Characteristics of an Iron-Reducing, Moderately Acidophilic Actinobacterium Isolated from Pyritic Mine Waste, and Its Potential Role in Mitigating Mineral Dissolution in Mineral Tailings Deposits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of C. ammoniigenes IT2
2.2. pH and Temperature Characteristics
2.3. Organic Nutrition of C. ammoniigenes IT2
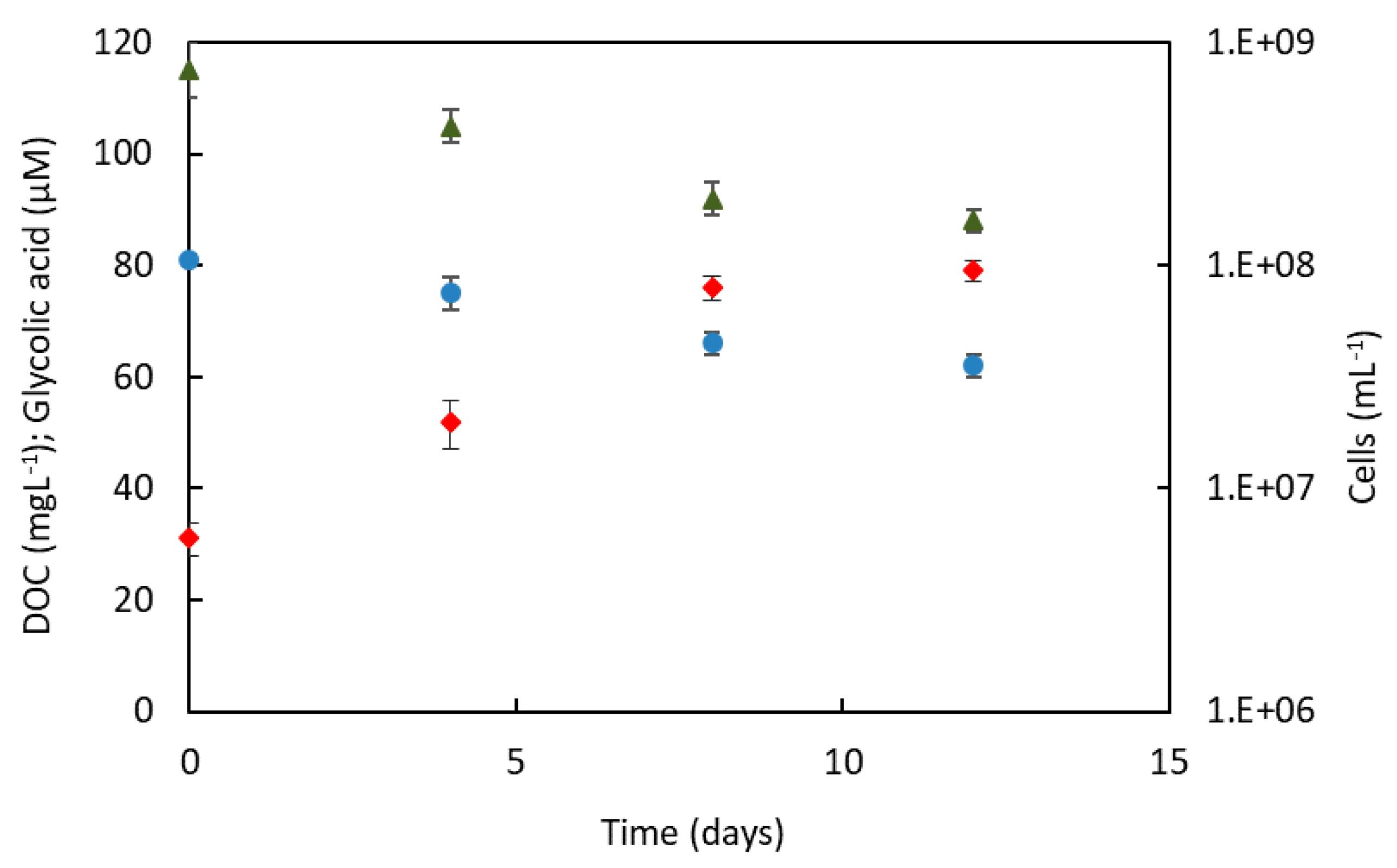
2.4. Oxidation of Iron and Reduced Sulfur
2.5. Growth of C. ammoniigenes IT2 in Spent Medium of Acidithiobacillus ferrooxidans
2.6. Tolerance of C. ammoniigenes IT2 to Some Transition Metals
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nancucheo, I.; Bitencourt, J.; Sahoo, P.K.; Alves, J.; Siqueira, J.O.; Oliveira, G. Recent developments for remediating acidic mine waters using sulfidogenic bacteria. BioMed Res. Int. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dold, B. Basic concepts in environmental geochemistry of sulfidic mine-waste management. In Waste Management; InTech: Rijeka, Croatia, 2010; pp. 173–198. [Google Scholar]
- Zanin, M.; Lambert, H.; Du Plessis, C. Lime use and functionality in sulphide mineral flotation: A review. Miner. Eng. 2019, 143, 105922. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- González, D.; Liu, Y.; Villa-Gómez, D.K.; Southam, G.; Hedrich, S.; Galleguillos, P.; Colipai, C.; Nancucheo, I. Performance of a sulfidogenic bioreactor inoculated with indigenous acidic communities for treating an extremely acidic mine water. Miner. Eng. 2019, 131, 370–375. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Schippers, A.; Breuker, A.; Blazejak, A.; Bosecker, K.; Kock, D.; Wright, T. The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy 2010, 104, 342–350. [Google Scholar] [CrossRef]
- Diaby, N.; Dold, B.; Pfeifer, H.-R.; Holliger, C.; Johnson, D.B.; Hallberg, K.B. Microbial communities in a porphyry copper tailings impoundment and their impact on the geochemical dynamics of the mine waste. Environ. Microbiol. 2007, 9, 298–307. [Google Scholar] [CrossRef]
- Bryan, C.; Hallberg, K.B.; Johnson, D.B. Mobilisation of metals in mineral tailings at the abandoned São Domingos copper mine (Portugal) by indigenous acidophilic bacteria. Hydrometallurgy 2006, 83, 184–194. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Anand, R.R.; Reith, F.; Gregg, A.L.; Noble, R.R.P.; Goldfarb, K.C.; Andersen, G.L.; Desantis, T.Z.; Piceno, Y.M.; Brodie, E.L. Bacterial communities associated with a mineral weathering profile at a sulfidic mine tailings dump in arid Western Australia. FEMS Microbiol. Ecol. 2012, 79(2), 298–311. [Google Scholar] [CrossRef]
- González, D.; Huber, K.J.; Tindall, B.; Hedrich, S.; Rojas-Villalobos, C.; Quatrini, R.; Dinamarca, M.A.; Ibacache-Quiroga, C.; Schwarz, A.; Canales, C.; et al. Acidiferrimicrobium australe gen. nov., sp. nov., an acidophilic and obligately heterotrophic, member of the Actinobacteria that catalyses dissimilatory oxido-reduction of iron isolated from metal-rich acidic water in Chile. Int. J. Syst. Evol. Microbiol. 2020, 70, 3348–3354. [Google Scholar] [CrossRef]
- Johnson, D.B. Recent developments in microbiological approaches for securing mine wastes and for recovering metals from mine waters. Miner. 2014, 4, 279–292. [Google Scholar] [CrossRef]
- Nancucheo, I.; Johnson, D.B. Significance of microbial communities and interactions in safeguarding reactive mine tailings by ecological engineering. Appl. Environ. Microbiol. 2011, 77, 8201–8208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aizawa, T.; Ve, N.B.; Kimoto, K.-I.; Iwabuchi, N.; Sumida, H.; Hasegawa, I.; Sasaki, S.; Tamura, T.; Kudo, T.; Suzuki, K.-I.; et al. Curtobacterium ammoniigenes sp. nov., an ammonia-producing bacterium isolated from plants inhabiting acidic swamps in actual acid sulfate soil areas of Vietnam. Int. J. Syst. Evol. Microbiol. 2007, 57, 1447–1452. [Google Scholar] [CrossRef] [Green Version]
- Nancucheo, I.; Rowe, O.F.; Hedrich, S.; Johnson, D.B. Solid and liquid media for isolating and cultivating acidophilic and acid-tolerant sulfate-reducing bacteria. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, O.F.; Sánchez-España, J.; Hallberg, K.B.; Johnson, D.B. Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ. Microbiol. 2007, 9, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Stookey, L. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. EE.UU 1970, 42, 779–781. [Google Scholar]
- Calkins, V. Microdetermination of glycolic and oxalic acids. Ind. Eng. Chem. Anal. Ed. 1943, 15, 762–763. [Google Scholar] [CrossRef]
- Nancucheo, I.; Johnson, D.B. Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microb. Biotechnol. 2011, 5, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Kosako, Y.; Tano, T.; Kishimoto, N. Acidobacterium capsulatum gen. nov., sp. nov.: An acidophilic chemoorganotrophic bacterium containing menaquinone from acidic mineral environment. Curr. Microbiol. 1991, 22, 1–7. [Google Scholar] [CrossRef]
- Urschel, M.R.; Hamilton, T.L.; Roden, E.E.; Boyd, E.S. Substrate preference, uptake kinetics and bioenergetics in a facultatively autotrophic, thermoacidophilic crenarchaeote. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, N.R.; Banerjee, P. Extreme tolerance to cadmium and high resistance to copper, nickel and zinc in different Acidiphilium strains. Lett. Appl. Microbiol. 1996, 23, 393–397. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Carbon, iron and sulfur metabolism in acidophilic micro-organisms. Adv. Microb. Physiol. 2008, 54, 201–255. [Google Scholar] [CrossRef]
- Kermer, R.; Hedrich, S.; Taubert, M.; Baumann, S.; Schlömann, M.; Johnson, D.B.; von Bergen, M.; Seifert, J. Elucidation of carbon transfer in a mixed culture of Acidiphilium cryptum and Acidithiobacillus ferrooxidans using protein-based stable isotope probing. J. Integr. OMICS. 2012, 2, 37–45. [Google Scholar]
- Johnson, D.B. Microbial communities and interactions in low pH environments. In Acidophiles: Life in Extremely Acidic Environments; Caister Academic Press: Wymondham, UK, 2016; pp. 121–137. [Google Scholar]
- Schnaitman, C.; Lundgren, D.G. Organic compounds in the spent medium of Ferrobacillus ferrooxidans. Can. J. Microbiol. 1965, 11, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Dold, B.; Blowes, D.W.; Dickhout, R.; Spangenberg, J.E.; Pfeifer, H.-R. Low molecular weight carboxylic acids in oxidizing porphyry copper tailings. Environ. Sci. Technol. 2005, 39, 2515–2521. [Google Scholar] [CrossRef]
- Nancucheo, I.; Johnson, D.B. Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia. Appl. Environ. Microbiol. 2009, 76, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Dold, B. Evolution of acid mine drainage formation in sulphidic mine tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.B.; Kanao, T.; Hedrich, S. Redox transformations of iron at extremely low pH: Fundamental and applied aspects. Front. Microbiol. 2012, 3, 3–96. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.B.; Hedrich, S.; Pakostova, E. Indirect redox transformations of iron, copper, and chromium catalyzed by extremely acidophilic bacteria. Front. Microbiol. 2017, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Quatrini, R.; Johnson, D.B. Acidophiles: Life in Extremely Acidic; Caister Academic Press: Haverhill, UK, 2016; pp. 265–284. [Google Scholar]
- Jones, R.; Johnson, D.B. Acidithrix ferrooxidans gen. nov., sp. nov.; a filamentous and obligately heterotrophic, acidophilic member of the Actinobacteria that catalyzes dissimilatory oxido-reduction of iron. Res. Microbiol. 2015, 166, 111–120. [Google Scholar] [CrossRef]

| Substrate | IT2 | Acidobacterium capsulatum a | Acidiphilium cryptum a |
|---|---|---|---|
| Glucose | ++ | ++ | ++ |
| Galactose | ++ | ++ | ++ |
| Fructose | ++ | Nd | Nd |
| Xylose | ++ | ++ | ++ |
| Mannose | ++ | ++ | + |
| Arabinose | − | ++ | ++ |
| Rhamnose | − | Nd | Nd |
| Maltose | + | ++ | + |
| Lactose | + | ++ | + |
| Glycerol | ++ | − | ++ |
| Mannitol | + | − | ++ |
| Sorbitol | − | Nd | Nd |
| Acetic acid | − | Nd | Nd |
| Citric acid | − | Nd | Nd |
| Glutamic acid | − | Nd | Nd |
| Asparagine | − | Nd | Nd |
| Arginine | − | Nd | Nd |
| Lysine | − | Nd | Nd |
| Leucine | − | Nd | Nd |
| Proline | − | Nd | Nd |
| Ethanol | − | − | − |
| Methanol | − | Nd | Nd |
| Tryptone soy broth | ++ | Nd | Nd |
| Yeast extract | ++ | Nd | Nd |
| Metal | MIC (mM) |
|---|---|
| Cu2+ | 15 (10) |
| Fe2+ | 250 a (200) |
| Zn2+ | 125 (100) |
| Ni2+ | 100 (75) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nancucheo, I.; Johnson, D.B. Characteristics of an Iron-Reducing, Moderately Acidophilic Actinobacterium Isolated from Pyritic Mine Waste, and Its Potential Role in Mitigating Mineral Dissolution in Mineral Tailings Deposits. Microorganisms 2020, 8, 990. https://doi.org/10.3390/microorganisms8070990
Nancucheo I, Johnson DB. Characteristics of an Iron-Reducing, Moderately Acidophilic Actinobacterium Isolated from Pyritic Mine Waste, and Its Potential Role in Mitigating Mineral Dissolution in Mineral Tailings Deposits. Microorganisms. 2020; 8(7):990. https://doi.org/10.3390/microorganisms8070990
Chicago/Turabian StyleNancucheo, Ivan, and D. Barrie Johnson. 2020. "Characteristics of an Iron-Reducing, Moderately Acidophilic Actinobacterium Isolated from Pyritic Mine Waste, and Its Potential Role in Mitigating Mineral Dissolution in Mineral Tailings Deposits" Microorganisms 8, no. 7: 990. https://doi.org/10.3390/microorganisms8070990





