Trystram, G. Small-scale food process engineering—Challenges and perspectives. Innov. Food Sci. Emerg. Technol. 2017, 46, 122–130. [Google Scholar] [CrossRef]
- Fava, F.; Totaro, G.; Diels, L.; Reis, M.; Duarte, J.; Carioca, O.B.; Poggi-Varaldo, H.M.; Ferreira, B.S. Biowaste biorefinery in Europe: Opportunities and research & development needs. New Biotechnol. 2015, 32, 100–108. [Google Scholar]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, G.D. Single-Cell Protein. In Biochemical Engineering and Biotechnology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 332–341. [Google Scholar]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63 (Suppl. C), 51–59. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Rocha, J.M.; Guerra, M.A. On the valorisation of lactose and its derivatives from cheese whey as a dairy industry by-product: An overview. Eur. Food Res. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Vilalbai, F.B. Fragmentação mecânica de amêndoas de cupuaçu (Theobroma grandiflorum) por meio de um beneficiador de cilindros [Mechanical fragmentation of cupuaçu almonds (Theobroma grandiflorum) using a cylinder processor]. Master’s Thesis, Universidade Estadual de Campinas [State University of Campinas], Campinas, Brazil, 2003; p. 78. [Google Scholar]
- Williams, I.D.; Schneider, F.; Syversen, F. The “food waste challenge” can be solved. Waste Manag. 2015, 41 (Suppl. C), 1–2. [Google Scholar] [CrossRef]
- Neves, M.; Antunes, M.; Fernandes, W.; Azevedo, Z.M.; Freitas, V.; Rocha, J.M.; Tecelão, C. Physicochemical and nutritional characterization of the leaves, flowers and fruits of Chorão da praia (Carpobrotus edulis) from Portuguese west shores. Food Res. Int. 2020. submitted. [Google Scholar]
- Aboukhalaf, A.; El Amraoui, B.; Tabatou, M.; Rocha, J.M.; Belahsen, R. Screening of the antimicrobial activity of some extracts of edible wild plants in Morocco. J. Funct. Food Health Dis. 2020, 6, 265–273. [Google Scholar] [CrossRef]
- Skendi, A.; Zinoviadou, K.G.; Papageorgiou, M.; Rocha, J.M. Advances on the valorisation and functionalization of by-products and wastes from cereal-based processing industry. Foods 2020, in press. [Google Scholar]
- TWC (The World Counts). 2020. Available online: https://www.theworldcounts.com/challenges/consumption/foods-and-beverages/food-waste-facts (accessed on 29 July 2020).
- European Union (EU). Farm to Fork Strategy—for a Fair, Healthy and Environmentally-Friendly Food System. 2020. Available online: https://ec.europa.eu/food/farm2fork_en;PDFreport (accessed on 29 July 2020).
- European Union (EU). European Green Deal—Striving to Be the First Climate-Neutral Continent. 2020. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 25 July 2020).
- European Union (EU). Directive 2008/98/EC on Waste (Waste Framework Directive). 2020. Available online: https://ec.europa.eu/environment/waste/framework/ (accessed on 25 July 2020).
- United Nations (UN). Transforming Our World: The 2030 Agenda for Sustainable Development. Vol. General Assembly Resolution A/RES/70/1 2015; Division for Sustainable Development, United Nations Department of Economic and Social Affairs, 2015; Available online: https://www.un.org/en/development/desa/population/migration/generalassembly/docs/globalcompact/A_RES_70_1_E.pdf (accessed on 27 August 2020).
- RECA Project 2020. Available online: https://www.projetoreca.com.br/site/sistemas-agroflorestais-safs/ (accessed on 30 July 2020).





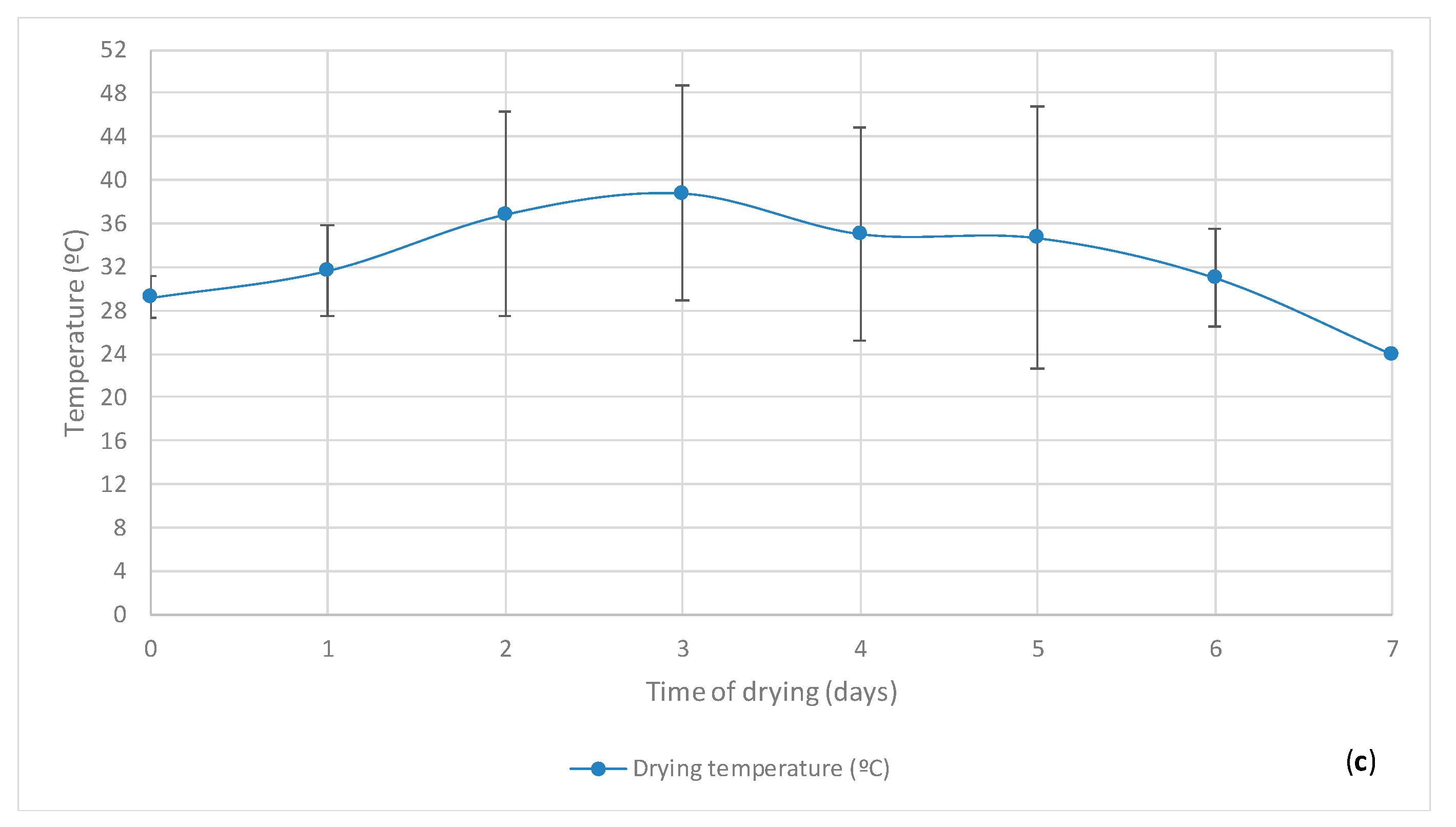

| Fermentation Time (days) | Temperature of the Seeds during Fermentation (°C) |
|---|---|
| Day Period: Morning | |
| 0 | 27.47 ± 1.62 |
| 1 | 32.28 ± 2.37 |
| 2 | 39.66 ± 5.79 |
| 3 | 45.23 ± 2.02 |
| 4 | 42.79 ± 3.52 |
| 5 | 40.98 ± 4.58 |
| 6 | 34.74 ± 4.32 |
| 7 | 31.18 ± 4.13 |
| Day Period: Afternoon | |
| 0 | 29.62 ± 1.28 |
| 1 | 33.54 ± 2.81 |
| 2 | 41.06 ± 4.71 |
| 3 | 43.35 ± 2.51 |
| 4 | 42.09 ± 4.34 |
| 5 | 39.09 ± 4.58 |
| 6 | 35.03 ± 3.11 |
| 7 | --- |
| Day Period: Morning + Afternoon | |
| 0 | 28.33 ± 1.81 |
| 1 | 32.98 ± 2.65 |
| 2 | 40.44 ± 5.16 |
| 3 | 44.29 ± 5.47 |
| 4 | 42.4 ± 3.94 |
| 5 | 39.93 ± 3.85 |
| 6 | 34.89 ± 3.69 |
| 7 | 31.18 ± 4.13 |
| Statistical Analysis | |
| Factor | Variance analysis of principal and interaction effects (F test) |
| Time of fermentation | 0.0013 NS |
| Mean values ±STDV | |
| Morning | 36.79 ± 6.28 a |
| Afternoon | 37.68 ± 5.07 a |
| Morning + Afternoon | 36.80 ± 5.76 a |
| Overall average | 37.9 ± 0.51 |
| CV | 27.45 |
| Drying Time(Days) | Temperature of the Seeds during Drying (°C) |
|---|---|
| Day Period: Morning | |
| 0 | 29.21 ± 1.92 |
| 1 | 30.26 ± 2.04 |
| 2 | 27.82 ± 1.48 |
| 3 | 30.47 ± 3.72 |
| 4 | 27.4 ± 0.35 |
| 5 | 33.32 ± 11.06 |
| 6 | 29.72 ± 3.51 |
| 7 | 24.0 ± 0.00 |
| Day Period: Afternoon | |
| 0 | 32.00 ± 0.00 |
| 1 | 33.12 ± 5.45 |
| 2 | 42.54 ± 7.36 |
| 3 | 42.98 ± 8.67 |
| 4 | 38.92 ± 9.99 |
| 5 | 36.08 ± 11.06 |
| 6 | 37.6 ± 0.00 |
| 7 | --- |
| Day Period: Morning + Afternoon | |
| 0 | 29.21 ± 1.92 |
| 1 | 31.69 ± 4.16 |
| 2 | 36.88 ± 9.38 |
| 3 | 38.81 ± 10.56 |
| 4 | 35.08 ± 9.78 |
| 5 | 34.70 ± 12.02 |
| 6 | 34.37 ± 11.81 |
| 7 | 24.00 ± 0.00 |
| STATISTICAL Analysis | |
| Factor | Variance analysis of principal and interaction effects (F test) |
| Time of drying | 6.07 NS |
| Mean values ±STDV | |
| Morning | 29. 02 ± 2.72 b |
| Afternoon | 40.46 ± 8.67 a |
| Morning + Afternoon | 33.09 ± 4.70 a,b |
| Overall average | 34.19 ± 5.80 |
| CV | 4.16 |
| Treatment Time (days) Treatment (ti) | pH | TTA (Total Titratable Acidity) (% Citric Acid) | Water Activity (aw) | L (Lightness) | a * [Chromaticity (Red-Green)] | b * [Chromaticity (Yellow-Blue)] | ΔE (Total Color Difference) | Moisture (Weight Loss) (%, w/w) | Ashes (%, w/w) | Total Proteins (%, w/w) | Total Lipids (Ether Extract) (%, w/w) | Total Fiber (%, w/w) | Total Carbohydrates (%, w/w) | Energy Value (kCal) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (t1) | 5.34 ± 0.12 | 1.56 ± 0.27 b | 0.53 ± 0.01 b | 36.68 ± 4.03 b | 15.06 ± 2.17 a | 20.72 ± 2.40 a | 65.18 ± 2.58 a | 7.49 ± 1.45 a,b | 3.25 ± 0.55 a | 10.26 ± 0.17 a | 31.52 ± 2.29 c | 44.61 ± 1.96 b | 2.87 ± 0.79 a | 157.75 ± 21.17 c |
| 30 (t2) | 5.31 ± 0.04 | 3.49 ± 0.18 a | 0.70 ± 0.02 a | 37.42 ± 4.66 b | 14.34 ± 1.09 a | 20.14 ± 2.29 a | 64.15 ± 3.39 a | 6.72 ± 0.55 b | 3.28 ± 0.32 a | 10.27 ± 0.19 a | 28.64 ± 1.75 c | 50.00 ± 0.90 a | 1.09 ± 0.05 b | 103.17 ± 18.25 d |
| 60 (t3) | 5.40 ± 0.07 | 3.15 ± 0.2 a | 0.72 ± 0.03 a | 34.63 ± 2.61 b | 13.32 ± 0.65 a | 18.51 ± 1.26 a | 66.05 ± 2.02 a | 8.59 ± 0.2 a | 3.88 ± 0.14 a | 10.05 ± 0.33 a | 45.34 ± 0.44 a | 27.00 ± 1.06 d | 5.13 ± 1.34 a | 360.80 ± 11.40 a |
| 90 (t4) | 5.51 ± 0.06 | 3.81 ± 0.37 a | 0.73 ± 0.01 a | 51.11 ± 0.51 a | 7.37 ± 0.59 b | 4.61 ± 0.15 b | 46.65 ± 0.58 b | 8.74 ± 0.13 a | 3.39 ± 0.11 a | 10.62 ± 0.80 a | 42.79 ± 0.57 b | 31.37 ± 0.43 c | 3.09 ± 0.44 a | 314.44 ± 5.50 b |
| Statistical Analysis | ||||||||||||||
| Factor | Variance analysis of the principal and interaction effects (F test) | |||||||||||||
| Time of storage | 5.31 NS | 45.25 ** | 79.40 ** | 26.74 ** | 38.86 ** | 204.98 ** | 60.53 ** | 5.48 * | 3.26 NS | 0.75 NS | 177.47 ** | 248.78 ** | 94.45 ** | 262.88 ** |
| Overall average | 5.39 ± 0.09 | 3.00 ± 1.00 | 0.67 ± 0.09 | 39.96 ± 7.53 | 12.52 ± 3.51 | 15.99 ± 7.65 | 60.51 ± 9.27 | 7.88 ±0.95 | 3.45 ± 0.29 | 10.30 ± 0.23 | 37.07 ± 8.23 | 38.25 ±1 0.84 | 3.05 ± 1.65 | 234.04 ± 123.13 |
| CV | 0.34 | 7.28 | 7.45 | 20.39 | 3.70 | 3.33 | 0.91 | 24.15 | 25,19 | 1.86 | 7.86 | 1.10 | 9.71 | 5.45 |
| Treatment (ti)– Replicate (Ri) | Time (Days) | Replicate | Water Activity (aw) | Total Mesophilic Viable Counts (log CFU/g) | Total Yeasts and Molds (log CFU/g) | Total Thermophilic Coliforms (MPN/g) |
|---|---|---|---|---|---|---|
| t1-R1 | 0 | 1 | 0.6437 | Countless | 3.75 | >1100 |
| t1-R2 | 0 | 2 | 0.6382 | Countless | 3.80 | 295.80 |
| t1-R3 | 0 | 3 | 0.6398 | Countless | 3.82 | 357.00 |
| t2-R1 | 30 | 1 | 0.6745 | Countless | 3.49 | 295.80 |
| t2-R2 | 30 | 2 | 0.6582 | Countless | 3.73 | 244.80 |
| t2-R3 | 30 | 3 | 0.6573 | Countless | 3.15 | 153.00 |
| t3-R1 | 60 | 1 | 0.7207 | 4.34 | 5.05 | >100 |
| t3-R2 | 60 | 2 | 0.6435 | 4.64 | 4.67 | 153.00 |
| t3-R3 | 60 | 3 | 0.6893 | 4.08 | 4.28 | >1100 |
| t4-R1 | 90 | 1 | 0.6271 | 4.66 | 3.85 | >1100 |
| t4-R2 | 90 | 2 | 0.6297 | 4.41 | 3.00 | 153.00 |
| t4-R3 | 90 | 3 | 0.6269 | 4.56 | 3.90 | >1100 |
| t5-R1 | 120 | 1 | 0.6154 | 4.04 | 0.00 | >1100 |
| t5-R2 | 120 | 2 | 0.6241 | 4.76 | 3.00 | 460.00 |
| t5-R3 | 120 | 3 | 0.6351 | 4.64 | 3.90 | >1100 |
| t6-R1 | 150 | 1 | 0.6386 | Countless | 3.70 | 120.00 |
| t6-R2 | 150 | 2 | 0.7029 | Countless | 3.70 | 112.20 |
| t6-R3 | 150 | 3 | 0.6896 | Countless | 4.58 | 112.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, J.M.L.; Rocha, J.M.; Cartaxo, C.B.C.; Vasconcelos, M.A.M.; Álvares, V.S.; Nascimento, M.M.; Yomura, R.T.B.; Kaefer, S. Monitoring and Optimization of Cupuaçu Seed Fermentation, Drying and Storage Processes. Microorganisms 2020, 8, 1314. https://doi.org/10.3390/microorganisms8091314
Souza JML, Rocha JM, Cartaxo CBC, Vasconcelos MAM, Álvares VS, Nascimento MM, Yomura RTB, Kaefer S. Monitoring and Optimization of Cupuaçu Seed Fermentation, Drying and Storage Processes. Microorganisms. 2020; 8(9):1314. https://doi.org/10.3390/microorganisms8091314
Chicago/Turabian StyleSouza, Joana M. L., João M. Rocha, Cleísa B. C. Cartaxo, Marcus A. M. Vasconcelos, Virginia S. Álvares, Matheus M. Nascimento, Renata T. B. Yomura, and Simara Kaefer. 2020. "Monitoring and Optimization of Cupuaçu Seed Fermentation, Drying and Storage Processes" Microorganisms 8, no. 9: 1314. https://doi.org/10.3390/microorganisms8091314
APA StyleSouza, J. M. L., Rocha, J. M., Cartaxo, C. B. C., Vasconcelos, M. A. M., Álvares, V. S., Nascimento, M. M., Yomura, R. T. B., & Kaefer, S. (2020). Monitoring and Optimization of Cupuaçu Seed Fermentation, Drying and Storage Processes. Microorganisms, 8(9), 1314. https://doi.org/10.3390/microorganisms8091314