Assessing the Effect of Humic Substances and Fe(III) as Potential Electron Acceptors for Anaerobic Methane Oxidation in a Marine Anoxic System
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Incubations with Suspended Particulate Matter
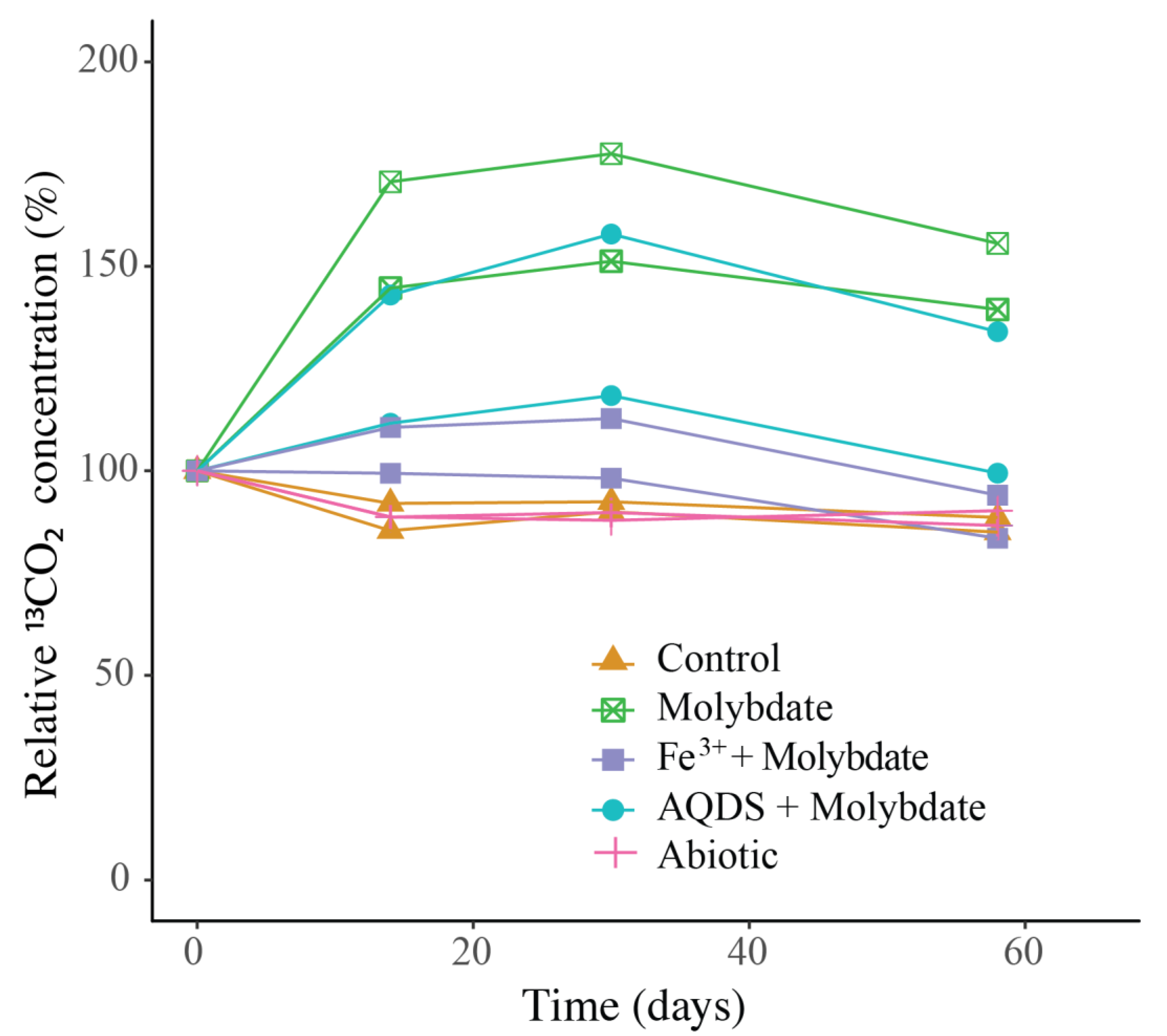
2.3. 13CO2 Analysis
2.4. DNA Extraction and Analysis
2.5. Quantitative PCR 16S rRNA Gene
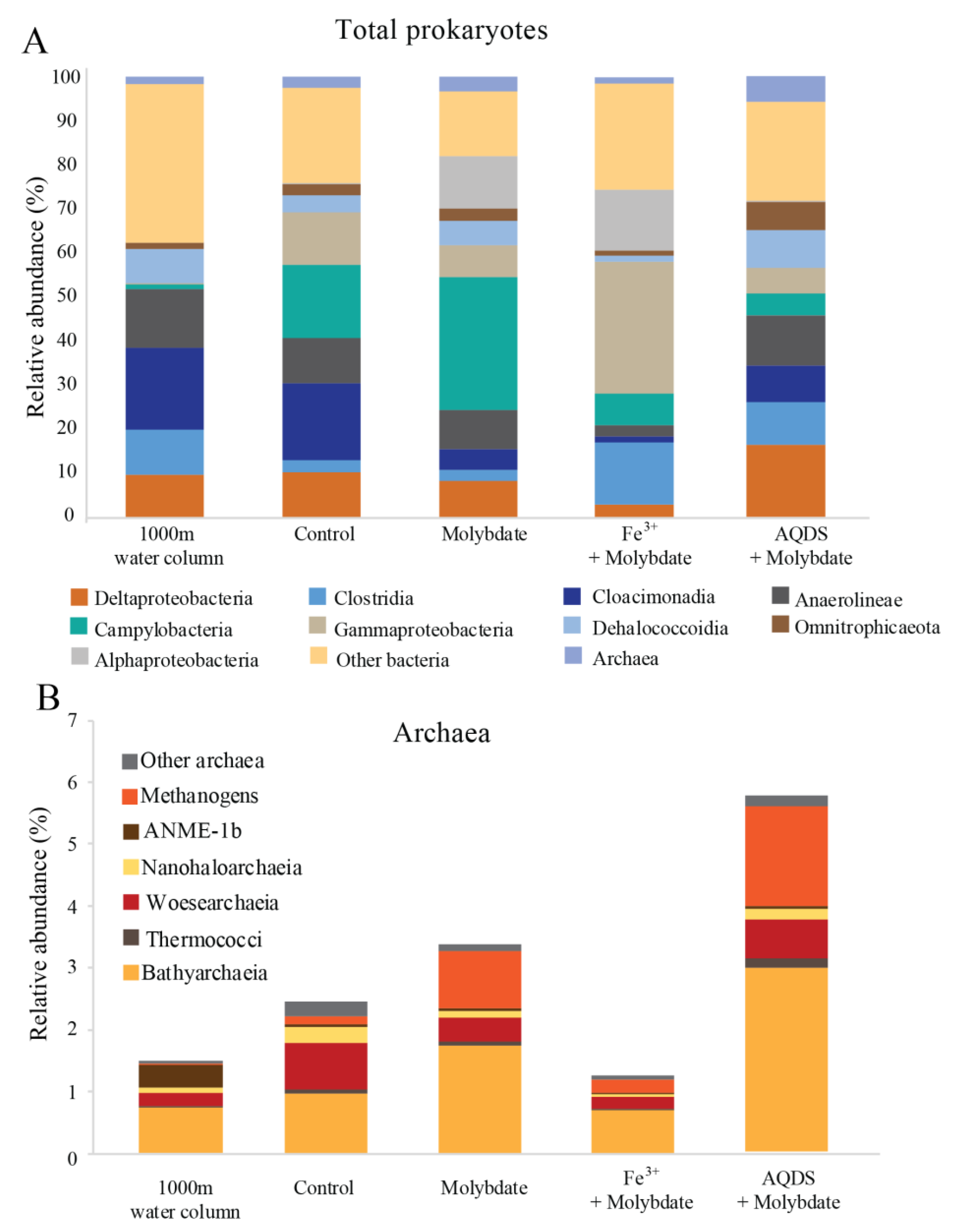
3. Results
3.1. Water Column Physicochemical Conditions and In Situ Microbial Community
3.2. Abiotic and Control Incubations
3.3. Incubations with Sodium Molybdate
3.4. Incubations with Sodium Molybdate and Soluble Fe3+ Complexes
3.5. Incubations with Sodium Molybdate and AQDS
4. Discussion
4.1. Enhanced Methane Oxidation by ANME-1b in Molybdate and in AQDS Incubations
4.2. Potential Role of Sulfur Cycling Organisms
4.3. Potential Methanogenesis by Methanogens, ANME and Bathyarchaeota
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.M.B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC, 2013: Summary for policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge Univ. Press: Cambridge, UK, 2013. [Google Scholar]
- Conrad, R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Reeburgh, W.S. Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef] [PubMed]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, R.; Jùrgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Orphan, V.J.; Turk, K.A.; Green, A.M.; House, C.H. Patterns of 15N assimilation and growth of methanotrophic ANME-2 archaea and sulfate-reducing bacteria within structured syntrophic consortia revealed by FISH-SIMS. Environ. Microbiol. 2009, 11, 1777–1791. [Google Scholar] [CrossRef]
- Nauhaus, K.; Albrecht, M.; Elvert, M.; Boetius, A.; Widdel, F. In vitro cell growth of marine archaeal-bacterial consortia during anaerobic oxidation of methane with sulfate. Environ. Microbiol. 2007, 9, 187–196. [Google Scholar] [CrossRef]
- Segarra, K.E.A.; Comerford, C.; Slaughter, J.; Joye, S.B. Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochim. Cosmochim. Acta 2013, 115, 15–30. [Google Scholar] [CrossRef]
- Ettwig, K.F.; Zhu, B.; Speth, D.; Keltjens, J.T.; Jetten, M.S.M.; Kartal, B. Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc. Natl. Acad. Sci. USA 2016, 113, 12792–12796. [Google Scholar] [CrossRef]
- Egger, M.; Rasigraf, O.; Sapart, C.J.; Jilbert, T.; Jetten, M.S.M.; Röckmann, T.; Van Der Veen, C.; Banda, N.; Kartal, B.; Ettwig, K.F.; et al. Iron-mediated anaerobic oxidation of methane in brackish coastal sediments. Environ. Sci. Technol. 2014, 49, 277–283. [Google Scholar] [CrossRef]
- Scheller, S.; Yu, H.; Chadwick, G.L.; Mcglynn, S.E. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science 2016, 351, 703–707. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Avendaño, K.A.; Balagurusamy, N.; Arriaga, S.; Nieto-Delgado, C.; Thalasso, F.; Cervantes, F.J. Electron shuttling mediated by humic substances fuels anaerobic methane oxidation and carbon burial in wetland sediments. Sci. Total Environ. 2019, 650, 2674–2684. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Wu, J.; Lu, Y.; Fu, L.; Zhang, F.; Lau, T.; Zeng, R.J. Humic substances as electron acceptors for anaerobic oxidation of methane driven by ANME-2d. Water Res. 2019, 164, 114935. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, K.U.; Hayes, J.M.; Sylva, S.P.; Brewert, P.G.; DeLong, E.F. Methane-consuming archaebacteria in marine sediments. Nature 1999, 398, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.J.; Putnam, N.; Preston, C.M.; Detter, J.C.; Rokhsar, D.; Richardson, P.M.; DeLong, E.F. Reverse methanogenesis: Testing the hypothesis with environmental genomics. Science 2004, 305, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Orphan, V.J.; House, C.H.; Hinrichs, K. Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 2001, 293, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Lösekann, T.; De Beer, D.; Elvert, M.; Nadalig, T.; Knittel, K.; Amann, R.; Sauter, E.J.; Schlüter, M.; Klages, M.; et al. Novel microbial communities of the Haakon Mosby mud volcano and their role as a methane sink. Nature 2006, 443, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Knittel, K.; Losekann, T.; Boetius, A.; Kort, R.; Amann, R. Diversity and distribution of methantrophic acrhaea at cold seeps. Appl. Environ. Microbiol. 2005, 71, 467–479. [Google Scholar] [CrossRef]
- Cui, M.; Ma, A.; Qi, H.; Zhuang, X.; Zhuang, G. Anaerobic oxidation of methane: An “active” microbial process. Microbiologyopen 2015, 4, 1–11. [Google Scholar] [CrossRef]
- Milucka, J.; Ferdelman, T.G.; Polerecky, L.; Franzke, D.; Wegener, G.; Schmid, M.; Lieberwirth, I.; Wagner, M.; Widdel, F.; Kuypers, M.M.M. Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 2012, 491, 541–546. [Google Scholar] [CrossRef]
- Moran, J.J.; Beal, E.J.; Vrentas, J.M.; Orphan, V.J.; Freeman, K.H.; House, C.H. Methyl sulfides as intermediates in the anaerobic oxidation of methane. Environ. Microbiol. 2008, 10, 162–173. [Google Scholar] [CrossRef]
- Valentine, D.L.; Reeburgh, W.S. New perspectives on anaerobic methane oxidation. Environ. Microbiol. 2000, 2, 477–484. [Google Scholar] [CrossRef]
- Wegener, G.; Krukenberg, V.; Riedel, D.; Tegetmeyer, H.E.; Boetius, A. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 2015, 526, 587–590. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, S.E.; Chadwick, G.L.; Kempes, C.P.; Orphan, V.J. Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature 2015, 526, 531–535. [Google Scholar] [CrossRef]
- Krukenberg, V.; Riedel, D.; Gruber-Vodicka, H.R.; Buttigieg, P.L.; Tegetmeyer, H.E.; Boetius, A.; Wegener, G. Gene expression and ultrastructure of meso- and thermophilic methanotrophic consortia. Environ. Microbiol. 2018, 20, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Meyerdierks, A.; Kube, M.; Kostadinov, I.; Teeling, H.; Glöckner, F.O.; Reinhardt, R.; Amann, R. Metagenome and mRNA expression analyses of anaerobic methanotrophic archaea of the ANME-1 group. Environ. Microbiol. 2010, 12, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Reeburgh, W.S.; Ward, B.B.; Whalen, S.C.; Sandbeck, K.A.; Kilpatrickt, K.A.; Kerkhof, L.J. Black Sea methane geochemistry. Deep Sea Res. Part A Oceanogr. Res. Pap. 1991, 38, S1189–S1210. [Google Scholar] [CrossRef]
- Schubert, C.J.; Coolen, M.J.L.; Neretin, L.N.; Schippers, A.; Abbas, B.; Durisch-Kaiser, E.; Wehrli, B.; Hopmans, E.C.; Damsté, J.S.S.; Wakeham, S.; et al. Aerobic and anaerobic methanotrophs in the Black Sea water column. Environ. Microbiol. 2006, 8, 1844–1856. [Google Scholar] [CrossRef]
- Durisch-Kaiser, E.; Klauser, L.; Wehrli, B.; Schubert, C. Evidence of Intense Archaeal and Bacterial Methanotrophic Activity in the Black Sea Water Column Evidence of Intense Archaeal and Bacterial Methanotrophic Activity in the Black Sea Water Column. Appl. Environ. Microbiol. 2005, 71, 8099–8106. [Google Scholar] [CrossRef]
- Wakeham, S.G.; Lewis, C.M.; Hopmans, E.C.; Schouten, S.; Damsté, J.S.S. Archaea mediate anaerobic oxidation of methane in deep euxinic waters of the Black Sea. Geochim. Cosmochim. Acta 2003, 67, 1359–1374. [Google Scholar] [CrossRef]
- Sollai, M.; Villanueva, L.; Hopmans, E.C.; Reichart, G.J.; Damsté, J.S.S. A combined lipidomic and 16S rRNA gene amplicon sequencing approach reveals archaeal sources of intact polar lipids in the stratified Black Sea water column. Geobiology 2019, 17, 91–109. [Google Scholar] [CrossRef]
- Schubert, C.J.; Durisch-Kaiser, E.; Holzner, C.P.; Klauser, L.; Wehrli, B.; Schmale, O.; Greinert, J.; McGinnis, D.F.; De Batist, M.; Kipfer, R. Methanotrophic microbial communities associated with bubble plumes above gas seeps in the Black Sea. Geochem. Geophys. Geosyst. 2006, 7. [Google Scholar] [CrossRef]
- Yanagawa, K.; Sunamura, M.; Lever, M.A.; Morono, Y.; Hiruta, A.; Ishizaki, O.; Matsumoto, R.; Urabe, T.; Inagaki, F. Niche separation of methanotrophic archaea (ANME-1 and -2) in methane-seep sediments of the Eastern Japan Sea offshore Joetsu. Geomicrobiol. J. 2011, 28, 118–129. [Google Scholar] [CrossRef]
- Suominen, S.; Dombrowski, N.; Damsté, J.S.S.; Villanueva, L. A diverse uncultivated microbial community is responsible for organic matter degradation in the Black Sea sulphidic zone. Environ. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Besseling, M.A.; Hopmans, E.C.; Christine Boschman, R.; Damsté, J.S.S.; Villanueva, L. Benthic archaea as potential sources of tetraether membrane lipids in sediments across an oxygen minimum zone. Biogeosciences 2018, 15, 4047–4064. [Google Scholar] [CrossRef]
- Asbun, A.A.; Besseling, M.A.; Balzano, S.; van Bleijswijk, J.; Witte, H.; Villanueva, L.; Engelmann, J.C. Cascabel: A flexible, scalable and easy-to-use amplicon sequence data analysis pipeline. BioRxiv 2019, 809384. [Google Scholar]
- Andrews, S.; Krueger, F.; Seconds-Pichon, A.; Biggins, F.; Wingett, S. FastQC. A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinformatics. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 14 July 2020).
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Haroon, M.F.; Hu, S.; Shi, Y.; Imelfort, M.; Keller, J.; Hugenholtz, P.; Yuan, Z.; Tyson, G.W. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 2013, 500, 567–570. [Google Scholar] [CrossRef]
- Beal, E.J.; House, C.H.; Orphan, V.J. Manganese- and iron-dependent marine methane oxidation. Science 2009, 325, 184–187. [Google Scholar] [CrossRef]
- Sivan, O.; Adler, M.; Pearson, A.; Gelman, F.; Bar-Or, I.; John, S.G.; Eckert, W. Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol. Oceanogr. 2011, 56, 1536–1544. [Google Scholar] [CrossRef]
- Reitner, J.; Peckmann, J.; Reimer, A.; Schumann, G.; Thiel, V. Methane-derived carbonate build-ups and associated microbial communities at cold seeps on the lower Crimean shelf (Black Sea). Facies 2005, 51, 66–79. [Google Scholar] [CrossRef]
- Gründger, F.; Carrier, V.; Svenning, M.M.; Panieri, G.; Vonnahme, T.R.; Klasek, S.; Niemann, H. Methane-fuelled biofilms predominantly composed of methanotrophic ANME-1 in Arctic gas hydrate-related sediments. Sci. Rep. 2019, 9, 9725. [Google Scholar] [CrossRef] [PubMed]
- Cassarini, C.; Zhang, Y.; Lens, P.N.L. Pressure Selects Dominant Anaerobic Methanotrophic Phylotype and Sulfate Reducing Bacteria in Coastal Marine Lake Grevelingen Sediment. Front. Environ. Sci. 2019, 6, 162. [Google Scholar] [CrossRef]
- Wilson, L.G.; Bandurski, R.S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J. Biol. Chem. 1958, 233, 975–981. [Google Scholar] [PubMed]
- Campbell, A.M.; Del Campillo-Campbell, A.; Villaret, D.B. Molybdate reduction by Escherichia coli K-12 and its chl mutants. Proc. Natl. Acad. Sci. USA 1985. [Google Scholar] [CrossRef]
- Shukor, M.Y.; Rahman, M.F.; Shamaan, N.A.; Syed, M.S. Reduction of molybdate to molybdenum blue by Enterobacter sp. strain Dr.Y13. J. Basic Microbiol. 2009. [Google Scholar] [CrossRef]
- Shukor, M.Y.; Ahmad, S.A.; Nadzir, M.M.M.; Abdullah, M.P.; Shamaan, N.A.; Syed, M.A. Molybdate reduction by Pseudomonas sp. strain DRY2. J. Appl. Microbiol. 2010. [Google Scholar] [CrossRef]
- Lloyd, K.G.; Alperin, M.J.; Teske, A. Environmental evidence for net methane production and oxidation in putative ANaerobic MEthanotrophic (ANME) archaea. Environ. Microbiol. 2011, 13, 2548–2564. [Google Scholar] [CrossRef]
- Bertram, S.; Blumenberg, M.; Michaelis, W.; Siegert, M.; Krüger, M.; Seifert, R. Methanogenic capabilities of ANME-archaea deduced from 13C-labelling approaches. Environ. Microbiol. 2013, 15, 2384–2393. [Google Scholar] [CrossRef]
- Albers, C.N.; Jensen, A.; Bælum, J.; Jacobsen, C.S. Inhibition of DNA Polymerases Used in Q-PCR by Structurally Different Soil-Derived Humic Substances. Geomicrobiol. J. 2013, 30, 675–681. [Google Scholar] [CrossRef]
- Sidstedt, M.; Jansson, L.; Nilsson, E.; Noppa, L.; Forsman, M.; Rådström, P.; Hedman, J. Humic substances cause fluorescence inhibition in real-time polymerase chain reaction. Anal. Biochem. 2015, 487, 30–37. [Google Scholar] [CrossRef]
- Vigneron, A.; Alsop, E.B.; Cruaud, P.; Philibert, G.; King, B.; Baksmaty, L. Contrasting Pathways for Anaerobic Methane Oxidation in Gulf of Mexico Cold Seep Sediments. Appl. Environ. Sci. 2019, 4, e00091-18. [Google Scholar] [CrossRef] [PubMed]
- Straub, K.L.; Schink, B. Ferrihydrite-Dependent Growth of Sulfurospirillum deleyianum through Electron Transfer via Sulfur Cycling. Society 2004, 70, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Goris, T.; Diekert, G. The genus Sulfurospirillum. In Organohalide-Respiring Bacteria; Springer: Berlin/Heidelberg, Germany, 2016; pp. 209–234. ISBN 9783662498750. [Google Scholar]
- Mino, S.; Kudo, H.; Arai, T.; Sawabe, T.; Takai, K.; Nakagawa, S. Sulfurovum aggregans sp. nov., A hydrogenoxidizing, Thiosulfate-reducing chemolithoautotroph within the Epsilonproteobacteria isolated from a deep-sea hydrothermal vent chimney, And an emended description of the genus Sulfurovum. Int. J. Syst. Evol. Microbiol. 2014, 64, 3195–3201. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Takai, K.; Inagaki, F.; Hirayama, H.; Nunoura, T.; Horikoshi, K.; Sako, Y. Distribution, phylogenetic diversity and physiological characteristics of epsilon-Proteobacteria in a deep-sea hydrothermal field. Environ. Microbiol. 2005, 7, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Perner, M. The globally widespread genus Sulfurimonas: Versatile energy metabolisms and adaptations to redox clines. Front. Microbiol. 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Goris, T.; Schubert, T.; Gadkari, J.; Wubet, T.; Tarkka, M.; Buscot, F.; Adrian, L.; Diekert, G. Insights into organohalide respiration and the versatile catabolism of Sulfurospirillum multivorans gained from comparative genomics and physiological studies. Environ. Microbiol. 2014, 16, 3562–3580. [Google Scholar] [CrossRef]
- Ross, D.E.; Norman, R.; Marshall, C.W.; May, H.D. Comparative genomic analysis of sulfurospirillum cavolei MES reconstructed from the metagenome of an electrosynthetic microbiome. PLoS ONE 2016, 11, e0151214. [Google Scholar] [CrossRef]
- Takai, K.; Suzuki, M.; Nakagawa, S.; Miyazaki, M.; Suzuki, Y.; Inagaki, F.; Horikoshi, K. Sulfurimonas paralvinellae sp. nov., a novel mesophilic, hydrogen- and sulfur-oxidizing chemolithoautotroph within the Epsilonproteo-bacteria isolated from a deep-sea hydrothermal vent polychaete nest, reclassification of Thiomicrospira denitrificans as S. Int. J. Syst. Evol. Microbiol. 2006, 56, 1725–1733. [Google Scholar] [CrossRef]
- Fadhlaoui, K.; Hania, W.B.; Postec, A.; Fauque, G.; Hamdi, M.; Ollivier, B.; Fardeau, M.L. Fusibacter fontis sp. Nov., a sulfur-reducing, anaerobic bacterium isolated from a mesothermic Tunisian spring. Int. J. Syst. Evol. Microbiol. 2015, 65, 3501–3506. [Google Scholar] [CrossRef]
- Ravot, G.; Magot, M.; Fardeau, M.L.; Patel, B.K.C.; Thomas, P.; Garcia, J.L.; Ollivier, B. Fusibacter paucivorans gen. nov., sp. nov., an anaerobic, thiosulfate- reducing bacterium from an oil-producing well. Int. J. Syst. Bacteriol. 1999, 49, 1141–1147. [Google Scholar] [CrossRef]
- Suzuki, D.; Li, Z.; Cui, X.; Zhang, C.; Katayama, A. Reclassification of Desulfobacterium anilini as Desulfatiglans anilini comb. nov. within Desulfatiglans gen. nov., And description of a 4-chlorophenol-degrading sulfate-reducing bacterium, Desulfatiglans parachlorophenolica sp. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 3081–3086. [Google Scholar] [CrossRef] [PubMed]
- Kevorkian, R.; Callahan, S.; Winstead, R.; Lloyd, K.G. ANME-1 archaea drive methane accumulation and removal in estuarine sediments. BioRxiv Prepr. 2020. [Google Scholar] [CrossRef]
- Evans, P.N.; Parks, D.H.; Chadwick, G.L.; Robbins, S.J.; Orphan, V.J.; Golding, S.D.; Tyson, G.W. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 2015, 350, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.; Rinna, J.; Roussel, E.G.; Fry, J.C.; Weightman, A.J.; Parkes, R.J. Prokaryotic functional diversity in different biogeochemical depth zones in tidal sediments of the Severn Estuary, UK, revealed by stable-isotope probing. FEMS Microbiol. Ecol. 2010, 72, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, K.G.; Schreiber, L.; Petersen, D.G.; Kjeldsen, K.U.; Lever, M.A.; Steen, A.D.; Stepanauskas, R.; Richter, M.; Kleindienst, S.; Lenk, S.; et al. Predominant archaea in marine sediments degrade detrital proteins. Nature 2013, 496, 215–218. [Google Scholar] [CrossRef]
- Seyler, L.M.; McGuinness, L.M.; Kerkhof, L.J. Crenarchaeal heterotrophy in salt marsh sediments. ISME J. 2014, 8, 1534–1543. [Google Scholar] [CrossRef]
- Zhou, Z.; Pan, J.; Wang, F.; Gu, J.D.; Li, M. Bathyarchaeota: Globally distributed metabolic generalists in anoxic environments. FEMS Microbiol. Rev. 2018, 42, 639–655. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, W.; Yang, B.; Tian, R.; Gu, S.; Luo, H.; Qian, P.Y. Genomic and transcriptomic evidence for carbohydrate consumption among microorganisms in a cold seep brine pool. Front. Microbiol. 2016, 7, 1825. [Google Scholar] [CrossRef]
- Niu, M.; Fan, X.; Zhuang, G.; Liang, Q.; Wang, F. Methane-metabolizing microbial communities in sediments of the Haima cold seep area, northwest slope of the South China Sea. FEMS Microbiol. Ecol. 2017, 93, 1–13. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Prieto-davó, A.; López-lozano, N.E.; García-gonzález, A.S.; López, M.G.; Cervantes, J. Anaerobic Methane Oxidation Driven by Microbial Reduction of Natural Organic Matter in a Tropical Wetland. Appl. Environ. Microbiol. 2017, 83, 1–15. [Google Scholar] [CrossRef]
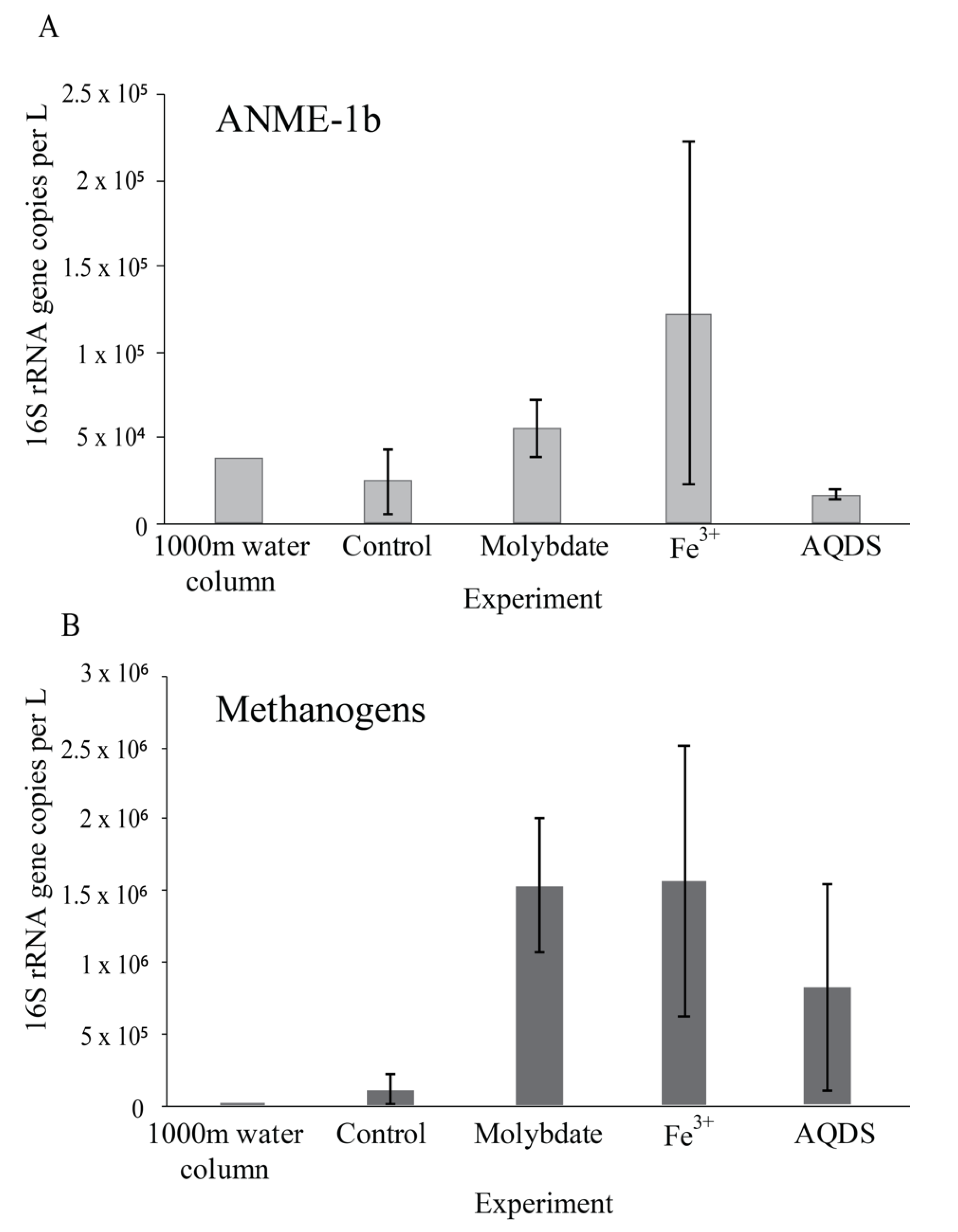
| Treatment | Total 16S rRNA Copies × µL−1 | Archaea | Bacteria | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ANME-1b | Methanogens * | Desulfatiglans | Fusibacter | SEEP-SRB | Sulfurimonas | Sulfurospirillum | Sulfurovum | ||
| 1000 m water column | 1.2 × 107 | 3.8 × 104 | 4.0 × 103 | 5.6 × 105 | 8.4 × 103 | 4.5 × 105 | 5.9 × 104 | 2.0 × 104 | 1.7 × 104 |
| Control | 7.8 × 107 | 2.4 × 104 | 1.2 × 105 | 2.1 × 106 | 1.2 × 106 | 4.4 × 105 | 5.4 × 106 | 4.6 × 106 | 2.6 × 106 |
| Molybdate | 1.7 × 108 | 5.5 × 104 | 1.5 × 106 | 1.1 × 107 | 3.1 × 106 | 9.1 × 105 | 4.1 × 106 | 4.5 × 107 | 4.0 × 105 |
| Fe3+ | 7.6 × 108 | 1.2 × 105 | 1.6 × 106 | 1.2 × 107 | 1.5 × 107 | 1.4 × 106 | 1.5 × 107 | 3.5 × 107 | 3.5 × 106 |
| AQDS | 4.7 × 107 | 1.7 × 104 | 8.2 × 105 | 6.1 × 106 | 3.2 × 106 | 8.2 × 105 | 1.6 × 106 | 2.6 × 105 | 1.8 × 105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Grinsven, S.; Sinninghe Damsté, J.S.; Villanueva, L. Assessing the Effect of Humic Substances and Fe(III) as Potential Electron Acceptors for Anaerobic Methane Oxidation in a Marine Anoxic System. Microorganisms 2020, 8, 1288. https://doi.org/10.3390/microorganisms8091288
van Grinsven S, Sinninghe Damsté JS, Villanueva L. Assessing the Effect of Humic Substances and Fe(III) as Potential Electron Acceptors for Anaerobic Methane Oxidation in a Marine Anoxic System. Microorganisms. 2020; 8(9):1288. https://doi.org/10.3390/microorganisms8091288
Chicago/Turabian Stylevan Grinsven, Sigrid, Jaap S. Sinninghe Damsté, and Laura Villanueva. 2020. "Assessing the Effect of Humic Substances and Fe(III) as Potential Electron Acceptors for Anaerobic Methane Oxidation in a Marine Anoxic System" Microorganisms 8, no. 9: 1288. https://doi.org/10.3390/microorganisms8091288
APA Stylevan Grinsven, S., Sinninghe Damsté, J. S., & Villanueva, L. (2020). Assessing the Effect of Humic Substances and Fe(III) as Potential Electron Acceptors for Anaerobic Methane Oxidation in a Marine Anoxic System. Microorganisms, 8(9), 1288. https://doi.org/10.3390/microorganisms8091288




