Decreased Expression of the High Mobility Group Box 1 (HMGB1) Gene in Peripheral Blood in Patients with Mild or Moderate Clostridioides difficile Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Tests
2.3. Gene Expression Assay
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. The Impact of Genetic Factors on the Course of Clostridioides Difficile Infection
4.2. The Role of HMGB1 in Clostridioides Difficile Infection
4.3. HMGB1 Gene Expression in Different Infections
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lawson, P.A.; Citron, D.M.; Tyrrell, K.L.; Finegold, S.M. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Preevot 1938. Anaerobe 2016, 40, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Penichea, A.G.; Savidge, T.C.; Dann, S.M. Recent insights into Clostridium difficile pathogenesis. Curr. Opin. Infect. Dis. 2013, 26, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Clabots, C.R.; Johnson, S.; Olson, M.; Peterson, L.R.; Gerding, D.N. Acquisition of Clostridium difficile by hospitalized patients: Evidence for colonized new admissions as a source of infection. J. Infect. Dis. 1992, 166, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, C.; Chen, D.; Stein, A.C.; Semik, P.E. Prevalence of Clostridium difficile colonization at admission to rehabilitation. Arch. Phys. Med. Rehabil. 2006, 87, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Lee, L.C.; Lin, H.J.; Liu, H.C.; Wu, Y.H.; Tsai, P.J.; Ko, W.C. Clinical impact of Clostridium difficile colonization. J. Microbiol. Immunol. Infect. 2015, 48, 241–248. [Google Scholar] [CrossRef]
- Hensgens, M.P.M.; Keessen, E.C.; Squire, M.M.; Riley, T.V.; Koene, M.G.; de Boer, E.; Lipman, L.J.; Kuijper, E.J. Clostridium difficile infection in the community: A zoonotic disease? Clin. Microbiol. Infect. 2012, 18, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 373, 287–288. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ma, Y.; Sun, C.L.; Li, S.; Wang, J.F. High Mobility Group Box1 protein is involved in endoplasmic reticulum stress induced by Clostridium difficile toxin A. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Bellussi, L.M.; Cocca, S.; Passali, G.C.; Passali, D. HMGB1 in the pathogenesis of nasal inflammatory diseases and its inhibition as new therapeutic approach: A review from the literature. Int. Arch. Otorhinolaryngol. 2017, 21, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Maruyama, I. HMGB1, a novel inflammatory cytokine. Clin. Chim. Acta 2007, 375, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Efron, P.A.; Liu, H.; Lottenberg, L.; Cuenca, A.G.; Gentile, L.F.; Miggins, M.V.; Bihorac, A.; Baker, H.V.; Moore, F.A.; Moldawer, L.L.; et al. A genomic analysis of Clostridium difficile infections in blunt trauma patients. J. Trauma Acute Care Surg. 2013, 74, 334–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J.; ESCMID. European Society of Clinical Microbiology and Infectious Diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol, Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Chu, C.; Li, Y.; Li, G.; Lei, X.; Zhou, W.; Chen, Z. High expression of HMGB1 in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect. 2018, 18, 439. [Google Scholar] [CrossRef]
- Majumdar, M.; Ratho, R.; Chawla, Y.; Singh, M.P. High levels of circulating HMGB1 as a biomarker of acute liver failure in patients with viral hepatitis E. Liver Int. 2013, 33, 1341–1348. [Google Scholar] [CrossRef]
- Mohajertehran, F.; Ayatollahi, H.; Khazaeni, K.; Shakeri, M.T.; Mohtasham, N. Overexpression of High-Mobility Motor Box 1 in the blood and tissues of patients with head and neck squamous cell carcinoma. Iran. J. Otorhinolaryngol. 2018, 30, 261–271. [Google Scholar]
- Garey, K.W.; Jiang, Z.D.; Ghantoji, S.; Tam, V.H.; Arora, V.; DuPont, H.L. A common polymorphism in the Interleukin-8 gene promoter is associated with an increased risk for recurrent Clostridium difficile infection. Clin. Infect. Dis. 2010, 51, 1406–1410. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.D.; DuPont, H.L.; Garey, K.; Price, M.; Graham, G.; Okhuysen, P.; Dao-Tran, T.; LaRocco, M. A common polymorphism in the interleukin 8 gene promoter is associated with Clostridium difficile diarrhea. Am. J. Gastroenterol. 2006, 101, 1112–1116. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Garey, K.W.; Price, M.; Graham, G.; Okhuysen, P.; Dao-Tran, T.; LaRocco, M.; DuPont, H.L. Association of interleukin-8 polymorphism and immunoglobulin G anti-toxin A in patients with Clostridium difficile–associated diarrhea. Clin. Gastroenterol. Hepatol. 2007, 5, 964–968. [Google Scholar] [CrossRef]
- Czepiel, J.; Biesiada, G.; Dróżdż, M.; Gdula-Argasińska, J.; Żurańska, J.; Marchewka, J.; Perucki, W.; Wołkow, P.; Garlicki, A. The presence of IL-8 +781 T/C polymorphism is associated with the parameters of severe Clostridium difficile infection. Microb. Pathog. 2018, 114, 281–285. [Google Scholar] [CrossRef]
- Madan, R.; Petri, W.A., Jr. Immune responses to Clostridium difficile infection. Trends Mol. Med. 2012, 18, 658–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connelly, T.M.; Koltun, W.A.; Sangster, W.; Berg, A.S.; Hegarty, J.P.; Harris, L., III; Deiling, S.; Stewart, D.B. An interleukin-4 polymorphism is associated with susceptibility to Clostridium difficile infection in patients with inflammatory bowel disease: Results of a retrospective cohort study. Surgery 2014, 156, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Abhyankar, M.M.; Mukherjee, A.; Xue, J.; Andersen, H.; Haslam, D.B.; Madan, R. Leptin receptor Q223R polymorphism influences neutrophil mobilization after Clostridium difficile infection. Mucosal Immunol. 2018, 11, 947–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fages, C.; Nolo, R.; Huttunen, H.J.; Eskelinen, E.; Rauvala, H. Regulation of cell migration by amphoterin. J. Cell Sci. 2000, 113, 611–620. [Google Scholar] [PubMed]
- Degryse, B.; Bonaldi, T.; Scaffidi, P.; Müller, S.; Resnati, M.; Sanvito, F.; Arrigoni, G.; Bianchi, M.E. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J. Cell Biol. 2001, 152, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, H.J.; Fages, C.; Kuja-Panula, J.; Ridley, A.J.; Rauvala, H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002, 62, 4805–4811. [Google Scholar]
- Rouhiainen, A.; Kuja-Panula, J.; Wilkman, E.; Pakkanen, J.; Stenfors, J.; Tuominen, R.K.; Lepäntalo, M.; Carpén, O.; Parkkinen, J.; Rauvala, H. Regulation of monocyte migration by amphoterin (HMGB1). Blood 2004, 104, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Orlova, V.V.; Choi, E.Y.; Xie, C.; Chavakis, E.; Bierhaus, A.; Ihanus, E.; Ballantyne, C.M.; Gahmberg, C.G.; Bianchi, M.E.; Nawroth, P.P.; et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007, 26, 1129–1139. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Li, W.; Ward, M.F.; Sama, A.E.; Wang, H. High mobility group box 1 protein as a potential drug target for infection- and injury-elicited inflammation. Inflamm. Allergy Drug Targets 2010, 9, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Yamoah, K.; Brebene, A.; Baliram, R.; Inagaki, K.; Dolios, G.; Arabi, A.; Majeed, R.; Amano, H.; Wang, R.; Yanagisawa, R.; et al. High-mobility group box proteins modulate tumor necrosis factor-alpha expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol. Endocrinol. 2008, 22, 1141–1153. [Google Scholar] [CrossRef] [Green Version]
- Pedrazzi, M.; Patrone, M.; Passalacqua, M.; Ranzato, E.; Colamassaro, D.; Sparatore, B.; Pontremoli, S.; Melloni, E. Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J. Immunol. 2007, 179, 8525–8532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiuza, C.; Bustin, M.; Talwar, S.; Tropea, M.; Gerstenberger, E.; Shelhamer, J.H.; Suffredini, A.F. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003, 101, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Wang, H.; Tang, Y.; Fan, Z.; Chen, F.; Xiao, X. High-mobility group box 1 protein induces tissue factor expression in vascular endothelial cells via activation of NF-kappaB and Egr-1. Thromb. Haemost. 2009, 102, 352–359. [Google Scholar] [PubMed] [Green Version]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Wittemann, B.; Neuer, G.; Michels, H.; Truckenbrodt, H.; Bautz, F.A. Autoantibodies to nonhistone chromosomal proteins HMG-1 and HMG-2 in sera of patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1990, 33, 1378–1383. [Google Scholar] [CrossRef]
- Popovic, K.; Ek, M.; Espinosa, A.; Padyukov, L.; Harris, H.E.; Wahren-Herlenius, M.; Nyberg, F. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005, 52, 3639–3645. [Google Scholar] [CrossRef]
- Porto, A.; Palumbo, R.; Pieron, M.; Aprigliano, G.; Chiesa, R.; Sanvito, F.; Maseri, A.; Bianchi, M.E.; Porto, A.; Palumbo, R.; et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility protein box 1. FASEB J. 2006, 20, E1–E9. [Google Scholar] [CrossRef]
- Vakkila, J.; Lotze, M.T. Inflammation and necrosis promote tumor growth. Nat. Rev. Immunol. 2004, 4, 641–648. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.L.; Sun, C.L.; Wang, J.; Li, S.; Wang, J.F. High mobility group box 1 protein is involved in acute inflammation induced by Clostridium difficile toxin A. Acta Biochim. Biophys. Sin. 2016, 48, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Liu, J.; Chen, S.; Qi, H.; Shi, K.; Li, S.; Ma, Y.; Wang, J. High-mobility group box 1 protein contributes to the immunogenicity of rTcdB-treated CT26 cells. Acta Biochim. Biophys. Sin. 2018, 50, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Chumbler, N.M.; Farrow, M.A.; Lapierre, L.A.; Franklin, J.L.; Haslam, D.; Goldenring, J.R.; Lacy, D.B. Clostridium difficile toxin B causes epithelial cell necrosis through an autoprocessing-independent mechanism. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef]
- Mukherjee, R.M.; Shravanti, G.V.; Jakkampudi, A.; Kota, R.; Jangala, A.L.; Reddy, P.B.; Rao, P.N.; Gupta, R.; Reddy, D.N. Reduced expression of DNA damage repair genes High Mobility Group Box1 and Poly(ADP-ribose) Polymerase1 in inactive carriers of hepatitis B virus infection—A possible stage of viral integration. J. Clin. Exp. Hepatol. 2013, 3, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lui, G.; Wong, C.K.; Ip, M.; Chu, Y.J.; Yung, I.M.; Cheung, C.S.; Zheng, L.; Lam, J.S.; Wong, K.T.; Sin, W.W.; et al. HMGB1/RAGE signaling and pro-inflammatory cytokine responses in non-HIV adults with active pulmonary tuberculosis. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, Y.; Lu, J.; Yan, X.J.; Yu, Y.; Sheng, Z.Y. Recombinant bactericidal/permeability–increasing protein inhibits endotoxin-induced High Mobility Group Box1 gene expression in sepsis. Shock 2008, 29, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Borde, C.; Barnay-Verdier, S.; Gaillard, C.; Hocini, H.; Maréchal, V.; Gozlan, J. Stepwise release of biologically active HMGB1 during HSV-2 infection. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Vitali, R.; Stronati, L.; Negroni, A.; Di Nardo, G.; Pierdomenico, M.; Del Giudice, E.; Rossi, P.; Cucchiara, S. Fecal HMGB1 is a novel marker of intestinal mucosal inflammation in pediatric inflammatory bowel disease. Am. J. Gastroenterol. 2011, 106, 2029–2040. [Google Scholar] [CrossRef]
- Palone, F.; Vitali, R.; Cucchiara, S.; Pierdomenico, M.; Negroni, A.; Aloi, M.; Nuti, F.; Felice, C.; Armuzzi, A.; Stronati, L. Role of HMGB1 as a suitable biomarker of subclinical intestinal inflammation and mucosal healing in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 1448–1457. [Google Scholar] [CrossRef]
- Kornblit, B.; Munthe-Fog, L.; Petersen, S.L.; Madsen, H.O.; Vindeløv, L.; Garred, P. The genetic variation of the human HMGB1 gene. Tissue Antigens 2007, 70, 151–156. [Google Scholar] [CrossRef]
- Song, W.; Tan, H.; Wang, S.; Zhang, Y.; Ding, Y. Association of High Mobility Group Box Protein B1 gene polymorphisms with pneumonia susceptibility and severity. Genet. Test. Mol. Biomark. 2019, 23, 3–11. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Mao, D.H.; Zhu, S.; Mi, X.G.; Yu, Q. An indel polymorphism in the 3’ untranslated region of HMGB1 confers risk for hepatocellular carcinoma by regulating HMGB1 transcriptional activity in a Chinese population. Neoplasma 2020, 67, 61–67. [Google Scholar] [CrossRef]
- Issac, M.S.M.; Girgis, M.; Haroun, M.; Shalaby, A. Association of genetic polymorphism of pre-microRNA-146a rs2910164 and serum High-Mobility Group Box 1 with febrile seizures in Egyptian children. J. Child Neurol. 2015, 30, 437–444. [Google Scholar] [CrossRef] [PubMed]
| Parameter | CDI Group | Control Group | P | ||
|---|---|---|---|---|---|
| n | Median (Q25–Q75) | n | Median (Q25–Q75) | ||
| Age (years) | 27 | 71 (67–84) | 28 | 69 (65–72) | 0.17 |
| WBC (×103/µL) | 27 | 8.9 (6.8–12.3) | 28 | 5.8 (5.1–6.8) | <0.001 |
| neutrophils (×103/µL) | 26 | 6.5 (4.4–9.7) | 28 | 2.9 (2.4–3.9) | <0.001 |
| creatinine (µmol/L) | 27 | 82 (58–121) | 28 | 76 (69–90) | 0.49 |
| albumin (g/L) | 25 | 28.6 (24.1–33) | 20 | 40.3 (34.9–41.9) | <0.001 |
| CRP (mg/L) | 27 | 74 (18–120) | 26 | 2.4 (1–3.8) | <0.001 |
| ΔCq | 27 | 3.71 (3.44–4.09) | 28 | 3.25 (3.02–3.35) | <0.001 |
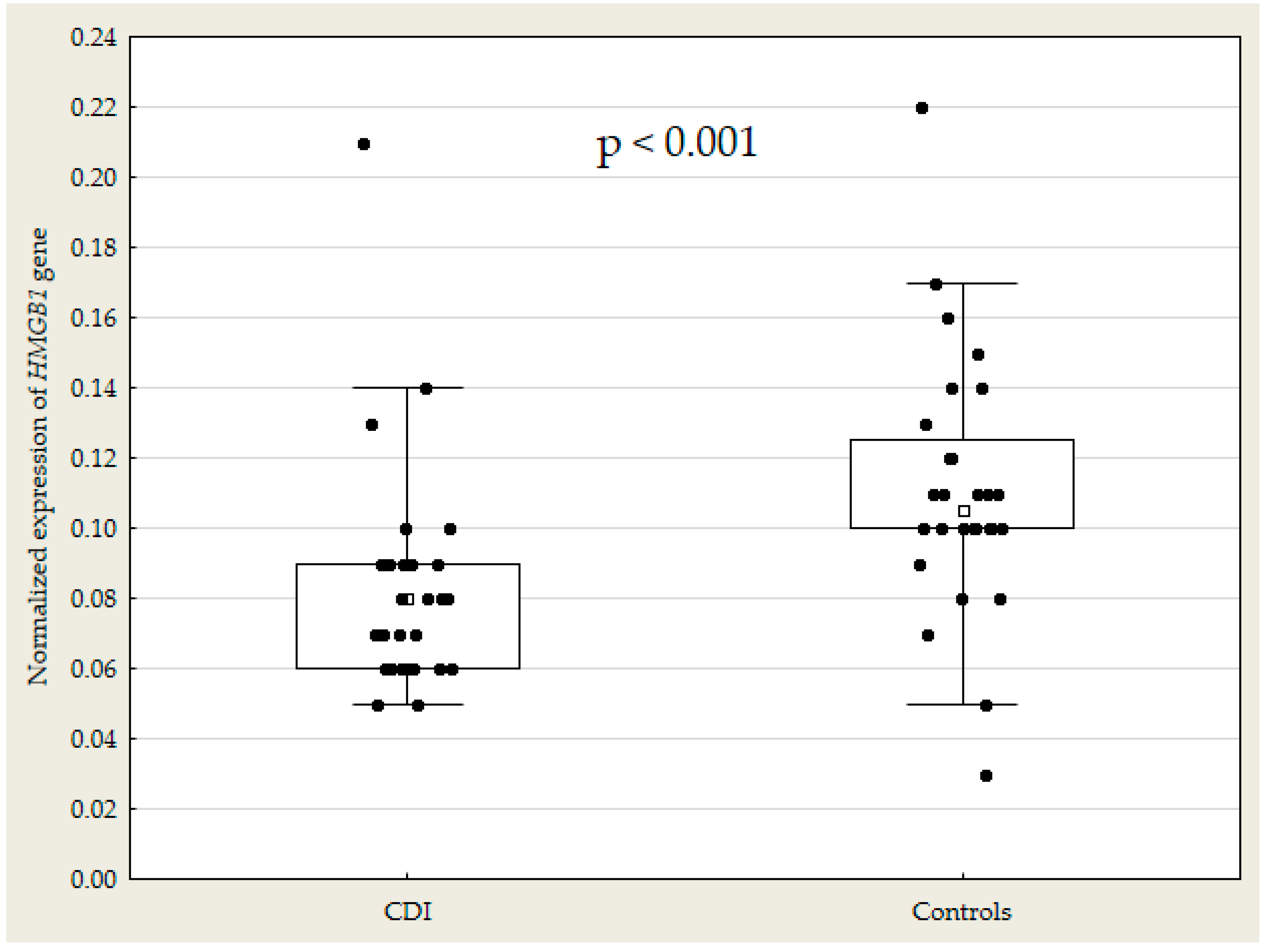
| Normalized Expression of HMGB1 gene | 27 | 0.08 (0.06–0.09) | 28 | 0.11 (0.10–0.13) | <0.001 |
| Parameter Correlated with HGMB1 Gene Expression | CDI Group | Control Group | ||
|---|---|---|---|---|
| Parameters compared | r | p | r | p |
| WBC | −0.29 | 0.14 | −0.16 | 0.41 |
| Neutrophils | −0.18 | 0.39 | −0.28 | 0.16 |
| Creatinine | −0.02 | 0.91 | −0.13 | 0.50 |
| Albumin | 0.40 | 0.07 | 0.42 | 0.07 |
| CRP | −0.42 | 0.03 | −0.09 | 0.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czepiel, J.; Biesiada, G.; Pitera, E.; Wołkow, P.P.; Michalak, M.; Garlicki, A. Decreased Expression of the High Mobility Group Box 1 (HMGB1) Gene in Peripheral Blood in Patients with Mild or Moderate Clostridioides difficile Infection. Microorganisms 2020, 8, 1217. https://doi.org/10.3390/microorganisms8081217
Czepiel J, Biesiada G, Pitera E, Wołkow PP, Michalak M, Garlicki A. Decreased Expression of the High Mobility Group Box 1 (HMGB1) Gene in Peripheral Blood in Patients with Mild or Moderate Clostridioides difficile Infection. Microorganisms. 2020; 8(8):1217. https://doi.org/10.3390/microorganisms8081217
Chicago/Turabian StyleCzepiel, Jacek, Grażyna Biesiada, Ewelina Pitera, Paweł P. Wołkow, Mateusz Michalak, and Aleksander Garlicki. 2020. "Decreased Expression of the High Mobility Group Box 1 (HMGB1) Gene in Peripheral Blood in Patients with Mild or Moderate Clostridioides difficile Infection" Microorganisms 8, no. 8: 1217. https://doi.org/10.3390/microorganisms8081217
APA StyleCzepiel, J., Biesiada, G., Pitera, E., Wołkow, P. P., Michalak, M., & Garlicki, A. (2020). Decreased Expression of the High Mobility Group Box 1 (HMGB1) Gene in Peripheral Blood in Patients with Mild or Moderate Clostridioides difficile Infection. Microorganisms, 8(8), 1217. https://doi.org/10.3390/microorganisms8081217





