Spoilage of Chilled Fresh Meat Products during Storage: A Quantitative Analysis of Literature Data
Abstract
:1. Introduction
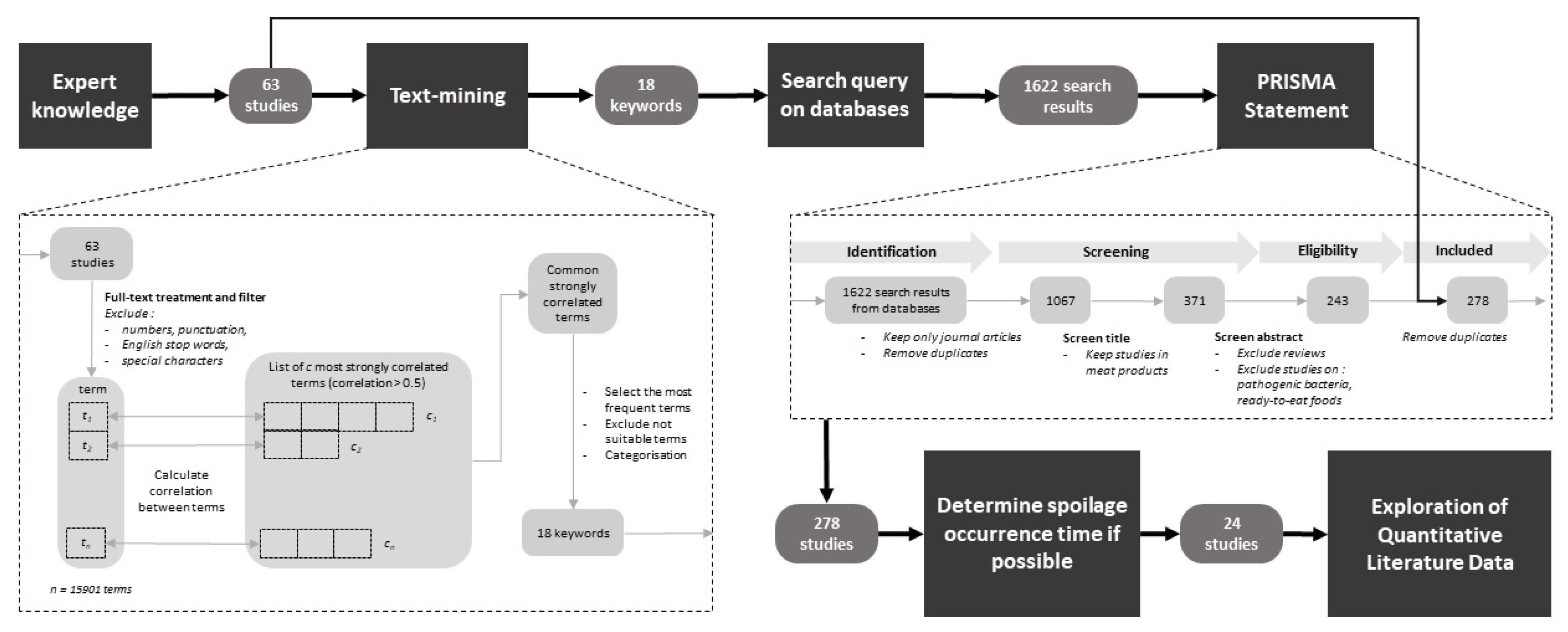
2. Materials and Methods
2.1. Query Process of Literature Data
2.2. Collection of Quantitative Data Associated with Spoilage Occurrence in Meat Products
2.2.1. Determination of Spoilage Occurrence Time
2.2.2. Factors Considered as Influencing Spoilage
2.2.3. Microbiological Data
2.3. Statistical Analyses
3. Results and Discussion
3.1. Measurement of Spoilage in Meat Products and Control Strategies
3.1.1. Types of Meat and Meat Products Associated with Spoilage
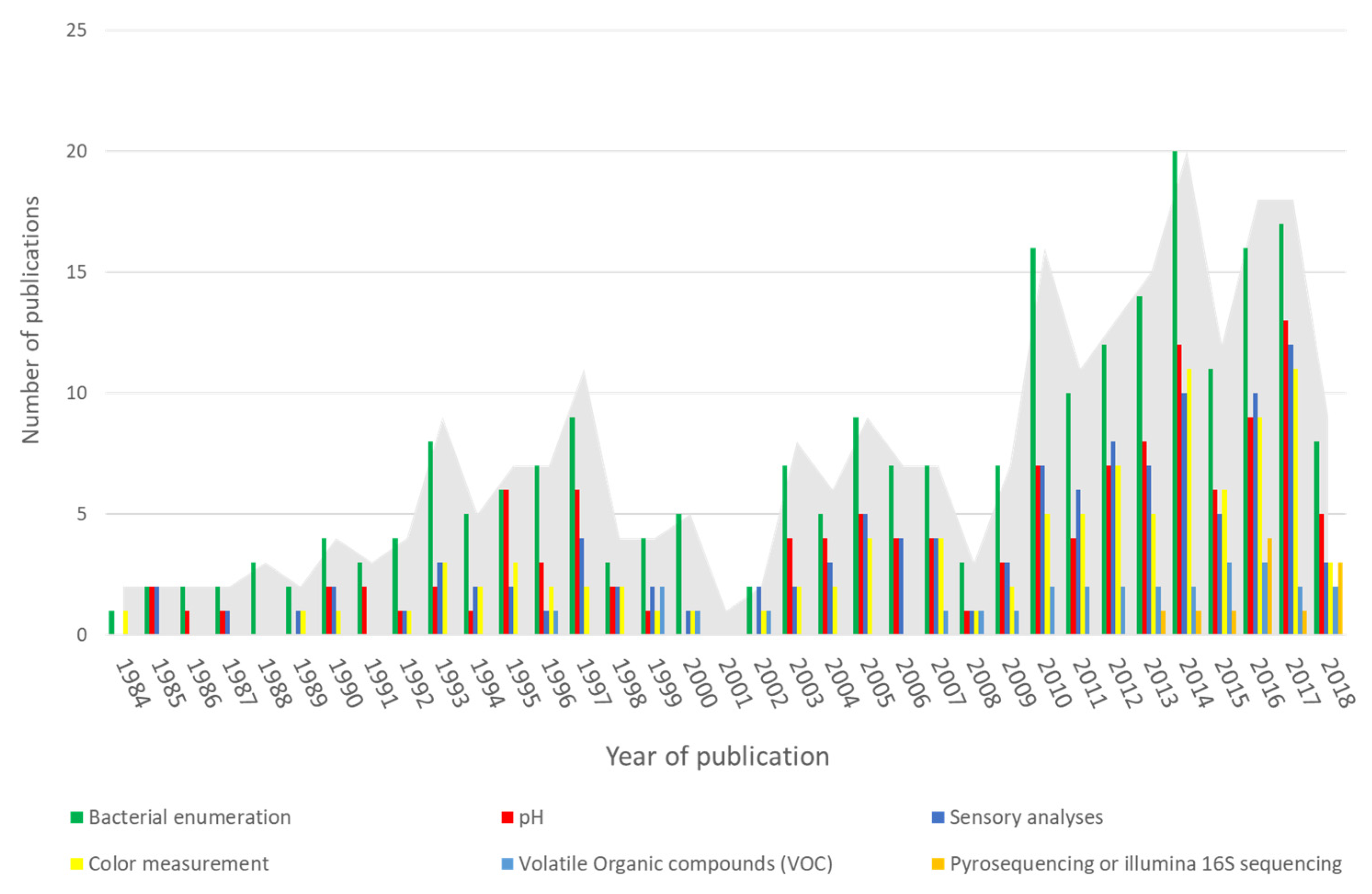
3.1.2. Measurement of Spoilage in Meat Products
Microbiological Analysis
Evaluation of the Organoleptic Quality
Evaluation of Physicochemical Properties
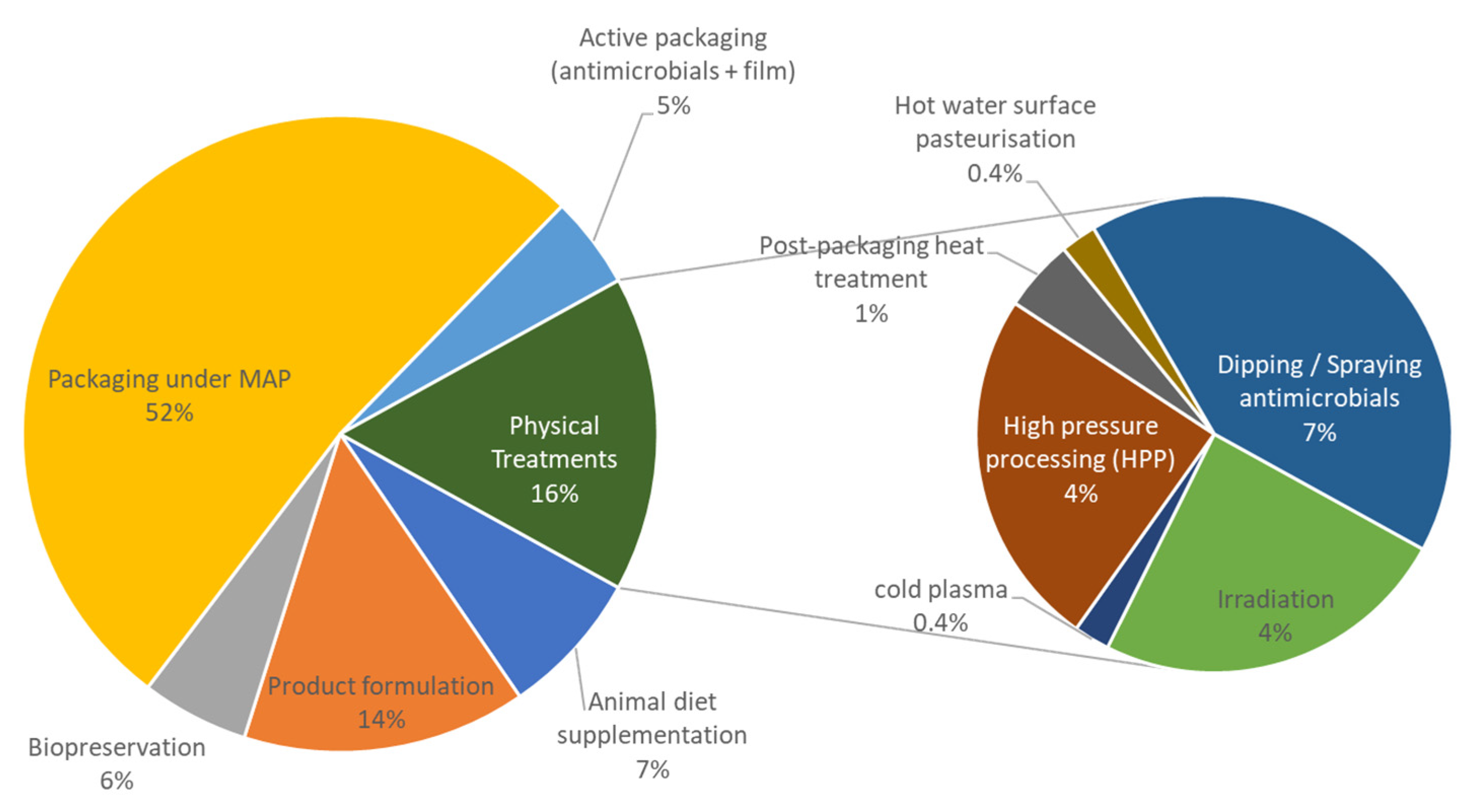
3.1.3. Preservation and Spoilage Control Strategies
Active and Modified Atmosphere Packaging
Formulation
Physical Treatments
Bio-Preservation
Animal Diet Supplementation
3.2. Spoilage Occurrence Time in Meat Products
3.2.1. Spoilage Occurrence Time in Red and White Meat Products
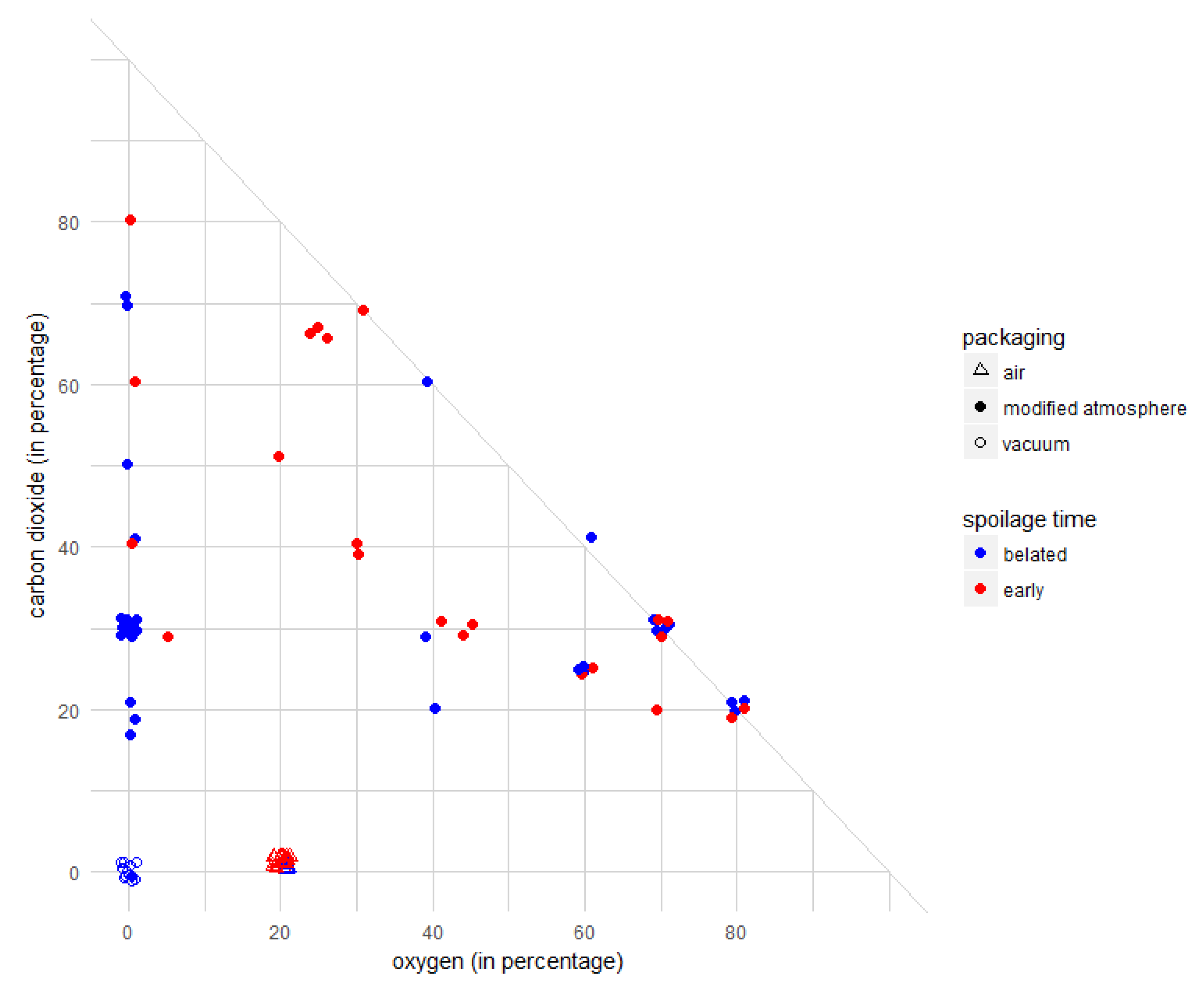
3.2.2. Influence of Initial Gas Composition in the Packaging
3.2.3. Influence of Chilled Storage Temperature
3.2.4. Relationship between Spoilage Time and Microbiological Indicators
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bohrer, B.M. Review: Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- Henchion, M.; McCarthy, M.; Resconi, V.C.; Troy, D. Meat consumption: Trends and quality matters. Meat Sci. 2014, 98, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Faostat. Food and Agriculture Organisation of the United Nations; FAO: Roma, Italy, 2015. [Google Scholar]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage–interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Borch, E.; Kant-Muermans, M.L.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef]
- Dainty, R.H.; Mackey, B.M. The relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes. J. Appl. Bacteriol. 1992, 73, 103s–114s. [Google Scholar] [CrossRef]
- Gray, J.I. Measurement of lipid oxidation: A review. J. Am. Oil Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Love, J.D.; Pearson, A.M. Lipid oxidation in meat and meat products: A review. J. Am. Oil Chem. Soc. 1971, 48, 547–549. [Google Scholar] [CrossRef]
- Tauro, P.; Kapoor, K.K.; Yadav, K.S. An Introduction to microbiology; Wiley Eastern: New Delhi, India, 1986; p. 412. [Google Scholar]
- Cerveny, J.; Meyer, J.D.; Hall, P.A. Microbiological spoilage of meat and poultry products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA, 2009; pp. 69–86. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef] [Green Version]
- Dousset, X.; Jaffrès, E.; Zagorec, M. Spoilage: Bacterial Spoilage, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 5, pp. 106–112. [Google Scholar]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-C.; Lu, Z. Community challenges in biomedical text mining over 10 years: Success, failure and the future. Brief. Bioinform. 2016, 17, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Onan, A.; Korukoğlu, S.; Bulut, H. Ensemble of keyword extraction methods and classifiers in text classification. Expert Syst. Appl. 2016, 57, 232–247. [Google Scholar] [CrossRef]
- Feinerer, I.; Hornik, K. Tm: Text Mining Package. R Package Version 0.7–4. 2008. Available online: http://tm.r-forge.r-project.org/ (accessed on 23 July 2020).
- Williams, G. Hands-on data science with R text mining. Available online: https://fr.scribd.com/doc/252462619/Hands-on-Data-Science-with-R-Text-Mining (accessed on 23 July 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R. Core Team, D.T. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis (Use R!); Springer: Berlin, Germany, 2009. [Google Scholar]
- Höll, L.; Behr, J.; Vogel, R.F. Identification and growth dynamics of meat spoilage microorganisms in modified atmosphere packaged poultry meat by MALDI-TOF MS. Food Microbiol. 2016, 60, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hilgarth, M.; Fuertes, S.; Ehrmann, M.; Vogel, R.F. Photobacterium carnosum sp. nov., isolated from spoiled modified atmosphere packaged poultry meat. Syst. Appl. Microbiol. 2018, 41, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kiermeier, A.; Tamplin, M.; May, D.; Holds, G.; Williams, M.; Dann, A. Microbial growth, communities and sensory characteristics of vacuum and modified atmosphere packaged lamb shoulders. Food Microbiol. 2013, 36, 305–315. [Google Scholar] [CrossRef]
- Benson, A.K.; David, J.R.; Gilbreth, S.E.; Smith, G.; Nietfeldt, J.; Legge, R.; Kim, J.; Sinha, R.; Duncan, C.E.; Ma, J.; et al. Microbial successions are associated with changes in chemical profiles of a model refrigerated fresh pork sausage during an 80-day shelf life study. Appl. Environ. Microbiol. 2014, 80, 5178–5194. [Google Scholar] [CrossRef] [Green Version]
- Stoops, J.; Ruyters, S.; Busschaert, P.; Spaepen, R.; Verreth, C.; Claes, J.; Lievens, B.; Van Campenhout, L. Bacterial community dynamics during cold storage of minced meat packaged under modified atmosphere and supplemented with different preservatives. Food Microbiol. 2015, 48, 192–199. [Google Scholar] [CrossRef]
- Fontana, C.; Bassi, D.; López, C.; Pisacane, V.; Otero, M.C.; Puglisi, E.; Rebecchi, A.; Cocconcelli, P.S.; Vignolo, G. Microbial ecology involved in the ripening of naturally fermented llama meat sausages. A focus on lactobacilli diversity. Int. J. Food Microbiol. 2016, 236, 17–25. [Google Scholar] [CrossRef]
- Fougy, L.; Desmonts, M.-H.; Coeuret, G.; Fassel, C.; Hamon, E.; Hézard, B.; Champomier-Vergès, M.-C.; Chaillou, S. Reducing salt in raw pork sausages increases spoilage and correlates with reduced bacterial diversity. Appl. Environ. Microbiol. 2016, 82, 3928–3939. [Google Scholar] [CrossRef] [Green Version]
- Mann, E.; Wetzels, S.U.; Pinior, B.; Metzler-Zebeli, B.U.; Wagner, M.; Schmitz-Esser, S. Psychrophile spoilers dominate the bacterial microbiome in musculature samples of slaughter pigs. Meat Sci. 2016, 117, 36–40. [Google Scholar] [CrossRef]
- Stellato, G.; La Storia, A.; Filippis, F.D.; Borriello, G.; Villani, F.; Ercolini, D. Overlap of spoilage microbiota between meat and meat processing environment in small-scale vs large-scale retail distribution. Appl. Environ. Microbiol. 2016, 82, 4045–4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Säde, E.; Penttinen, K.; Björkroth, J.; Hultman, J. Exploring lot-to-lot variation in spoilage bacterial communities on commercial modified atmosphere packaged beef. Food Microbiol. 2017, 62, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondi, S.; Nappi, M.R.; Sirangelo, T.M.; Leonardi, A.; Amaretti, A.; Ulrici, A.; Magnani, R.; Montanari, C.; Tabanelli, G.; Gardini, F.; et al. Bacterial community of industrial raw sausage packaged in modified atmosphere throughout the shelf life. Int. J. Food Microbiol. 2018, 280, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Rouger, A.; Moriceau, N.; Prévost, H.; Remenant, B.; Zagorec, M. Diversity of bacterial communities in French chicken cuts stored under modified atmosphere packaging. Food Microbiol. 2018, 70, 7–16. [Google Scholar] [CrossRef]
- Vermeiren, L.; Devlieghere, F.; Debevere, J. Evaluation of meat born lactic acid bacteria as protective cultures for the biopreservation of cooked meat products. Int. J. Food Microbiol. 2004, 96, 149–164. [Google Scholar] [CrossRef]
- Ercolini, D.; Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Ferranti, P.; Mauriello, G.; Villani, F. Different molecular types of Pseudomonas fragi have the same overall behaviour as meat spoilers. Int. J. Food Microbiol. 2010, 142, 120–131. [Google Scholar] [CrossRef]
- Ellouze, M.; Augustin, J.C. Applicability of biological time temperature integrators as quality and safety indicators for meat products. Int. J. Food Microbiol. 2010, 138, 119–129. [Google Scholar] [CrossRef]
- Vaikousi, H.; Biliaderis, C.G.; Koutsoumanis, K.P. Applicability of a microbial Time Temperature Indicator (TTI) for monitoring spoilage of modified atmosphere packed minced meat. Int. J. Food Microbiol. 2009, 133, 272–278. [Google Scholar] [CrossRef]
- Wang, G.-y.; Wang, H.-h.; Han, Y.-w.; Xing, T.; Ye, K.-p.; Xu, X.-L.; Zhou, G.-H. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. 2017, 63, 139–146. [Google Scholar] [CrossRef]
- Nowak, A.; Kalemba, D.; Krala, L.; Piotrowska, M.; Czyzowska, A. The effects of thyme (Thymus vulgaris) and rosemary (Rosmarinus officinalis) essential oils on Brochothrix thermosphacta and on the shelf life of beef packaged in high-oxygen modified atmosphere. Food Microbiol. 2012, 32, 212–216. [Google Scholar] [CrossRef]
- Ntzimani, A.G.; Giatrakou, V.I.; Savvaidis, I.N. Combined natural antimicrobial treatments (EDTA, lysozyme, rosemary and oregano oil) on semi cooked coated chicken meat stored in vacuum packages at 4 °C: Microbiological and sensory evaluation. Innov. Food Sci. Emerg. Technol. 2010, 11, 187–196. [Google Scholar] [CrossRef]
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Effect of preformed konjac gels, with and without olive oil, on the technological attributes and storage stability of merguez sausage. Meat Sci. 2013, 93, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Vasilatos, G.C.; Savvaidis, I.N. Chitosan or rosemary oil treatments, singly or combined to increase turkey meat shelf-life. Int. J. Food Microbiol. 2013, 166, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, J.S.; Martens, M.; Turkki, P. Analysis of sensory quality changes during storage of a modified atmosphere packaged meat product (pizza topping) by an electronic nose system. Lebensm-Wiss. Technol. 2007, 40, 1083–1094. [Google Scholar] [CrossRef]
- del Río, E.; Panizo-Morán, M.; Prieto, M.; Alonso-Calleja, C.; Capita, R. Effect of various chemical decontamination treatments on natural microflora and sensory characteristics of poultry. Int. J. Food Microbiol. 2007, 115, 268–280. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Gómez, M. Shelf life of fresh foal meat under MAP, overwrap and vacuum packaging conditions. Meat Sci. 2012, 92, 610–618. [Google Scholar] [CrossRef]
- Luño, M.; Beltrán, J.A.; Roncalés, P. Shelf-life extension and colour stabilisation of beef packaged in a low O2 atmosphere containing CO: Loin steaks and ground meat. Meat Sci. 1998, 48, 75–84. [Google Scholar] [CrossRef]
- Muermans, M.L.T.; Stekelenburg, F.K.; Zwietering, M.H.; Huis in ‘t Veld, J.H.J. Modelling of the microbiological quality of meat. Food Control 1993, 4, 216–221. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Bañón, S. Antioxidant and antimicrobial effects of dietary supplementation with rosemary diterpenes (carnosic acid and carnosol) vs vitamin E on lamb meat packed under protective atmosphere. Meat Sci. 2015, 110, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; O’Grady, M.N.; Kerry, J.P. Effect of varying the gas headspace to meat ratio on the quality and shelf-life of beef steaks packaged in high oxygen modified atmosphere packs. Meat Sci. 2013, 94, 447–454. [Google Scholar] [CrossRef]
- Ahnström, M.L.; Seyfert, M.; Hunt, M.C.; Johnson, D.E. Dry aging of beef in a bag highly permeable to water vapour. Meat Sci. 2006, 73, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Torrieri, E.; Russo, F.; Di Monaco, R.; Cavella, S.; Villani, F.; Masi, F. Shelf life prediction of fresh Italian pork sausage modified atmosphere packed. Food Sci. Technol. Int. 2011, 17, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.G.; Moorhead, S.M.; Broda, D.M. Influence of heat shrink treatments on the onset of clostridial “blown pack” spoilage of vacuum packed chilled meat. Food Res. Int. 2001, 34, 271–275. [Google Scholar] [CrossRef]
- Broda, D.M.; Delacy, K.M.; Bell, R.G.; Braggins, T.J.; Cook, R.L. Psychrotrophic Clostridium spp. associated with ‘blown pack’ spoilage of chilled vacuum-packed red meats and dog rolls in gas-impermeable plastic casings. Int. J. Food Microbiol. 1996, 29, 335–352. [Google Scholar] [CrossRef]
- Tsakanikas, P.; Pavlidis, D.; Panagou, E.; Nychas, G.-J. Exploiting multispectral imaging for non-invasive contamination assessment and mapping of meat samples. Talanta 2016, 161, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Argyri, A.A.; Mallouchos, A.; Panagou, E.Z.; Nychas, G.-J.E. The dynamics of the HS/SPME–GC/MS as a tool to assess the spoilage of minced beef stored under different packaging and temperature conditions. Int. J. Food Microbiol. 2015, 193, 51–58. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Holm, E.S.; Schäfer, A.; Koch, A.G.; Petersen, M.A. Investigation of spoilage in saveloy samples inoculated with four potential spoilage bacteria. Meat Sci. 2013, 93, 687–695. [Google Scholar] [CrossRef]
- Blixt, Y.; Borch, E. Using an electronic nose for determining the spoilage of vacuum-packaged beef. Int. J. Food Microbiol. 1999, 46, 123–134. [Google Scholar] [CrossRef]
- Papadopoulou, O.S.; Tassou, C.C.; Schiavo, L.; Nychas, G.-J.E.; Panagou, E.Z. Rapid Assessment of Meat Quality by Means of an Electronic Nose and Support Vector Machines. Procedia Food Sci. 2011, 1, 2003–2006. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Tian, L.; Zhao, G.; Zhang, Q.; Gao, X.; Huang, X.; Sun, L. Formation of biogenic amines and growth of spoilage-related microorganisms in pork stored under different packaging conditions applying PCA. Meat Sci. 2014, 96, 843–848. [Google Scholar] [CrossRef]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Chapter 17 - Spoilage bacteria and meat quality. In Meat Quality Analysis; Biswas, A.K., Mandal, P.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 307–334. [Google Scholar] [CrossRef]
- Balamatsia, C.C.; Patsias, A.; Kontominas, M.G.; Savvaidis, I.N. Possible role of volatile amines as quality-indicating metabolites in modified atmosphere-packaged chicken fillets: Correlation with microbiological and sensory attributes. Food Chem. 2007, 104, 1622–1628. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Riel, G.; Boulaaba, A.; Popp, J.; Klein, G. Effects of parsley extract powder as an alternative for the direct addition of sodium nitrite in the production of mortadella-type sausages—Impact on microbiological, physicochemical and sensory aspects. Meat Sci. 2017, 131, 166–175. [Google Scholar] [CrossRef]
- Rodrigues, J.B.M.; Sarantópoulos, C.I.G.L.; Bromberg, R.; Andrade, J.C.; Brunelli, K.; Miyagusku, L.; Marquezini, M.G.; Yamada, E.A. Evaluation of the effectiveness of non-irradiated and chlorine-free packaging for fresh beef preservation. Meat Sci. 2017, 125, 30–36. [Google Scholar] [CrossRef]
- Ercolini, D.; Russo, F.; Blaiotta, G.; Pepe, O.; Mauriello, G.; Villani, F. Simultaneous detection of Pseudomonas fragi, P. lundensis, and P. putida from meat by use of a multiplex PCR assay targeting the carA gene. Appl. Environ. Microbiol. 2007, 73, 2354–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, M.; Bertelsen, G. The use of CO2 in packaging of fresh red meats and its effect on chemical quality changes in the meat: A review. J. Muscle Foods 2002, 13, 143–168. [Google Scholar] [CrossRef]
- Tremonte, P.; Sorrentino, E.; Succi, M.; Reale, A.; Maiorano, G.; Coppola, R. Shelf life offresh sausages stored under modified atmospheres. J. Food Prot. 2005, 68, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- McMillin, K.W. Where is MAP Going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 2008, 80, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Skandamis, P.N.; Nychas, G.-J.E. Preservation of fresh meat with active and modified atmosphere packaging conditions. Int. J. Food Microbiol. 2002, 79, 35–45. [Google Scholar] [CrossRef]
- Sirocchi, V.; Devlieghere, F.; Peelman, N.; Sagratini, G.; Maggi, F.; Vittori, S.; Ragaert, P. Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chem. 2017, 221, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Ammor, M.S.; Argyri, A.A.; Nychas, G.-J.E. Rapid monitoring of the spoilage of minced beef stored under conventionally and active packaging conditions using Fourier transform infrared spectroscopy in tandem with chemometrics. Meat Sci. 2009, 81, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Doulgeraki, A.I.; Paramithiotis, S.; Kagkli, D.M.; Nychas, G.-J.E. Lactic acid bacteria population dynamics during minced beef storage under aerobic or modified atmosphere packaging conditions. Food Microbiol. 2010, 27, 1028–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argyri, A.A.; Doulgeraki, A.I.; Blana, V.A.; Panagou, E.Z.; Nychas, G.-J.E. Potential of a simple HPLC-based approach for the identification of the spoilage status of minced beef stored at various temperatures and packaging systems. Int. J. Food Microbiol. 2011, 150, 25–33. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Paramithiotis, S.; Nychas, G.-J.E. Characterization of the Enterobacteriaceae community that developed during storage of minced beef under aerobic or modified atmosphere packaging conditions. Int. J. Food Microbiol. 2011, 145, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Doulgeraki, A.I.; Nychas, G.-J.E. Monitoring the succession of the biota grown on a selective medium for pseudomonads during storage of minced beef with molecular-based methods. Food Microbiol. 2013, 34, 62–69. [Google Scholar] [CrossRef]
- Blana, V.A.; Nychas, G.-J.E. Presence of quorum sensing signal molecules in minced beef stored under various temperature and packaging conditions. Int. J. Food Microbiol. 2014, 173, 1–8. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Cayré, M.E.; Garro, O.; Vignolo, G.; Cayre, M.E.; Garro, O.; Vignolo, G.; Cayré, M.E.; Garro, O.; Vignolo, G.; Cayre, M.E.; et al. Effect of storage temperature and gas permeability of packaging film on the growth of lactic acid bacteria and Brochothrix thermosphacta in cooked meat emulsions. Food Microbiol. 2005, 22, 505–512. [Google Scholar] [CrossRef]
- Ferrentino, G.; Balzan, S.; Spilimbergo, S. Optimization of supercritical carbon dioxide treatment for the inactivation of the natural microbial flora in cubed cooked ham. Int. J. Food Microbiol. 2013, 161, 189–196. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Antimicrobial packaging of chicken fillets based on the release of carvacrol from chitosan/cyclodextrin films. Int. J. Food Microbiol. 2014, 188, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hasapidou, A.; Savvaidis, I.N. The effects of modified atmosphere packaging, EDTA and oregano oil on the quality of chicken liver meat. Food Res. Int. 2011, 44, 2751–2756. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Devlieghere, F.; Sampers, I. Combined use of cinnamon essential oil and MAP/vacuum packaging to increase the microbial and sensorial shelf life of lean pork and salmon. Food Packag. Shelf Life 2017, 12, 51–58. [Google Scholar] [CrossRef]
- Georgantelis, D.; Ambrosiadis, I.; Katikou, P.; Blekas, G.; Georgakis, S.A. Effect of rosemary extract, chitosan and alpha-tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4 °C. Meat Sci. 2007, 76, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Baldin, J.C.; Michelin, E.C.; Polizer, Y.J.; Rodrigues, I.; de Godoy, S.H.S.; Fregonesi, R.P.; Pires, M.A.; Carvalho, L.T.; Fávaro-Trindade, C.S.; de Lima, C.G.; et al. Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Sci. 2016, 118, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Coutinho de Oliveira, T.L.; Malfitano de Carvalho, S.; de Araújo Soares, R.; Andrade, M.A.; Cardoso, M.d.G.; Ramos, E.M.; Piccoli, R.H. Antioxidant effects of Satureja montana L. essential oil on TBARS and color of mortadella-type sausages formulated with different levels of sodium nitrite. Lebensm-Wiss. Technol. 2012, 45, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Vihavainen, E.J.; Björkroth, J. Spoilage of value-added, high-oxygen modified-atmosphere packaged raw beef steaks by Leuconostoc gasicomitatum and Leuconostoc gelidum. Int. J. Food Microbiol. 2007, 119, 340–345. [Google Scholar] [CrossRef]
- Serrano, R.; Bañón, S. Reducing SO2 in fresh pork burgers by adding chitosan. Meat Sci. 2012, 92, 651–658. [Google Scholar] [CrossRef]
- Sadler, D.N.; Swan, J.E. Effect of NaCl, polydextrose, and storage conditions on the functional characteristics and microbial quality of pre- and post-rigor salted beef. Meat Sci. 1997, 46, 329–338. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Interactive inhibition of meat spoilage and pathogenic bacteria by lysozyme, nisin and EDTA in the presence of nitrite and sodium chloride at 24 [deg]C. Int. J. Food Microbiol. 2003, 80, 251–259. [Google Scholar] [CrossRef]
- Samapundo, S.; Ampofo-Asiama, J.; Anthierens, T.; Xhaferi, R.; Van Bree, I.; Szczepaniak, S.; Goemaere, O.; Steen, L.; Dhooge, M.; Paelinck, H.; et al. Influence of NaCl reduction and replacement on the growth of Lactobacillus sakei in broth, cooked ham and white sauce. Int. J. Food Microbiol. 2010, 143, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, S.A. The growth of Aeromonas hydrophila K144 in ground pork at 5 °C. Int. J. Food Microbiol. 1988, 7, 41–48. [Google Scholar] [CrossRef]
- Crist, C.A.; Williams, J.B.; Schilling, M.W.; Hood, A.F.; Smith, B.S.; Campano, S.G. Impact of sodium lactate and vinegar derivatives on the quality of fresh Italian pork sausage links. Meat Sci. 2014, 96, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, B.C.; Langsrud, S. A dissolving CO2 headspace combined with organic acids prolongs the shelf-life of fresh pork. Meat Sci. 2010, 85, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Bradford, D.D.; Huffman, D.L.; Egbert, W.R.; Jones, W.R. Low fat fresh pork sausage patty stability in refrigerated storage with potassium lactate. J. Food Sci. 1993, 58, 488–491. [Google Scholar] [CrossRef]
- Guillou, S.; Lerasle, M.; Simonin, H.; Anthoine, V.; Chéret, R.; Federighi, M.; Membré, J.M. Multi-criteria framework as an innovative tradeoff approach to determine the shelf-life of high pressure-treated poultry. Int. J. Food Microbiol. 2016, 233, 60–72. [Google Scholar] [CrossRef]
- Devlieghere, F.; Geeraerd, A.H.; Versyck, K.J.; Bernaert, H.; Van Impe, J.F.; Debevere, J. Shelf life of modified atmosphere packed cooked meat products: Addition of Na-lactate as a fourth shelf life determinative factor in a model and product validation. Int. J. Food Microbiol. 2000, 58, 93–106. [Google Scholar] [CrossRef]
- Deumier, F.; Collignan, A. The effects of sodium lactate and starter cultures on pH, lactic acid bacteria, Listeria monocytogenes and Salmonella spp. levels in pure chicken dry fermented sausage. Meat Sci. 2003, 65, 1165–1174. [Google Scholar] [CrossRef]
- Friedrich, L.; Siró, I.; Dalmadi, I.; Horváth, K.; Ágoston, R.; Balla, C. Influence of various preservatives on the quality of minced beef under modified atmosphere at chilled storage. Meat Sci. 2008, 79, 332–343. [Google Scholar] [CrossRef]
- Magrinya, N.; Terjung, N.; Loeffler, M.; Gibis, M.; Bou, R.; Weiss, J. Influence of fat addition on the antimicrobial activity of sodium lactate, lauric arginate and methylparaben in minced meat. Int. J. Food Microbiol. 2015, 215, 86–94. [Google Scholar] [CrossRef]
- Lambropoulou, K.A.; Drosinos, E.H.; Nychas, G.J.E. The effect of glucose supplementation on the spoilage microflora and chemical composition of minced beef stored aerobically or under a modified atmosphere at 4 °C. Int. J. Food Microbiol. 1996, 30, 281–291. [Google Scholar] [CrossRef]
- Lerasle, M.; Federighi, M.; Simonin, H.; Anthoine, V.; Rezé, S.; Chéret, R.; Guillou, S. Combined use of modified atmosphere packaging and high pressure to extend the shelf-life of raw poultry sausage. Innov. Food Sci. Emerg. Technol. 2014, 23, 54–60. [Google Scholar] [CrossRef]
- Masana, M.O.; Barrio, Y.X.; Palladino, P.M.; Sancho, A.M.; Vaudagna, S.R. High pressure treatments combined with sodium lactate to inactivate Escherichia coli O157:H7 and spoilage microbiota in cured beef carpaccio. Food Microbiol. 2015, 46, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.F.; McKay, A.M.; Connolly, M.; Linton, M. Effect of high pressure on the microbiological quality of cooked chicken during storage at normal and abuse refrigeration temperatures. Food Microbiol. 2010, 27, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Ferrocino, I.; Cavallero, M.C.; Riva, S.; Giordano, M.; Cocolin, L. Potentially active spoilage bacteria community during the storage of vacuum packaged beefsteaks treated with aqueous ozone and electrolyzed water. Int. J. Food Microbiol. 2018, 266, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, J.; Andrássy, É. Interaction of ionising radiation and acidulants on the growth of the microflora of a vacuum-packaged chilled meat product. Int. J. Food Microbiol. 1993, 19, 145–152. [Google Scholar] [CrossRef]
- Grandison, A.S.; Jennings, A. Extension of the shelf life of fresh minced chicken meat by electron beam irradiation combined with modified atmosphere packaging. Food Control 1993, 4, 83–88. [Google Scholar] [CrossRef]
- Gamage, S.D.; Faith, N.G.; Luchansky, J.B.; Buege, D.R.; Ingham, S.C. Inhibition of microbial growth in chub-packed ground beef by refrigeration (2 °C) and medium-dose (2.2 to 2.4 kGy) irradiation. Int. J. Food Microbiol. 1997, 37, 175–182. [Google Scholar] [CrossRef]
- Kamat, A.S.; Khare, S.; Doctor, T.; Nair, P.M. Control of Yersinia enterocolitica in raw pork and pork products by γ-irradiation. Int. J. Food Microbiol. 1997, 36, 69–76. [Google Scholar] [CrossRef]
- Sørheim, O.; Nissen, H.; Nesbakken, T. The storage life of beef and pork packaged in an atmosphere with low carbon monoxide and high carbon dioxide. Meat Sci. 1999, 52, 157–164. [Google Scholar] [CrossRef]
- Lacroix, M.; Smoragiewicz, W.; Jobin, M.; Latreille, B.; Krzystyniak, K. Protein quality and microbiological changes in aerobically- or vacuum-packaged, irradiated fresh pork loins. Meat Sci. 2000, 56, 31–39. [Google Scholar] [CrossRef]
- Lacroix, M.L.; Smoragiewicz, W.; Jobin, M.; Latreille, B.; Krzystyniak, K. The effect of irradiation of fresh pork loins on the protein quality and microbiological changes in aerobically—or vacuum-packaged. Radiat. Phys. Chem. 2002, 63, 317–322. [Google Scholar] [CrossRef]
- Nortjé, K.; Buys, E.M.; Minnaar, A. Effect of γ-irradiation on the sensory quality of moist beef biltong. Meat Sci. 2005, 71, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Min, J.-S.; Lee, S.-O.; Jang, A.; Jo, C.; Lee, M. Irradiation and organic acid treatment for microbial control and the production of biogenic amines in beef and pork. Food Chem. 2007, 104, 791–799. [Google Scholar] [CrossRef]
- Xavier, M.d.l.P.; Dauber, C.; Mussio, P.; Delgado, E.; Maquieira, A.; Soria, A.; Curuchet, A.; Márquez, R.; Méndez, C.; López, T. Use of mild irradiation doses to control pathogenic bacteria on meat trimmings for production of patties aiming at provoking minimal changes in quality attributes. Meat Sci. 2014, 98, 383–391. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, H.; Hinton, A.; Zhang, J. Influence of in-package cold plasma treatment on microbiological shelf life and appearance of fresh chicken breast fillets. Food Microbiol. 2016, 60, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Narasimha Rao, D.; Sreenivasamurthy, V. A preliminary study of the effect of urea in the preservation of meat. Meat Sci. 1986, 17, 251–265. [Google Scholar] [CrossRef]
- Zeitoun, A.A.M.; Debevere, J.M. The effect of treatment with buffered lactic acid on microbial decontamination and on shelf life of poultry. Int. J. Food Microbiol. 1990, 11, 305–311. [Google Scholar] [CrossRef]
- Zeitoun, A.A.M.; Debevere, J.M. Inhibition, survival and growth of Listeria monocytogenes on poultry as influenced by buffered lactic acid treatment and modified atmosphere packaging. Int. J. Food Microbiol. 1991, 14, 161–169. [Google Scholar] [CrossRef]
- Greer, G.G.; Dilts, B.D. Lactic acid inhibition of the growth of spoilage bacteria and cold tolerant pathogens on pork. Int. J. Food Microbiol. 1995, 25, 141–151. [Google Scholar] [CrossRef]
- Guerrero, I.; Mendiolea, R.; Ponce, E.; Prado, A. Inoculation of lactic acid bacteria on meat surfaces as a means of decontamination in semitropical conditions. Meat Sci. 1995, 40, 397–411. [Google Scholar] [CrossRef]
- Djenane, D.; Sánchez-Escalante, A.; Beltrán, J.A.; Roncalés, P. The shelf-life of beef steaks treated with dl-lactic acid and antioxidants and stored under modified atmospheres. Food Microbiol. 2003, 20, 1–7. [Google Scholar] [CrossRef]
- Nattress, F.M.; Baker, L.P. Effects of treatment with lysozyme and nisin on the microflora and sensory properties of commercial pork. Int. J. Food Microbiol. 2003, 85, 259–267. [Google Scholar] [CrossRef]
- Economou, T.; Pournis, N.; Ntzimani, A.; Savvaidis, I.N. Nisin–EDTA treatments and modified atmosphere packaging to increase fresh chicken meat shelf-life. Food Chem. 2009, 114, 1470–1476. [Google Scholar] [CrossRef]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Latou, E.; Mexis, S.F.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Combined effect of chitosan and modified atmosphere packaging for shelf life extension of chicken breast fillets. Lebensm-Wiss. Technol. 2014, 55, 263–268. [Google Scholar] [CrossRef]
- Pavelková, A.; Kačániová, M.; Horská, E.; Rovná, K.; Hleba, L.; Petrová, J. The effect of vacuum packaging, EDTA, oregano and thyme oils on the microbiological quality of chicken’s breast. Anaerobe 2014, 29, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Paparella, A.; Mazzarrino, G.; Chaves-López, C.; Rossi, C.; Sacchetti, G.; Guerrieri, O.; Serio, A. Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016, 59, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, W.; Pothakos, V.; De Vuyst, L.; Leroy, F. Diversity of the dominant bacterial species on sliced cooked pork products at expiration date in the Belgian retail. Food Microbiol. 2017, 65, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Saucier, L. Microbial spoilage, quality and safety within the context of meat sustainability. Meat Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hauge, S.J.; Wahlgren, M.; Røtterud, O.-J.; Nesbakken, T. Hot water surface pasteurisation of lamb carcasses: Microbial effects and cost-benefit considerations. Int. J. Food Microbiol. 2011, 146, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Noonpakdee, W.; Santivarangkna, C.; Jumriangrit, P.; Sonomoto, K.; Panyim, S. Isolation of nisin-producing Lactococcus lactis WNC 20 strain from nham, a traditional Thai fermented sausage. Int. J. Food Microbiol. 2003, 81, 137–145. [Google Scholar] [CrossRef]
- Papamanoli, E.; Tzanetakis, N.; Litopoulou-Tzanetaki, E.; Kotzekidou, P. Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 2003, 65, 859–867. [Google Scholar] [CrossRef]
- Vermeiren, L.; Devlieghere, F.; Vandekinderen, I.; Debevere, J. The interaction of the non-bacteriocinogenic Lactobacillus sakei 10A and lactocin S producing Lactobacillus sakei 148 towards Listeria monocytogenes on a model cooked ham. Food Microbiol. 2006, 23, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulos, C.; De Mey, E.; Dewulf, L.; Paelinck, H.; De Smedt, A.; Vandendriessche, F.; De Vuyst, L.; Leroy, F. Interactions between bacterial isolates from modified-atmosphere-packaged artisan-type cooked ham in view of the development of a bioprotective culture. Food Microbiol. 2010, 27, 1086–1094. [Google Scholar] [CrossRef]
- Kalschne, D.L.; Geitenes, S.; Veit, M.R.; Sarmento, C.M.P.; Colla, E. Growth inhibition of lactic acid bacteria in ham by nisin: A model approach. Meat Sci. 2014, 98, 744–752. [Google Scholar] [CrossRef]
- Rubio, R.; Martín, B.; Aymerich, M.T.; Garriga, M. The potential probiotic Lactobacillus rhamnosus CTC1679 survives the passage through the gastrointestinal tract and its use as starter culture results in safe nutritionally enhanced fermented sausages. Int. J. Food Microbiol. 2014, 186, 55–60. [Google Scholar] [CrossRef]
- Osés, S.M.; Diez, A.M.; Gómez, E.M.; Wilches-Pérez, D.; Luning, P.A.; Jaime, I.; Rovira, J. Control of Escherichia coli and Listeria monocytogenes in suckling-lamb meat evaluated using microbial challenge tests. Meat Sci. 2015, 110, 262–269. [Google Scholar] [CrossRef]
- da Silva Sabo, S.; Pérez-Rodríguez, N.; Domínguez, J.M.; de Souza Oliveira, R.P. Inhibitory substances production by Lactobacillus plantarum ST16Pa cultured in hydrolyzed cheese whey supplemented with soybean flour and their antimicrobial efficiency as biopreservatives on fresh chicken meat. Food Res. Int. 2017, 99, 762–769. [Google Scholar] [CrossRef]
- Ramaroson, M.; Guillou, S.; Rossero, A.; Rezé, S.; Anthoine, V.; Moriceau, N.; Martin, J.-L.; Duranton, F.; Zagorec, M. Selection procedure of bioprotective cultures for their combined use with High Pressure Processing to control spore-forming bacteria in cooked ham. Int. J. Food Microbiol. 2018, 276, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, P.; Korkeala, H. Lactobacillus contamination of cooked ring sausages at sausage processing plants. Int. J. Food Microbiol. 1987, 5, 323–330. [Google Scholar] [CrossRef]
- Gill, C.O.; Bryant, J. The contamination of pork with spoilage bacteria during commercial dressing, chilling and cutting of pig carcasses. Int. J. Food Microbiol. 1992, 16, 51–62. [Google Scholar] [CrossRef]
- Gustavsson, P.; Borch, E. Contamination of beef carcasses by psychrotrophic Pseudomonas and Enterobacteriaceae at different stages along the processing line. Int. J. Food Microbiol. 1993, 20, 67–83. [Google Scholar] [CrossRef]
- Nerbrink, E.; Borch, E. Evaluation of bacterial contamination at separate processing stages in emulsion sausage production. Int. J. Food Microbiol. 1993, 20, 37–44. [Google Scholar] [CrossRef]
- Holley, R.A.; Doyon, G.; Fortin, J.; Rodrigue, N.; Carbonneau, M. Post-process, packaging-induced fermentation of delicatessen meats. Food Res. Int. 1996, 29, 35–48. [Google Scholar] [CrossRef]
- Greer, G.G.; Jones, S.D.M. Quality and bacteriological consequences of beef carcass spray-chilling: Effects of spray duration and boxed beef storage temperature. Meat Sci. 1997, 45, 61–73. [Google Scholar] [CrossRef]
- Voidarou, C.; Vassos, D.; Rozos, G.; Alexopoulos, A.; Plessas, S.; Tsinas, A.; Skoufou, M.; Stavropoulou, E.; Bezirtzoglou, E. Microbial challenges of poultry meat production. Anaerobe 2011, 17, 341–343. [Google Scholar] [CrossRef]
- Bañón, S.; Méndez, L.; Almela, E. Effects of dietary rosemary extract on lamb spoilage under retail display conditions. Meat Sci. 2012, 90, 579–583. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Jordán, M.J.; Bañón, S. Shelf life of meat from lambs given essential oil-free rosemary extract containing carnosic acid plus carnosol at 200 or 400 mgkg−1. Meat Sci. 2014, 96, 1452–1459. [Google Scholar] [CrossRef]
- Serrano, R.; Jordán, M.J.; Bañón, S. Use of dietary rosemary extract in ewe and lamb to extend the shelf life of raw and cooked meat. Small Rumin. Res. 2014, 116, 144–152. [Google Scholar] [CrossRef]
- Chulayo, A.Y.; Bradley, G.; Muchenje, V. Effects of transport distance, lairage time and stunning efficiency on cortisol, glucose, HSPA1A and how they relate with meat quality in cattle. Meat Sci. 2016, 117, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Leticia, M.; Paola, D.; Jordi, O.; Julio, O.; Sancho, B. Effects of sage distillation by-product (Salvia lavandulifolia Vahl.) dietary supplementation in light lambs fed on concentrates on meat shelf life and fatty acid composition. Meat Sci. 2017, 134, 44–53. [Google Scholar] [CrossRef]
- Balamatsia, C.C.; Paleologos, E.K.; Kontominas, M.G.; Savvaidis, I.N. Correlation between microbial flora, sensory changes and biogenic amines formation in fresh chicken meat stored aerobically or under modified atmosphere packaging at 4 degrees C: Possible role of biogenic amines as spoilage indicators. Antonie Leeuwenhoek 2006, 89, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Álvarez-González, T.; Alonso-Calleja, C. Effect of several packaging conditions on the microbiological, physicochemical and sensory properties of ostrich steaks during refrigerated storage. Food Microbiol. 2017, 72, 146–156. [Google Scholar] [CrossRef]
- Chouliara, E.; Karatapanis, A.; Savvaidis, I.N.N.; Kontominas, M.G.G. Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4 degrees C. Food Microbiol. 2007, 24, 607–617. [Google Scholar] [CrossRef]
- Herbert, U.; Rossaint, S.; Khanna, M.A.M.A.; Kreyenschmidt, J. Comparison of argon-based and nitrogen-based modified atmosphere packaging on bacterial growth and product quality of chicken breast fillets. Poult. Sci. 2013, 92, 1348–1356. [Google Scholar] [CrossRef]
- Jääskeläinen, E.; Johansson, P.; Kostiainen, O.; Nieminen, T.T.; Schmidt, G.; Somervuo, P.; Mohsina, M.; Vanninen, P.; Auvinen, P.; Björkroth, J. Significance of heme-based respiration in meat spoilage caused by Leuconostoc gasicomitatum. Appl. Environ. Microbiol. 2013, 79, 1078–1085. [Google Scholar] [CrossRef] [Green Version]
- Jääskeläinen, E.; Hultman, J.; Parshintsev, J.; Riekkola, M.-L.; Björkroth, J.; Jaaskelainen, E.; Hultman, J.; Parshintsev, J.; Riekkola, M.-L.; Bjorkroth, J.; et al. Development of spoilage bacterial community and volatile compounds in chilled beef under vacuum or high oxygen atmospheres. Int. J. Food Microbiol. 2016, 223, 25–32. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Agathaggelou, E.I.; Skandamis, P.N. Storage of pork meat under modified atmospheres containing vapors from commercial alcoholic beverages. Int. J. Food Microbiol. 2014, 178, 65–75. [Google Scholar] [CrossRef]
- Liu, F.; Yang, R.Q.; Li, Y.F. Correlations between growth parameters of spoilage micro-organisms and shelf-life of pork stored under air and modified atmosphere at −2, 4 and 10 °C. Food Microbiol. 2006, 23, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Djenane, D.; Cilla, I.; Beltran, J.A.; Roncalés, P. Effect of varying oxygen concentrations on the shelf-life of fresh pork sausages packaged in modified atmosphere. Food Chem. 2006, 94, 219–225. [Google Scholar] [CrossRef]
- Miks-Krajnik, M.; Yoon, Y.J.; Ukuku, D.O.; Yuk, H.G. Identification and quantification of volatile chemical spoilage indexes associated with bacterial growth dynamics in aerobically stored chicken. J. Food Sci. 2016, 81, M2006–M2014. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, T.T.; Dalgaard, P.; Björkroth, J. Volatile organic compounds and Photobacterium phosphoreum associated with spoilage of modified-atmosphere-packaged raw pork. Int. J. Food Microbiol. 2016, 218, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Rahkila, R.; Nieminen, T.T.; Johansson, P.; Säde, E.; Björkroth, J. Characterization and evaluation of the spoilage potential of Lactococcus piscium isolates from modified atmosphere packaged meat. Int. J. Food Microbiol. 2012, 156, 50–59. [Google Scholar] [CrossRef]
- Rossaint, S.; Klausmann, S.; Kreyenschmidt, J. Effect of high-oxygen and oxygen-free modified atmosphere packaging on the spoilage process of poultry breast fillets. Poult. Sci. 2015, 94, 96–103. [Google Scholar] [CrossRef]
- Pin, C.; García de Fernando, G.D.; Ordóñez, J.A. Effect of modified atmosphere composition on the metabolism of glucose by Brochothrix thermosphacta. Appl. Environ. Microbiol. 2002, 68, 4441–4447. [Google Scholar] [CrossRef] [Green Version]
- Senter, S.D.; Arnold, J.W.; Chew, V. APC values and volatile compounds formed in commercially processed, raw chicken parts during storage at 4 and 13 °C and under simulated temperature abuse conditions. J. Sci. Food Agric. 2000, 80, 1559–1564. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Han, Y.Q.; Cao, J.X.; Xu, X.-L.; Zhou, G.-H.; Zhang, W.Y. The spoilage of air-packaged broiler meat during storage at normal and fluctuating storage temperatures. Poult. Sci. 2012, 91, 208–214. [Google Scholar] [CrossRef]
- Grech, V.; Calleja, N. WASP (Write a Scientific Paper): Parametric vs. non-parametric tests. Early Hum. Dev. 2018, 123, 48–49. [Google Scholar] [CrossRef]
- Gill, J. The insignificance of null hypothesis significance testing. Polit. Res. Quart. 1999, 52, 647–674. [Google Scholar] [CrossRef]
- Gliner, J.A.; Morgan, G.A.; Leech, N.L.; Harmon, R.J. Problems with null hypothesis significance testing. J. Experim. Educ. 2002, 71, 83–92. [Google Scholar] [CrossRef]





| Formulations | Meat Products | Observed Effects | References |
|---|---|---|---|
| Oregano essential oil | Chicken liver meat | Maintenance of freshness and sensorial quality, limitation of lipid oxidation | [81] |
| Cinnamon essential oil | Pork meat | Increase of microbial shelf life but unacceptable discoloration in high-oxygen atmosphere | [82] |
| Rosemary extract or oil | Fresh pork sausages | In combination with chitosan, extension of shelf-life of meat products; inhibition of lipid oxidation and rancidity | [83] |
| Parsley extract | Mortadella-type sausages | Inhibition of L. monocytogenes; improvement of overall appearance (color, cohesiveness, taste, aroma and saltiness) | [63] |
| Microencapsulated jabuticaba extract (MJE) | Fresh sausages | Natural dye used in replacement of commercial while maintaining antioxidant and antimicrobial activity and sensory acceptance | [84] |
| Satureja montana L. essential oil | Mortadella-type sausages | Antioxidant activity | [85] |
| Mixed spices and marinade (various ingredients and preservatives) | Beef (minced meats or steaks; pork meat | Alteration of microbial counts due to preservative addition with decrease of microbial diversity dominated by L. algidus and Leuconostoc sp. irrespective of the preservative tested. Glucose and packaging under oxygen in favor of spoilage. | [25,86] |
| Chitosan | Pork (loins, burgers, sausages); turkey and chicken breasts | Extension of shelf-life of low-sulphite burgers and turkey fillets, with synergistic effect on low dose sulphite or rosemary oil on spoilage prevention. | [41,87] |
| Sodium nitrite | Minced beef | Combined with essential oil stabilization of red meat color even at low dose; inhibitory effect on bacterial growth, control of lipid oxidation. | [85,88] |
| NaCl | Pork (loins, ground meat, ham, sausages) | Acid and exudate production following salt reduction, combination of MAP and low salt concentration correlated with sulfurous off-odors and higher spoilage than under vacuum, Maintenance of bacterial richness, inhibition of pathogens. Inhibition of growth of Aeromonas hydrophila at 3%. | [27,89,90,91] |
| EDTA | Beefsteaks; chicken (breasts, liver meat) | Combined to oregano essential oil and MAP extended the shelf-life of fresh chicken liver. | [81] |
| Lysozyme | Synthetic media (target products: processed ham and bologna) | B. thermosphacta inhibited by 500 mg/l or less lysozyme. Lysozyme also effective against P. acidilactici, En. faecalis and W. viridescens. | [89] |
| Vinegar, acetic acid | Pork sausages, fresh pork | Bacteriostatic properties of vinegar/sodium lactate mixture with reduced bacterial growth. CO2, citric acid and acetic acid reduced total growth. | [92,93] |
| Lactate | Pork sausages, pork meat, chicken fermented sausages | Lactate-diacetate altered the dynamics dramatically, yielding growth of a single species of Lactobacillus (L. graminis). Psychrotrophic, coliform and lactic acid bacteria retarded by lactate. No effect on sensory properties. Shelf-life extension. Synergistic effect between lactate and carbon dioxide. No or slight effect on color. Reduced effect with increased level of fat. | [24,94,95,96,97,98,99] |
| Polydextrose/glucose supplement | Hot-boned, mixed hindquarter cuts; minced beef | Increase of functionality properties of batters made with pre-rigor salted mince with added Polydextrose@. Similar composition and bacterial numbers in mince supplemented with glucose. Glucose, glucose 6-phosphate and lactic acid consumed at slower rates by the flora under MAP than in air. | [88,100] |
| References | Author, Year | Experimental Conditions in Each Study (N = 93) | Studied Bacterial Indicators (Enumeration) | Spoilage Sensory Indicators | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [Red/White] Meat | Packaging | Replicate (n × r) | Mesophilic Aerobic Counts | LAB | Brochothrix Spp. | Enterobac-teriaceae | Pseudom-onas Spp. | Texture | Color | Odor | Flavor | Exudate/Drip Loss | ||
| [77] | (Alizadeh Sani, 2017) | [R] Lamb—Raw meat | Air | 2 × 1 | ||||||||||
| [153] | (Balamatsia, 2006) | [W] Chicken—Breast fillets | Air; MAP | 2 × 3 | ||||||||||
| [61] | (Balamatsia, 2007) | [W] Chicken—Breast fillets | Air; MAP; VP | 3 × 1 | ||||||||||
| [154] | (Capita, 2017) | [R] Ostrich—Steaks | MAP | 8 × 8 | ||||||||||
| [155] | (Chouliara, 2007) | [W] Chicken—Breast fillets | Air; MAP | 6 × 1 | ||||||||||
| [43] | (del Río, 2007) | [W] Chicken | Air | 3 × 6 | ||||||||||
| [34] | Ercolini, 2010 | [R/W] Beef, Pork, Chicken—Various | Air | 1 × 1 | ||||||||||
| [81] | (Hasapidou, 2011) | [W] Chicken—Liver breasts | Air; MAP | 6 × 1 | ||||||||||
| [156] | (Herbert, 2013) | [W] Chicken—Breast fillets | MAP | 6 × 1 | ||||||||||
| [157] | (Jääskeläinen, 2013) | [R] Pork—Raw meat | MAP | 1 × 1 | ||||||||||
| [158] | (Jääskeläinen, 2016) | [R] Beef—Raw meat | MAP; VP | 2 × 3 | ||||||||||
| [159] | (Kapetanakou, 2014) | [R] Pork—Steaks | MAP | 4 × 2 | ||||||||||
| [160] | (Liu, 2006 #4619) | [R] Pork—Legs | Air; MAP | 6 × 3 | ||||||||||
| [44] | (Lorenzo, 2012) | [R] Foal—Steaks | Air; MAP; VP | 4 × 3 | ||||||||||
| [161] | (Martinez, 2006) | [R] Pork—Forelegs | MAP | 5 × 2 | ||||||||||
| [162] | (Miks-Krajnik, 2016) | [W] Chicken—Breast fillets | Air | 1 × 3 | ||||||||||
| [163] | (Nieminen, 2016) | [R] Pork—Loin | MAP | 4 × 2 | ||||||||||
| [124] | (Petrou, 2012) | [W] Chicken—Breast fillets | MAP | 4 × 1 | ||||||||||
| [164] | (Rahkila, 2012) | [R] Pork—Various | MAP | 3 × 1 | ||||||||||
| [165] | (Rossaint, 2015) | [W] Chicken—Breast fillets | MAP | 2 × 2 | ||||||||||
| [25] | (Stoops, 2015) | [R] Beef—Raw meat | MAP | 3 × 3 | ||||||||||
| [67] | (Tremonte, 2005) | [R] Pork | MAP | 3 × 3 | ||||||||||
| [41] | (Vasilatos, 2013) | [W] Turkey | VP | 4 × 1 | ||||||||||
| [37] | (Wang, 2017) | [W] Chicken | Air | 3 × 1 | ||||||||||
| Mesophilic Aerobic Counts | Lactic Acid Bacteria | Brochothrix spp. | Pseudomonas spp. | Enterobacteriaceae | |
|---|---|---|---|---|---|
| N | 71 | 62 | 45 | 66 | 59 |
| [min-max] | [2.8–9.8] | [1.2–9.1] | [0.5–8.5] | [1.0–9.0] | [0.8–9.8] |
| early | [2.8–9.2] | [1.2–8.0] | [0.5–8.0] | [1.0–9.0] | [2.0–9.8] |
| belated | [2.9–9.8] | [2.5–9.1] | [2.0–8.5] | [1.0–8.0] | [0.8–8.6] |
| Mean ± sd | 6.52 ± 1.59 | 5.36 ± 1.89 | 5.18 ± 1.82 | 5.28 ± 1.89 | 4.61 ± 1.83 |
| early | 6.31 ± 1.70 | 4.46 ± 1.68 | 4.74 ± 2.08 | 5.43 ± 2.04 | 4.55 ± 1.90 |
| belated | 6.71 ± 1.43 | 6.05 ± 1.76 | 5.50 ± 1.56 | 5.13 ± 1.75 | 4.67 ± 1.80 |
| Correlation with spoilage time | 0.0279 | 0.3208 * | 0.1042 | −0.2046 | −0.0762 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luong, N.-D.M.; Coroller, L.; Zagorec, M.; Membré, J.-M.; Guillou, S. Spoilage of Chilled Fresh Meat Products during Storage: A Quantitative Analysis of Literature Data. Microorganisms 2020, 8, 1198. https://doi.org/10.3390/microorganisms8081198
Luong N-DM, Coroller L, Zagorec M, Membré J-M, Guillou S. Spoilage of Chilled Fresh Meat Products during Storage: A Quantitative Analysis of Literature Data. Microorganisms. 2020; 8(8):1198. https://doi.org/10.3390/microorganisms8081198
Chicago/Turabian StyleLuong, Ngoc-Du Martin, Louis Coroller, Monique Zagorec, Jeanne-Marie Membré, and Sandrine Guillou. 2020. "Spoilage of Chilled Fresh Meat Products during Storage: A Quantitative Analysis of Literature Data" Microorganisms 8, no. 8: 1198. https://doi.org/10.3390/microorganisms8081198
APA StyleLuong, N.-D. M., Coroller, L., Zagorec, M., Membré, J.-M., & Guillou, S. (2020). Spoilage of Chilled Fresh Meat Products during Storage: A Quantitative Analysis of Literature Data. Microorganisms, 8(8), 1198. https://doi.org/10.3390/microorganisms8081198





