How to RESPOND to Modern Challenges for People Living with HIV: A Profile for a New Cohort Consortium
Abstract
:1. Introduction
2. Materials and Methods:
2.1. Cohort Participation
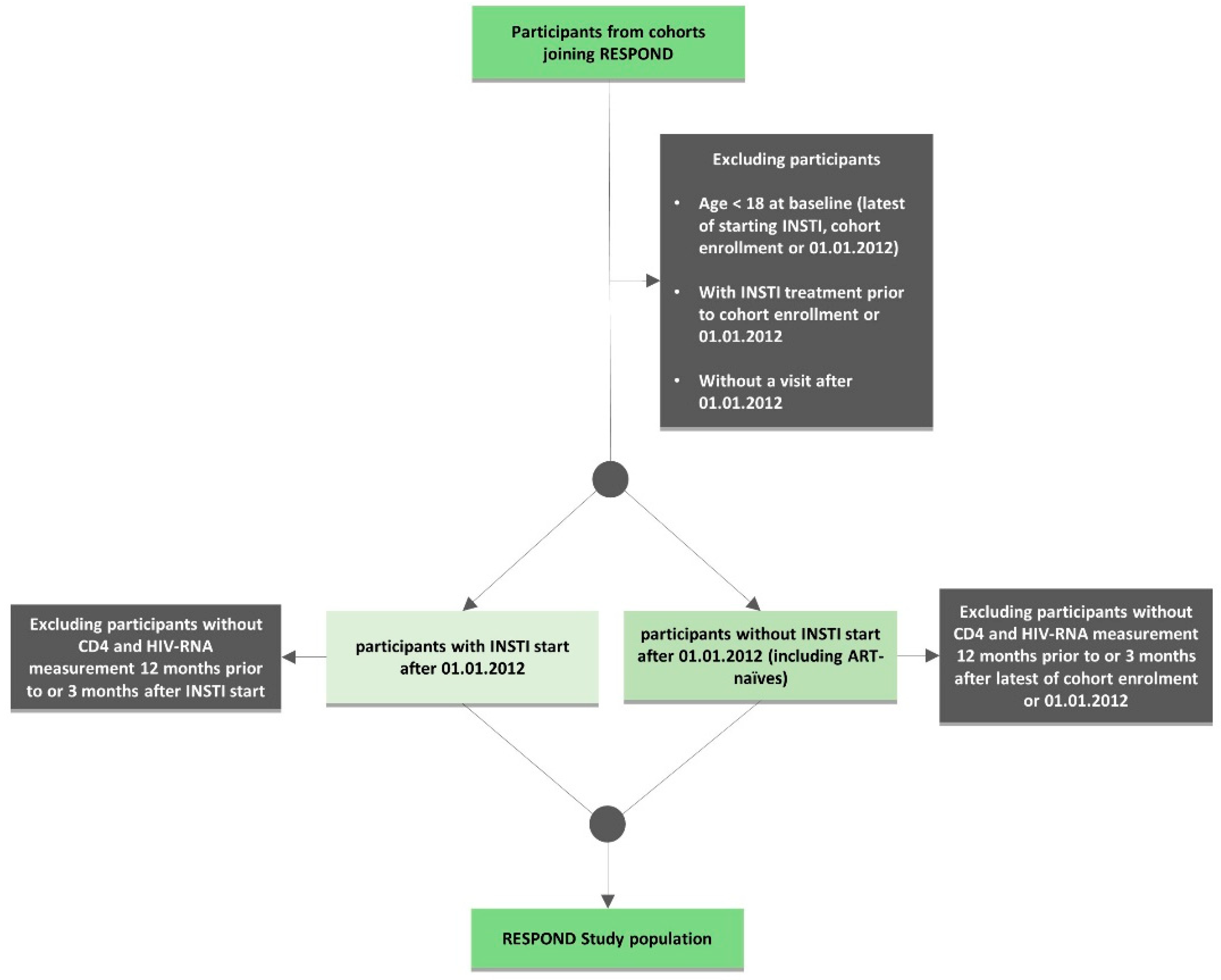
2.2. Inclusion Criteria
2.3. Ethical Considerations
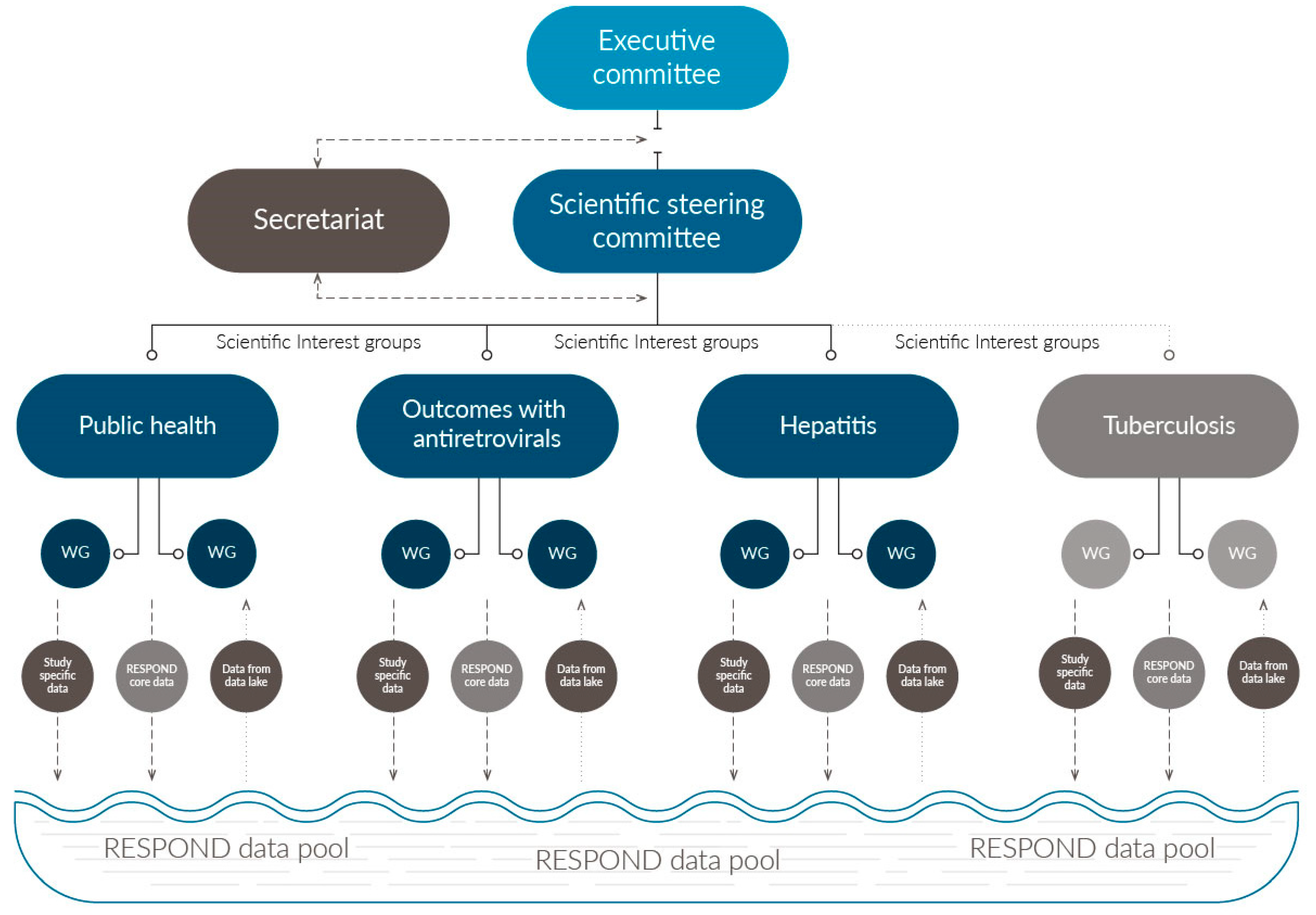
2.4. Consortium Organization and Governance:
2.5. Scientific Focus and Development of Scientific Agendas
2.6. Data Collection and Quality Assurance
3. Results
3.1. Baseline Demographics and Clinical Caracteristics
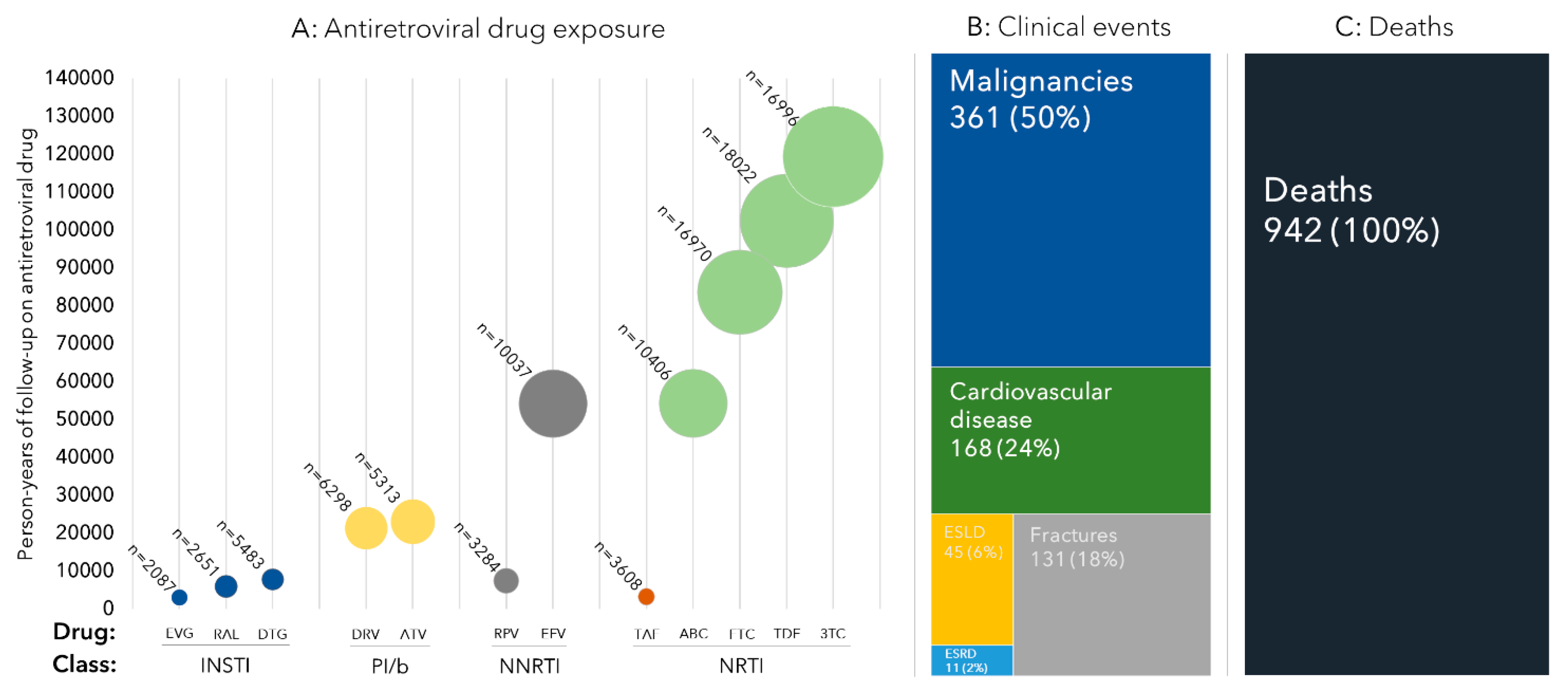
3.2. ART Exposure
3.3. Clinical Events
3.4. First RESPOND Findings
4. Discussion
4.1. Pharmacovigilance and Comorbidities in a Modern Era
4.2. Hepatitis and Other Infectious Diseases
4.3. Using Cohort Data to Support Public Health Challenges
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Writing Group Members
- CHIP, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark;
- Centre for Clinical Research, Epidemiology, Modelling and Evaluation (CREME), Institute for Global Health, University College London, London, UK;
- Department of Infectious Diseases, Bern University Hospital, University of Bern, Switzerland;
- Austrian HIV Cohort Study (AHIVCOS), Medizinische Universität Innsbruck, Innsbruch, Austria;
- Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich Switzerland;
- Royal Free HIV Cohort, London, England;
- Department of Infectious Diseases, St Pierre University Hospital Bruxelles, Brussels, Belgium;
- Modena HIV Cohort, Università degli Studi di Modena, Modena, Italy;
- San Raffaele Scientific Institute, Università Vita-Salute San Raffaele, Milano, Italy;
- Italian Cohort Naive Antiretrovirals (ICONA), ASST Santi Paolo e Carlo, Milano, Italy;
- University Hospital Cologne, Cologne, Germany;
- Universitätsklinikum Bonn und Medizinische Fakultät, Bonn, Germany;
- Nice HIV Cohort, Université Côte d’Azur et Centre Hospitalier Universitaire, Nice, France;
- Georgian National AIDS Health Information System (AIDS HIS), Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia;
- AIDS Therapy Evaluation in The Netherlands (ATHENA) cohort, Stichting HIV Monitoring, Amsterdam, The Netherlands;
- The Australian HIV Observational Database (AHOD), UNSW Sidney, Sydney, Austalia;
- Swedish InfCare HIV Cohort, Karolinska University Hospital, Stockholm, Sweden;
- PISCIS Cohort Study, Centre Estudis Epidemiologics de ITS i VIH de Catalunya (CEEISCAT), Badalona, Spain;
- Frankfurt HIV Cohort Study, Johann Wolfgang Goethe-University Hospital, Frankfurt, Germany;
- ViiV Healthcare, RTP, North Carolina, USA;
- Gilead Sciences, Foster City, California;
- Medical University of Warsaw, Poland;
- Hosp. Universitari Germans Trias i Pujol, Badalona, Spain;
- Austrian HIV Cohort Study (AHIVCOS), Medizinische Universität Wien, Vienna, Austria;
- European AIDS Treatment Group (EATG)
References
- The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med. 2003, 349, 1993–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friis-Moller, N.; Weber, R.; Reiss, P.; Thiebaut, R.; Kirk, O.; d’Arminio Monforte, A.; Pradier, C.; Morfeldt, L.; Mateu, S.; Law, M. Cardiovascular disease risk factors in HIV patients—Association with antiretroviral therapy. Results from the DAD study. AIDS 2003, 17, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Laut, K.; Kirk, O.; Rockstroh, J.; Phillips, A.; Ledergerber, B.; Gatell, J.; Gazzard, B.; Horban, A.; Karpov, I.; Losso, M.; et al. The EuroSIDA study: 25 years of scientific achievements. HIV Med. 2020, 21, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Chêne, G.; Phillips, A.; Costagliola, D.; Sterne, J.A.; Furrer, H.; Del Amo, J.; Mocroft, A.; d’Arminio Monforte, A.; Dabis, F.; Miro, J.M.; et al. Cohort profile: Collaboration of observational HIV epidemiological research europe (COHERE) in EuroCoord. Int. J. Epidemiol. 2017, 46, 797–797n. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RESPOND Consortium Description v1.0 2019. Available online: https://chip.dk/Portals/0/files/RESPOND/RESPOND_Consortium-description_V1.0_2019MAY29.pdf?ver=20191–0-02-144627-317 (accessed on 27 July 2020).
- RESPOND Governance and Procedures 2019. Available online: https://chip.dk/Portals/0/files/RESPOND/RESPOND%20governance%20and%20procedures_v6_2019SEP30.pdf?ver=2019-10-02-144419-230 (accessed on 27 July 2020).
- WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 27 July 2020).
- Commission Directive 2005/28/EC. Available online: https://eur-lex.europa.eu/eli/dir/2005/28/oj (accessed on 27 July 2020).
- RESPOND Project Proposal Template. Available online: https://chip.dk/Portals/0/files/RESPOND/RESPOND_study%20proposal%20template.doc?ver=2017-12-07-090247-223 (accessed on 27 July 2020).
- About HICDEP. Available online: https://hicdep.org/Wiki/About-HICDEP (accessed on 27 July 2020).
- CHIP CC. Standard Operating Procedurefor Data Transfer in RESPOND and EuroSIDA. vs 3.0. Available online: https://chip.dk/Portals/0/files/Eurosida/EuroSIDA/RESPOND_EuroSIDA_CARE_SOP_Electronic_Version3%200_2019_final.pdf?ver=2019-10-02-141500-817 (accessed on 27 July 2020).
- About Project REDCap. Available online: https://projectredcap.org/about/ (accessed on 27 July 2020).
- CHIP CC. RESPOND Electronic Submission Tool (REST) User Guide vs 1.0. Available online: https://chip.dk/Portals/0/files/RESPOND/RESPOND_EuroSIDA_D45_Electronic_Submission_Tool_User_guide_Version1.pdf?ver=2017-12-07-091717-467 (accessed on 27 July 2020).
- CHIP CC. RESPOND Manual of Operations (MOOP). vs 1.6. Available online: https://chip.dk/Portals/0/files/RESPOND/RESPOND%20Manual%20of%20Operations%20MOOP__Version%201.6.pdf?ver=2019-11-05-124535-643 (accessed on 27 July 2020).
- Greenberg, L.; Ryom, L.; Wandeler, G.; Grabmeier-Pfistershammer, K.; Öllinger, A.; Neesgaard, B.; Stephan, C.; Calmy, A.; Rauch, A.; Castagna, A.; et al. Uptake and discontinuation of integrase inhibitors (INSTIs) in a large cohort setting. J. Acquir. Immune Defic. Syndr. 2020, 83, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Neesgaard, B. Virologic and immunologic outcomes of integrase inhibitors (INSTIs). In Proceedings of the Conference on Retrovirus and Oppotunistic Infections, Seattle, WA, USA, 4–7 March 2019. [Google Scholar]
- Mocroft, A. Virologic, immunologic and clinical outcomes in antiretroviral treatment (ART) naïve individuals in the RESPOND cohort collaboration (PE2/40). In Proceedings of the 17th European AIDS Conference, EACS 2019, Basel, Switzerland, 6–9 November 2019. [Google Scholar]
- Greenberg, L. Clinical outcomes of two drug regimens (2DRs) Vs. In three drug regimens (3DRs) in HIV. In Proceedings of the Conference on Retrovirus and Opertunistic Infections, Boston, MA, USA, 8–11 March 2020. [Google Scholar]
- Raben, D. A simple tool to evaluate the effectiveness of HIV care for settings with gaps in data availability. HIV Med. 2019, 20, 27. [Google Scholar]
- Lazarus, J.V.; Nielsen, K.K. HIV and people over 50 years old in Europe. HIV Med. 2010, 11, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Gueler, A.; Moser, A.; Calmy, A.; Günthard, H.F.; Bernasconi, E.; Furrer, H.; Fux, C.A.; Battegay, M.; Cavassini, M.; Vernazza, P.; et al. Life expectancy in HIV-positive persons in Switzerland: matched comparison with general population. AIDS 2017, 31, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althoff, K.N.; Smit, M.; Reiss, P.; Justice, A.C. HIV and ageing: Improving quantity and quality of life. Curr. Opin. HIV AIDS 2016, 11, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Orlando, G.; Zona, S.; Menozzi, M.; Carli, F.; Garlassi, E.; Roverato, A.; Palella, F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 2011, 53, 1120–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtzman, C.; Armon, C.; Tedaldi, E.; Chmiel, J.S.; Buchacz, K.; Wood, K.; Brooks, J.T.; HOPS Investigators. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J. Gen. Intern. Med. 2013, 28, 1302–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troya, J.; Bascunana, J. Safety and tolerability: Current challenges to antiretroviral therapy for the long-term management of HIV infection. AIDS Rev. 2016, 18, 127–137. [Google Scholar] [PubMed]
- World Health Organization, Fact Sheets; Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death2018 (accessed on 27 July 2020).
- World Health Organization, Fact Sheets; Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis2020 (accessed on 27 July 2020).
- Podlekareva, D.N.; Efsen, A.M.; Schultze, A.; Post, F.A.; Skrahina, A.M.; Panteleev, A.; Furrer, H.; Robert, F.M.; Losso, M.H.; Toibaro, J.; et al. Tuberculosis-related mortality in people living with HIV in Europe and Latin America: an international cohort study. Lancet HIV 2016, 3, e120–e131. [Google Scholar] [CrossRef] [Green Version]
- (UNAIDS) UNPoHA. 90-90-90 An Ambitious Treatment Target to Help end the AIDS Epidemic United Nations Programme on HIV/AIDS (UNAIDS). Available online: http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/90-90-90_en.pdf2014 (accessed on 27 July 2020).
- The PrePaREstudy. Available online: https://chip.dk/Studies/PrEPaRe (accessed on 27 July 2020).
- CARE East Cohort Protocol (A Study in the RESPOND Consortium) Version 1.0. 2019. Available online: https://chip.dk/Portals/0/files/CARE/CARE-East_Cohort_Protocol_V1.0_2019AUG16.pdf?ver=2019-12-03-130 (accessed on 27 July 2020).
- Schultze, A.; Phillips, A.N.; Paredes, R.; Battegay, M.; Rockstroh, J.K.; Machala, L.; Tomazic, J.; Girard, P.M.; Januskevica, I.; Gronborg-Laut, K. HIV resistance testing and detected drug resistance in Europe. AIDS 2015, 29, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- 2Bbosa, N.; Kaleebu, P.; Ssemwanga, D. HIV subtype diversity worldwide. Curr. Opin. HIV AIDS 2019, 14, 153–160. [Google Scholar] [CrossRef]
| RESPOND Core Variables | Study Specific Variables | |
|---|---|---|
| Demographics and Basic Information | Date of birth | Ethnicity |
| Sex | Country of origin | |
| Date first seen at department | Non-intravenous illicit drug use | |
| Date of first positive HIV1-Ab/Ag test | Intravenous drug use | |
| Height | ||
| Weigth | ||
| Baseline smoking status | ||
| Current smoking status | ||
| Risk of HIV acquisition of infection | ||
| Risk of HCV acquisition of infection | ||
| Risk of HBV acquisition of infection | ||
| Infection Related Laboratory Values | HIV RNA | CD8 counts |
| CD4 counts | HLA B*57:01 | |
| HCV-antibodies | HCV genotype (and subtype) | |
| HCV RNA | Blood samples | |
| HBS Antigen | Tuberculosis diagnosis | |
| HBV DNA | Tuberculosis resistance | |
| Laboratory Values | Creatinin | ALT |
| Total cholesterol | AST | |
| HDL cholesterol | INR | |
| LDL cholesterol | Hemoglobin | |
| Triglycerides | Bilirubin | |
| HbA1C or blood glucose | Albumin | |
| Urine dipstick tests for proteinuria | ||
| Antiviral Treatment Treatment * | Nucleos(t)ide reverse transcriptase inhibitors | Hepatitis C treatment |
| Non-nucleotide reverse transcriptase inhibitors | ||
| Protease inhibitors (cobicistat/ritonavir boosted or upboosted) | ||
| Integrase strand transfer inhibitors Entry inhibitors | ||
| Fusion inhibitors | ||
| Non-Antiviral Treatment Treatment * | Anti-thrombotic drugs | Opioid substitution therapy |
| Anti-hypertensive drugs | Anti-Tuberculosis treatment | |
| Anti-diabetic drugs | ||
| Lipid lowering drugs | ||
| Other Paraclinical Data | Blood pressure | Bone Mass density: t-score |
| Bone Mass density: z-score | ||
| Bone Mass density area | ||
| Liver trans elastography (fibroscan) | ||
| Liver biopsy (metavir stage) | ||
| Acoustic radiation force impulse (ARFI) | ||
| HCC screening (Abdominal CT/MRI and ultrasound) | ||
| Clinical Events | Myocardial Infarctions ** | Pregnancy |
| Stroke ** | ||
| Invasive cardiovascular procedures (coronary angioplasty/stenting, coronary bypass surgery, and carotid endarterectomi) ** | ||
| Non-AIDS defining malignancies ** | ||
| End-stage liver disease ** | ||
| End-stage renal disease ** | ||
| Fracture ** | ||
| AIDS events (including AIDS defining cancers **) | ||
| Deaths *** |
| n | % | ||
|---|---|---|---|
| All | 26,258 | 100 | |
| Age (Years) | ≤40 | 6488 | 24.9 |
| 41–50 | 7909 | 30.4 | |
| 51–60 | 7811 | 30.0 | |
| >60 | 3810 | 14.6 | |
| Region | Southern Europe (plus Argentina) | 6933 | 26.6 |
| Western Europe | 13,320 | 51.2 | |
| Northern Europe (plus Australia) | 2524 | 9.7 | |
| Central Eastern Europe | 1421 | 5.5 | |
| Eastern Europe | 1820 | 7.0 | |
| Sex | Male | 19,329 | 74.3 |
| Female | 6679 | 25.7 | |
| Race | White | 18,834 | 72.4 |
| Black | 4368 | 16.8 | |
| Other/Unknown | 2816 | 10.8 | |
| HIV Acquisition Risk | Men-sex-with-men | 11,300 | 43.4 |
| Intraveneous drug use | 3883 | 14.9 | |
| Heterosexual | 8931 | 34.3 | |
| Other/Unknown | 1904 | 7.3 | |
| Viral load (copies/mL) * | <200 | 24,073 | 89.5 |
| ≥200 | 2185 | 10.5 | |
| <LOD, where LOD> 200 cp/mL ** | 165 | 0.6 | |
| ART naïve | No | 24,634 | 94.7 |
| Yes | 1384 | 5.3 | |
| CD4 (cells/μL) *** | ≤200 | 1410 | 5.4 |
| 201–350 | 2689 | 10.3 | |
| 351–500 | 4429 | 17.0 | |
| 501–750 | 8758 | 33.7 | |
| >750 | 8861 | 33.3 | |
| CD4 Nadir (cells/μL) | ≤50 | 4268 | 16.4 |
| 51–200 | 8233 | 31.6 | |
| 201–350 | 7813 | 30.0 | |
| >350 | 5544 | 21.3 | |
| Date of HIV diagnosis | ≤2011 | 20,723 | 79.6 |
| >2012 | 4494 | 17.3 | |
| Unknown | 801 | 3.1 | |
| HBV status | Negative | 21,088 | 81.1 |
| Positive | 1347 | 5.2 | |
| Unknown | 3583 | 13.8 | |
| HCV status | Negative | 16,727 | 64.3 |
| Positive | 6622 | 25.5 | |
| Unknown | 2669 | 10.3 | |
| Median | Interquartile Range | ||
| Age (years) | 48 | 40–56 | |
| CD4 (cells/μL) | 621 | 438–830 | |
| CD4 Nadir (cells/μL) | 208 | 91–327 | |
| Baseline date (month/year) | 6/17 | 12/15–9/17 | |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
The RESPOND Study Group. How to RESPOND to Modern Challenges for People Living with HIV: A Profile for a New Cohort Consortium. Microorganisms 2020, 8, 1164. https://doi.org/10.3390/microorganisms8081164
The RESPOND Study Group. How to RESPOND to Modern Challenges for People Living with HIV: A Profile for a New Cohort Consortium. Microorganisms. 2020; 8(8):1164. https://doi.org/10.3390/microorganisms8081164
Chicago/Turabian StyleThe RESPOND Study Group. 2020. "How to RESPOND to Modern Challenges for People Living with HIV: A Profile for a New Cohort Consortium" Microorganisms 8, no. 8: 1164. https://doi.org/10.3390/microorganisms8081164
APA StyleThe RESPOND Study Group. (2020). How to RESPOND to Modern Challenges for People Living with HIV: A Profile for a New Cohort Consortium. Microorganisms, 8(8), 1164. https://doi.org/10.3390/microorganisms8081164




