Cytomegalovirus and Inflammatory Bowel Diseases (IBD) with a Special Focus on the Link with Ulcerative Colitis (UC)
Abstract
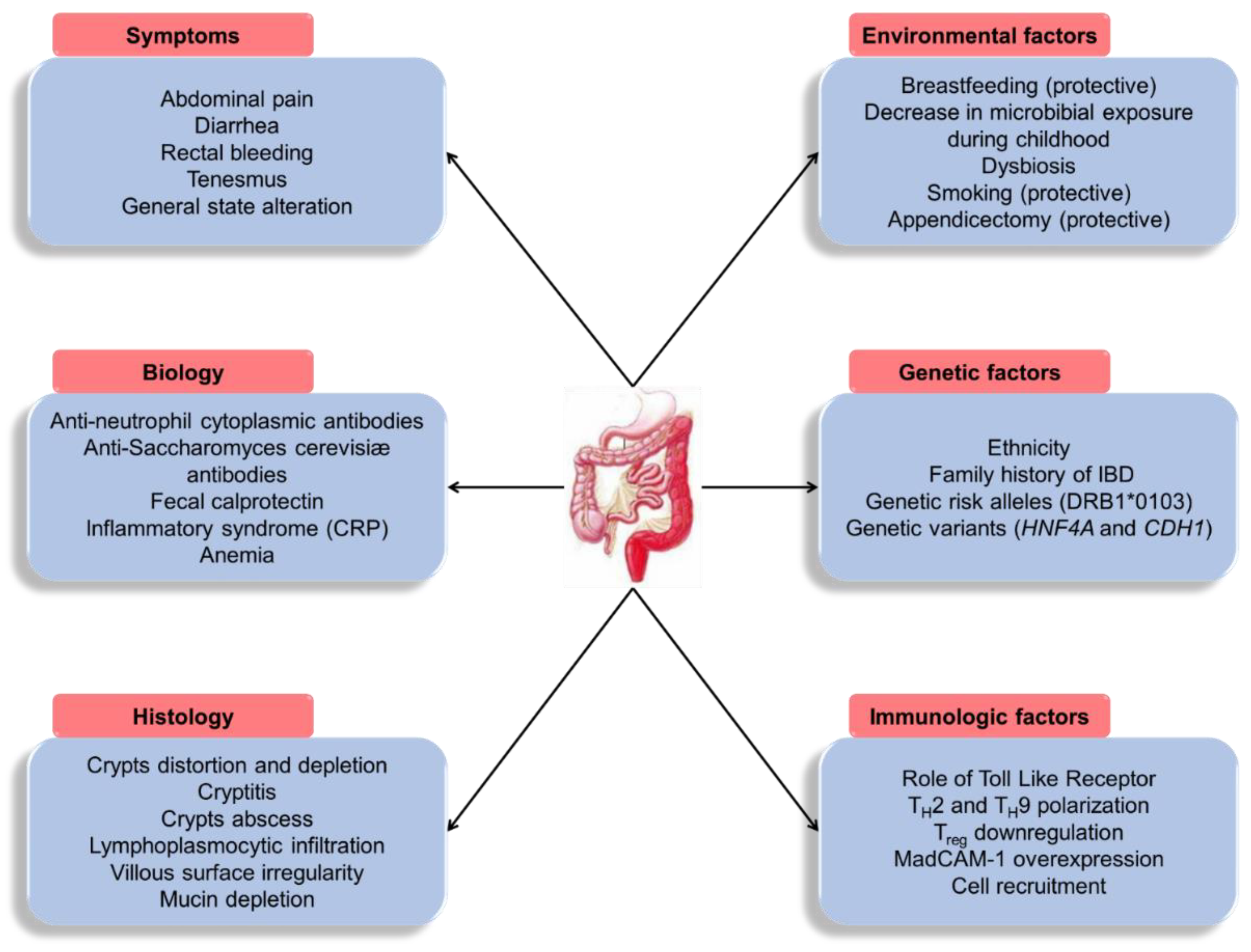
1. Introduction
2. Brief Recalls on the Pathophysiology of CMV Infection
2.1. Cell Tropism and Transmission of CMV
2.2. Lytic Replication Cycle
2.3. Latency and Reactivation
2.4. Infection vs. Disease
2.5. CMV and Inflammation
2.6. Antiviral Drugs against CMV
3. CMV Infection of the Digestive Tract in Immunocompromised and Immunocompetent Patients
4. Controversial Role of CMV Infection in IBD Inflammatory Flares—Analysis of Confounding Factors
4.1. Differences between Primary Infection and Tissue Reactivation
4.2. UC versus CD
4.3. Virological Diagnosis of CMV Colitis
4.4. Typing the CMV Strains
4.5. Other Assays for CMV Colitis Diagnosis
5. Inflammation and Immunosuppressive Therapies Contribute to Colonic Reactivation of CMV Infection during UC
5.1. Impact of Inflammation
5.2. Anti-Inflammatory and Immunosuppressive Drugs
6. Active Pejorative Role of CMV Reactivations in UC Flare-Ups
6.1. Association of CMV Infection with a Pejorative Evolution of UC
6.2. Association with Steroid Resistance and Resistance to Immunosuppressive Treatments
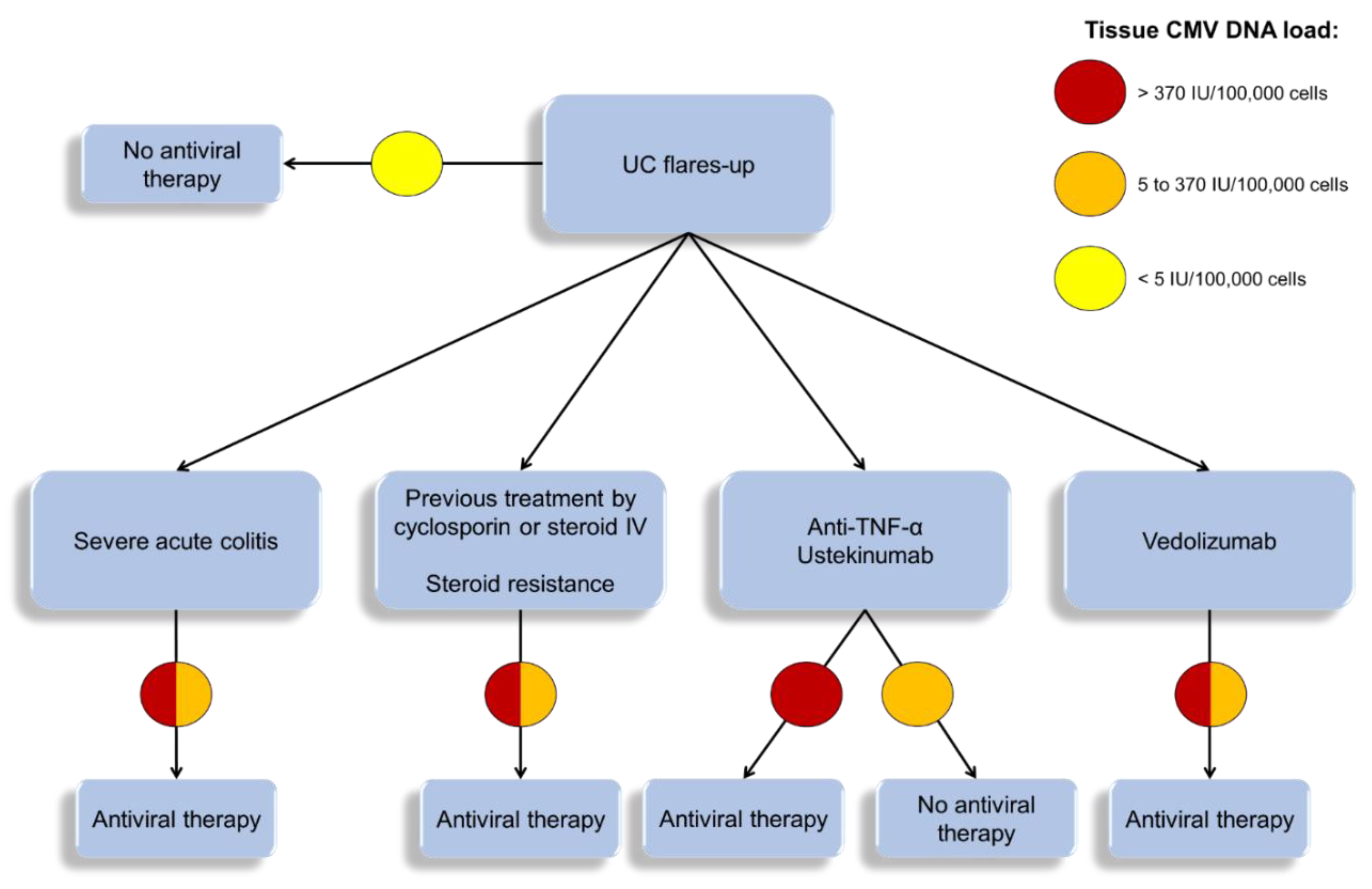
6.3. Indications of Viral Load Measurement During UC Flare-Ups
6.4. Place of Antiviral Treatment
6.5. Other Therapeutic Options
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hendler, S.A.; Barber, G.E.; Okafor, P.N.; Chang, M.S.; Limsui, D.; Limketkai, B.N. Cytomegalovirus infection is associated with worse outcomes in inflammatory bowel disease hospitalizations nationwide. Int. J. Colorectal Dis. 2020, 35, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet Lond. Engl. 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Ghione, S.; Sarter, H.; Fumery, M.; Armengol-Debeir, L.; Savoye, G.; Ley, D.; Spyckerelle, C.; Pariente, B.; Peyrin-Biroulet, L.; Turck, D.; et al. Dramatic Increase in Incidence of Ulcerative Colitis and Crohn’s Disease (1988-2011): A Population-Based Study of French Adolescents. Am. J. Gastroenterol. 2018, 113, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Tripathi, K.; Feuerstein, J.D. New developments in ulcerative colitis: Latest evidence on management, treatment, and maintenance. Drugs Context 2019, 8, 212572. [Google Scholar] [CrossRef]
- Varani, S.; Landini, M.P. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae 2011, 2, 6. [Google Scholar] [CrossRef]
- Park, S.C.; Jeen, Y.M.; Jeen, Y.T. Approach to cytomegalovirus infections in patients with ulcerative colitis. Korean J. Intern. Med. 2017, 32, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Fakhreddine, A.Y.; Frenette, C.T.; Konijeti, G.G. A Practical Review of Cytomegalovirus in Gastroenterology and Hepatology. Gastroenterol. Res. Pract. 2019, 2019, 6156581. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Watson, G.M.; Jonjic, S.; Degli-Esposti, M.A.; Rossjohn, J. Modulation of innate and adaptive immunity by cytomegaloviruses. Nat. Rev. Immunol. 2020, 20, 113–127. [Google Scholar] [CrossRef]
- Picarda, G.; Benedict, C.A. Cytomegalovirus: Shape-Shifting the Immune System. J. Immunol. 2018, 200, 3881–3889. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus infection during pregnancy: State of the science. Am. J. Obstet. Gynecol. 2020. [Google Scholar] [CrossRef]
- Crough, T.; Khanna, R. Immunobiology of human cytomegalovirus: From bench to bedside. Clin. Microbiol. Rev. 2009, 22, 76–98. [Google Scholar] [CrossRef]
- Collins-McMillen, D.; Buehler, J.; Peppenelli, M.; Goodrum, F. Molecular Determinants and the Regulation of Human Cytomegalovirus Latency and Reactivation. Viruses 2018, 10, 444. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D.; et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [CrossRef]
- Boeckh, M.; Geballe, A.P. Cytomegalovirus: Pathogen, paradigm, and puzzle. J. Clin. Invest. 2011, 121, 1673–1680. [Google Scholar] [CrossRef]
- Rossini, G.; Cerboni, C.; Santoni, A.; Landini, M.P.; Landolfo, S.; Gatti, D.; Gribaudo, G.; Varani, S. Interplay between human cytomegalovirus and intrinsic/innate host responses: A complex bidirectional relationship. Mediators Inflamm. 2012, 2012, 607276. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C. Treatment of cytomegalovirus infections beyond acute disease to improve human health. Expert Rev. Anti Infect. Ther. 2014, 12, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Couzi, L.; Pitard, V.; Moreau, J.-F.; Merville, P.; Déchanet-Merville, J. Direct and Indirect Effects of Cytomegalovirus-Induced γδ T Cells after Kidney Transplantation. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Chen, S.J.; Wang, L.J.; Du, Y.; Si, J.M. Cytomegalovirus: A probable cause of steroid-refractory ulcerative colitis. J. Dig. Dis. 2013, 14, 160–165. [Google Scholar] [CrossRef]
- Goodman, A.L.; Murray, C.D.; Watkins, J.; Griffiths, P.D.; Webster, D.P. CMV in the gut: A critical review of CMV detection in the immunocompetent host with colitis. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 13–18. [Google Scholar] [CrossRef][Green Version]
- Sezgin, E.; An, P.; Winkler, C.A. Host Genetics of Cytomegalovirus Pathogenesis. Front. Genet. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Britt, W.J.; Prichard, M.N. New therapies for human cytomegalovirus infections. Antiviral Res. 2018, 159, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Ligat, G.; Cazal, R.; Hantz, S.; Alain, S. The human cytomegalovirus terminase complex as an antiviral target: A close-up view. FEMS Microbiol. Rev. 2018, 42, 137–145. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Diamond, D.J.; La Rosa, C.; Chiuppesi, F.; Contreras, H.; Dadwal, S.; Wussow, F.; Bautista, S.; Nakamura, R.; Zaia, J.A. A fifty-year odyssey: Prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert Rev. Vaccines 2018, 17, 889–911. [Google Scholar] [CrossRef]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef]
- Sinzger, C.; Grefte, A.; Plachter, B.; Gouw, A.S.; The, T.H.; Jahn, G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 1995, 76 Pt 4, 741–750. [Google Scholar] [CrossRef]
- You, D.M.; Johnson, M.D. Cytomegalovirus infection and the gastrointestinal tract. Curr. Gastroenterol. Rep. 2012, 14, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Aomatsu, K.; Nakamura, M.; Aomatsu, N.; Aomatsu, K. Cytomegalovirus colitis followed by ischemic colitis in a non-immunocompromised adult: A case report. World J. Gastroenterol. 2015, 21, 3750–3754. [Google Scholar] [CrossRef]
- D’cruz, R.T.; Lau, C.C.-L.; Thamboo, T.P. Severe ischemic cytomegalovirus proctocolitis with multiple perforation. Arch. Virol. 2018, 163, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Smythies, L.E.; Grabski, R.; Li, M.; Ballestas, M.E.; Shimamura, M.; Sun, J.J.; Grams, J.; Stahl, R.; Niederweis, M.E.; et al. Cytomegalovirus promotes intestinal macrophage-mediated mucosal inflammation through induction of Smad7. Mucosal Immunol. 2018, 11, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Yamakawa, T.; Hirano, T.; Kazama, T.; Hirayama, D.; Wagatsuma, K.; Nakase, H. Current Diagnostic and Therapeutic Approaches to Cytomegalovirus Infections in Ulcerative Colitis Patients Based on Clinical and Basic Research Data. Int. J. Mol. Sci. 2020, 21, 2438. [Google Scholar] [CrossRef]
- Maidji, E.; Somsouk, M.; Rivera, J.M.; Hunt, P.W.; Stoddart, C.A. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog. 2017, 13, e1006202. [Google Scholar] [CrossRef]
- Bernard, S.; Germi, R.; Lupo, J.; Laverrière, M.-H.; Masse, V.; Morand, P.; Gavazzi, G. Symptomatic cytomegalovirus gastrointestinal infection with positive quantitative real-time PCR findings in apparently immunocompetent patients: A case series. Clin. Microbiol. Infect. 2015, 21, 1121.e1–1121.e7. [Google Scholar] [CrossRef]
- Inayat, F.; Hussain, Q.; Shafique, K.; Tasleem, S.H.; Hurairah, A. Cytomegalovirus Colitis in Immunocompetent Patients. Cureus 2016, 8, e869. [Google Scholar] [CrossRef]
- Goelz, R.; Hamprecht, K.; Klingel, K.; Poets, C.F. Intestinal manifestations of postnatal and congenital cytomegalovirus infection in term and preterm infants. J. Clin. Virol. 2016, 83, 29–36. [Google Scholar] [CrossRef]
- Dioverti, M.V.; Razonable, R.R. Cytomegalovirus. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulou, E.; Ternes, K.; Mechie, N.-C.; Bremer, S.C.B.; Kunsch, S.; Ellenrieder, V.; Neesse, A.; Amanzada, A. Cytomegalovirus colitis in inflammatory bowel disease and after haematopoietic stem cell transplantation: Diagnostic accuracy, predictors, risk factors and disease outcome. BMJ Open Gastroenterol. 2019, 6, e000258. [Google Scholar] [CrossRef] [PubMed]
- Tun, G.S.Z.; Raza, M.; Hale, M.F.; Lobo, A.J. Polymerase chain reaction for detection of mucosal cytomegalovirus infection in patients with acute ulcerative colitis. Ann. Gastroenterol. 2019, 32, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Pillet, S.; Oussalah, A.; Berthelot, P.; Del Tedesco, E.; Phelip, J.-M.; Chambonnière, M.-L.; Garraud, O.; Peyrin-Biroulet, L.; Pozzetto, B. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am. J. Gastroenterol. 2011, 106, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Wethkamp, N.; Nordlohne, E.-M.; Meister, V.; Helwig, U.; Respondek, M. Identification of clinically relevant cytomegalovirus infections in patients with inflammatory bowel disease. Mod. Pathol 2018, 31, 527–538. [Google Scholar] [CrossRef]
- Pillet, S.; Pozzetto, B.; Roblin, X. Cytomegalovirus and ulcerative colitis: Place of antiviral therapy. World J. Gastroenterol. 2016, 22, 2030–2045. [Google Scholar] [CrossRef]
- El-Matary, W.; Stefanovici, C.; Van Caeseele, P.; Deora, V.; McCurdy, J. Detection of Cytomegalovirus in Colonic Mucosa of Children With Inflammatory Bowel Disease: Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 221–224. [Google Scholar] [CrossRef]
- deBruyn, J.C.C.; Soon, I.S.; Fonseca, K.; Feng, S.; Purtzki, M.; Goedhart, C.; Kuhn, S.; Vanderkooi, O.G.; Wrobel, I. Serologic Status of Routine Childhood Vaccines, Cytomegalovirus, and Epstein-Barr Virus in Children With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1218–1226. [Google Scholar] [CrossRef]
- Yerushalmy-Feler, A.; Kern-Isaacs, S.; Cohen, S. CMV Infection in Pediatric IBD. Curr. Gastroenterol. Rep. 2018, 20, 13. [Google Scholar] [CrossRef]
- Cohen, S.; Martinez-Vinson, C.; Aloi, M.; Turner, D.; Assa, A.; de Ridder, L.; Wolters, V.M.; de Meij, T.; Alvisi, P.; Bronsky, J.; et al. Cytomegalovirus Infection in Pediatric Severe Ulcerative Colitis-A Multicenter Study from the Pediatric Inflammatory Bowel Disease Porto Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Pediatr. Infect. Dis. J. 2018, 37, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Abe, T.; Tsuda, S.; Ono, I. Cytomegalovirus infection associated with onset of ulcerative colitis. BMC Res. Notes 2013, 6, 40. [Google Scholar] [CrossRef]
- Lawlor, G.; Moss, A.C. Cytomegalovirus in inflammatory bowel disease: Pathogen or innocent bystander? Inflamm. Bowel Dis. 2010, 16, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Criscuoli, V.; Rizzuto, M.-R.; Cottone, M. Cytomegalovirus and inflammatory bowel disease: Is there a link? World J. Gastroenterol. 2006, 12, 4813–4818. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Iwao, Y.; Mori, T.; Sakuraba, A.; Yajima, T.; Hisamatsu, T.; Okamoto, S.; Morohoshi, Y.; Izumiya, M.; Ichikawa, H.; et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am. J. Gastroenterol. 2007, 102, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Domènech, E.; Vega, R.; Ojanguren, I.; Hernández, A.; Garcia-Planella, E.; Bernal, I.; Rosinach, M.; Boix, J.; Cabré, E.; Gassull, M.A. Cytomegalovirus infection in ulcerative colitis: A prospective, comparative study on prevalence and diagnostic strategy. Inflamm. Bowel Dis. 2008, 14, 1373–1379. [Google Scholar] [CrossRef]
- Rowan, C.; Judge, C.; Cannon, M.D.; Cullen, G.; Mulcahy, H.E.; Ryan, E.; De Gascun, C.F.; Doherty, G.A. Severe Symptomatic Primary CMV Infection in Inflammatory Bowel Disease Patients with Low Population Seroprevalence. Gastroenterol. Res. Pract. 2018, 2018, 1029401. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef]
- Roblin, X.; Pillet, S.; Berthelot, P.; Del Tedesco, E.; Phelip, J.-M.; Chambonnière, M.-L.; Peyrin-Biroulet, L.; Pozzetto, B. Prevalence of cytomegalovirus infection in steroid-refractory Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, E1396–E1397. [Google Scholar] [CrossRef]
- Römkens, T.E.H.; Bulte, G.J.; Nissen, L.H.C.; Drenth, J.P.H. Cytomegalovirus in inflammatory bowel disease: A systematic review. World J. Gastroenterol. 2016, 22, 1321–1330. [Google Scholar] [CrossRef]
- de Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmy-Feler, A.; Padlipsky, J.; Cohen, S. Diagnosis and Management of CMV Colitis. Curr. Infect. Dis. Rep. 2019, 21, 5. [Google Scholar] [CrossRef]
- Nowacki, T.M.; Bettenworth, D.; Meister, T.; Heidemann, J.; Lenze, F.; Schmidt, H.H.; Heinzow, H.S. Novel score predicts risk for cytomegalovirus infection in ulcerative colitis. J. Clin. Virol. 2018, 105, 103–108. [Google Scholar] [CrossRef]
- Paul, M.; Gupta, E.; Jain, P.; Rastogi, A.; Bhatia, V. Diagnostic utility of quantitative cytomegalovirus DNA polymerase chain reaction in intestinal biopsies from patients with inflammatory bowel disease. J. Lab. Physicians 2018, 10, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Roblin, X.; Cornillon, J.; Mariat, C.; Pozzetto, B. Quantification of cytomegalovirus viral load. Expert Rev. Anti Infect. Ther. 2014, 12, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; James, P.; Cordeiro, E.; Mallick, R.; Shukla, T.; McCurdy, J.D. Diagnostic Accuracy of Blood-Based Tests and Histopathology for Cytomegalovirus Reactivation in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2017, 23, 551–560. [Google Scholar] [CrossRef]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohns Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Johnson, J.; Affolter, K.; Boynton, K.; Chen, X.; Valentine, J.; Peterson, K. CMV Disease in IBD: Comparison of Diagnostic Tests and Correlation with Disease Outcome. Inflamm. Bowel Dis. 2018, 24, 1539–1546. [Google Scholar] [CrossRef]
- Oh, S.J.; Lee, C.K.; Kim, Y.-W.; Jeong, S.J.; Park, Y.M.; Oh, C.H.; Kim, J.-W.; Kim, H.J. True cytomegalovirus colitis is a poor prognostic indicator in patients with ulcerative colitis flares: The 10-year experience of an academic referral inflammatory bowel disease center. Scand. J. Gastroenterol. 2019, 54, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; McCurdy, J.D.; Smyrk, T.C. Is Standard Histology Sufficient to Detect Cytomegalovirus Reactivation in Inflammatory Bowel Disease? Am. J. Clin. Pathol. 2017, 148, 459–460. [Google Scholar] [CrossRef]
- Nguyen, M.; Bradford, K.; Zhang, X.; Shih, D.Q. Cytomegalovirus Reactivation in Ulcerative Colitis Patients. Ulcers 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Zidar, N.; Ferkolj, I.; Tepeš, K.; Štabuc, B.; Kojc, N.; Uršič, T.; Petrovec, M. Diagnosing cytomegalovirus in patients with inflammatory bowel disease--by immunohistochemistry or polymerase chain reaction? Virchows Arch. Int. J. Pathol. 2015, 466, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Mourad, F.H.; Hashash, J.G.; Kariyawasam, V.C.; Leong, R.W. Ulcerative Colitis and Cytomegalovirus Infection: From A to Z. J. Crohns Colitis 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.H.; Post, K.; Sen, J.D.; Chang, H.Y.; Zhao, Z.; Fan, R.; Chen, S.; Leland, D.; Cheng, L.; Lin, J. qPCR increases sensitivity to detect cytomegalovirus in formalin-fixed, paraffin-embedded tissue of gastrointestinal biopsies. Hum. Pathol. 2014, 45, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Burston, J.; van Hal, S.; Dubedat, S.; Lee, A. Inclusions or bystanders? CMV PCR sensitivity and specificity in tissue samples. J. Clin. Virol. 2017, 90, 38–39. [Google Scholar] [CrossRef]
- Suárez-Lledó, M.; Marcos, M.Á.; Cuatrecasas, M.; Bombi, J.A.; Fernández-Avilés, F.; Magnano, L.; Martínez-Cibrián, N.; Llobet, N.; Rosiñol, L.; Gutiérrez-García, G.; et al. Quantitative PCR Is Faster, More Objective, and More Reliable Than Immunohistochemistry for the Diagnosis of Cytomegalovirus Gastrointestinal Disease in Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 2281–2286. [Google Scholar] [CrossRef]
- Nakase, H.; Yoshino, T.; Matumura, K.; Honzawa, Y.; Yamamoto, S.; Matsuura, M.; Chiba, T. Positive finding of colonic polymerase chain reaction for cytomegalovirus DNA is not false positive but a warning for treating patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm. Bowel Dis. 2011, 17, E13–E14. [Google Scholar] [CrossRef]
- Jones, A.; McCurdy, J.D.; Loftus, E.V.; Bruining, D.H.; Enders, F.T.; Killian, J.M.; Smyrk, T.C. Effects of antiviral therapy for patients with inflammatory bowel disease and a positive intestinal biopsy for cytomegalovirus. Clin. Gastroenterol. Hepatol. 2015, 13, 949–955. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Racca, F.; Paolucci, S.; Campanini, G.; Pozzi, L.; Betti, E.; Riboni, R.; Vanoli, A.; Baldanti, F.; Corazza, G.R. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: Need for mucosal viral load measurement. World J. Gastroenterol. 2015, 21, 1915–1926. [Google Scholar] [CrossRef]
- Pillet, S.; Williet, N.; Pouvaret, A.; Del Tedesco, E.; Saint-Sardos, P.; Pozzetto, B.; Roblin, X. Distribution of Cytomegalovirus DNA Load in the Inflamed Colon of Ulcerative Colitis Patients. Am. J. Gastroenterol. 2016, 111, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Kambham, N.; Vij, R.; Cartwright, C.A.; Longacre, T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: A case-control study. Am. J. Surg. Pathol. 2004, 28, 365–373. [Google Scholar] [CrossRef]
- Al-Zafiri, R.; Gologan, A.; Galiatsatos, P.; Szilagyi, A. Cytomegalovirus complicating inflammatory bowel disease: A 10-year experience in a community-based, university-affiliated hospital. Gastroenterol. Hepatol. 2012, 8, 230–239. [Google Scholar]
- Maconi, G.; Lombardini, M.; Furfaro, F.; Bezzio, C.; Zerbi, P.; Ardizzone, S. Long-term outcome of inflammatory bowel diseases with cytomegalovirus colitis: Effect of antiviral treatment. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Zagórowicz, E.; Bugajski, M.; Wieszczy, P.; Pietrzak, A.; Magdziak, A.; Mróz, A. Cytomegalovirus Infection in Ulcerative Colitis is Related to Severe Inflammation and a High Count of Cytomegalovirus-positive Cells in Biopsy Is a Risk Factor for Colectomy. J. Crohns Colitis 2016, 10, 1205–1211. [Google Scholar] [CrossRef]
- Clos-Parals, A.; Rodríguez-Martínez, P.; Cañete, F.; Mañosa, M.; Ruiz-Cerulla, A.; Paúles, M.J.; Llaó, J.; Gordillo, J.; Fumagalli, C.; Garcia-Planella, E.; et al. Prognostic value of the burden of cytomegalovirus colonic reactivation evaluated by immunohistochemical staining in patients with active ulcerative colitis. J. Crohns Colitis 2018. [Google Scholar] [CrossRef]
- Yoshino, T.; Nakase, H.; Ueno, S.; Uza, N.; Inoue, S.; Mikami, S.; Matsuura, M.; Ohmori, K.; Sakurai, T.; Nagayama, S.; et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm. Bowel Dis. 2007, 13, 1516–1521. [Google Scholar] [CrossRef]
- Tsuchido, Y.; Nagao, M.; Matsuura, M.; Nakano, S.; Yamamoto, M.; Matsumura, Y.; Seno, H.; Ichiyama, S. Real-time quantitative PCR analysis of endoscopic biopsies for diagnosing CMV gastrointestinal disease in non-HIV immunocompromised patients: A diagnostic accuracy study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2389–2396. [Google Scholar] [CrossRef]
- McCurdy, J.D.; Enders, F.T.; Jones, A.; Killian, J.M.; Loftus, E.V.; Bruining, D.H.; Smyrk, T.C. Detection of Cytomegalovirus in Patients with Inflammatory Bowel Disease: Where to Biopsy and How Many Biopsies? Inflamm. Bowel Dis. 2015, 21, 2833–2838. [Google Scholar] [CrossRef]
- Lee, H.-S.; Park, S.H.; Kim, S.-H.; Kim, J.; Choi, J.; Lee, H.J.; Kim, W.S.; Lee, J.-M.; Kwak, M.S.; Hwang, S.W.; et al. Risk Factors and Clinical Outcomes Associated with Cytomegalovirus Colitis in Patients with Acute Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 912–918. [Google Scholar] [CrossRef]
- Hirayama, Y.; Ando, T.; Hirooka, Y.; Watanabe, O.; Miyahara, R.; Nakamura, M.; Yamamura, T.; Goto, H. Characteristic endoscopic findings and risk factors for cytomegalovirus-associated colitis in patients with active ulcerative colitis. World J. Gastrointest. Endosc. 2016, 8, 301–309. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, W.; Lv, H.; Wu, D.; Feng, Y.; Shu, H.; Jin, M.; Hu, L.; Wang, Q.; Wu, D.; et al. The Association Between CMV Viremia or Endoscopic Features and Histopathological Characteristics of CMV Colitis in Patients with Underlying Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, R.; Farshadpour, F.; Makvandi, M.; Hamidifard, M.; Esmailizadeh, M.; Ahmadi, B.; Heidari, H. Determination of cytomegalovirus prevalence and glycoprotein B genotypes among ulcerative colitis patients in Ahvaz, Iran. Jundishapur J. Microbiol. 2015, 8, e17458. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Hokama, A.; Iraha, A.; Ohira, T.; Kinjo, T.; Hirata, T.; Kinjo, T.; Parrott, G.L.; Fujita, J. Distribution of cytomegalovirus genotypes among ulcerative colitis patients in Okinawa, Japan. Intest. Res. 2018, 16, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Baradhi, K.M.; Aure, R.L.; El-Amm, J.-M. High-dose Valganciclovir Treatment for Resistant Cytomegalovirus Colitis due to UL97 and UL54 Mutations. Transplant. Proc. 2018, 50, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Kim, J.; Lee, H.-S.; Choi, J.; Jang, S.J.; Jung, J.; Kim, M.J.; Chong, Y.P.; Lee, S.-O.; Choi, S.-H.; et al. Clinical Implications of the CMV-Specific T-Cell Response and Local or Systemic CMV Viral Replication in Patients With Moderate to Severe Ulcerative Colitis. Open Forum Infect. Dis. 2019, 6, ofz526. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, H.-S.; Lee, H.-J.; Kim, S.-M.; Shin, S.; Park, S.-H.; Kim, K.-J.; Kim, Y.-H.; Sung, H.; Lee, S.-O.; et al. Clinical applications of interferon-γ releasing assays for cytomegalovirus to differentiate cytomegalovirus disease from bystander activation: A pilot proof-of-concept study. Korean J. Intern. Med. 2017, 32, 900–909. [Google Scholar] [CrossRef]
- Tarasewicz, A.; Dębska-Ślizień, A.; Rutkowski, B. Clinical Utility of QuantiFERON-Cytomegalovirus Test in Management of Kidney Transplant Recipients. Transplant. Proc. 2016, 48, 1650–1653. [Google Scholar] [CrossRef]
- Yong, M.K.; Cameron, P.U.; Slavin, M.; Morrissey, C.O.; Bergin, K.; Spencer, A.; Ritchie, D.; Cheng, A.C.; Samri, A.; Carcelain, G.; et al. Identifying Cytomegalovirus Complications Using the Quantiferon-CMV Assay After Allogeneic Hematopoietic Stem Cell Transplantation. J. Infect. Dis. 2017, 215, 1684–1694. [Google Scholar] [CrossRef]
- Boom, R.; Sol, C.; Weel, J.; Lettinga, K.; Gerrits, Y.; van Breda, A.; Wertheim-Van Dillen, P. Detection and quantitation of human cytomegalovirus DNA in faeces. J. Virol. Methods 2000, 84, 1–14. [Google Scholar] [CrossRef]
- Ganzenmueller, T.; Kluba, J.; Becker, J.U.; Bachmann, O.; Heim, A. Detection of cytomegalovirus (CMV) by real-time PCR in fecal samples for the non-invasive diagnosis of CMV intestinal disease. J. Clin. Virol. 2014, 61, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Herfarth, H.H.; Long, M.D.; Rubinas, T.C.; Sandridge, M.; Miller, M.B. Evaluation of a non-invasive method to detect cytomegalovirus (CMV)-DNA in stool samples of patients with inflammatory bowel disease (IBD): A pilot study. Dig. Dis. Sci. 2010, 55, 1053–1058. [Google Scholar] [CrossRef]
- Magdziak, A.; Szlak, J.; Mróz, A.; Wieszczy, P.; Zagórowicz, E. A stool test in patients with active ulcerative colitis helps exclude cytomegalovirus disease. Scand. J. Gastroenterol. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Q.; Xu, L.-P.; Han, T.-T.; Zhang, X.-H.; Wang, Y.; Han, W.; Wang, F.-R.; Wang, J.-Z.; Chen, H.; Chen, Y.-H.; et al. Detection of human cytomegalovirus (CMV) DNA in feces has limited value in predicting CMV enteritis in patients with intestinal graft-versus-host disease after allogeneic stem cell transplantation. Transpl. Infect. Dis. 2015, 17, 655–661. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Mohammed Vashist, N.; Samaan, M.; Mosli, M.H.; Parker, C.E.; MacDonald, J.K.; Nelson, S.A.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Jairath, V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst. Rev. 2018, 1, CD011450. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Honzawa, Y.; Toyonaga, T.; Yamada, S.; Minami, N.; Yoshino, T.; Matsuura, M. Diagnosis and treatment of ulcerative colitis with cytomegalovirus infection: Importance of controlling mucosal inflammation to prevent cytomegalovirus reactivation. Intest. Res. 2014, 12, 5–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gauss, A.; Rosenstiel, S.; Schnitzler, P.; Hinz, U.; Rehlen, T.; Kadmon, M.; Ehehalt, R.; Stremmel, W.; Zawierucha, A. Intestinal cytomegalovirus infection in patients hospitalized for exacerbation of inflammatory bowel disease: A 10-year tertiary referral center experience. Eur. J. Gastroenterol. Hepatol. 2015, 27, 712–720. [Google Scholar] [CrossRef]
- Shukla, T.; Singh, S.; Tandon, P.; McCurdy, J.D. Corticosteroids and Thiopurines, But Not Tumor Necrosis Factor Antagonists, are Associated With Cytomegalovirus Reactivation in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2016. [Google Scholar] [CrossRef]
- Inoue-Toyoda, M.; Kato, K.; Nagata, K.; Yoshikawa, H. Glucocorticoids facilitate the transcription from the human cytomegalovirus major immediate early promoter in glucocorticoid receptor- and nuclear factor-I-like protein-dependent manner. Biochem. Biophys. Res. Commun. 2015, 458, 180–185. [Google Scholar] [CrossRef]
- Van Damme, E.; Sauviller, S.; Lau, B.; Kesteleyn, B.; Griffiths, P.; Burroughs, A.; Emery, V.; Sinclair, J.; Van Loock, M. Glucocorticosteroids trigger reactivation of human cytomegalovirus from latently infected myeloid cells and increase the risk for HCMV infection in D+R+ liver transplant patients. J. Gen. Virol. 2015, 96, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dou, Y.; Yang, H.; Ni, A.; Zhang, R.; Qian, J. Alteration of glucocorticoid receptors and exacerbation of inflammation during lytic cytomegalovirus infection in THP-1 cells. FEBS Open Bio 2017, 7, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Ohta, M.; Ohkura, T.; Ando, T.; Ohmiya, N.; Niwa, Y.; Goto, H. Cytomegalovirus infection in severe ulcerative colitis patients undergoing continuous intravenous cyclosporine treatment in Japan. World J. Gastroenterol. 2007, 13, 754–760. [Google Scholar] [CrossRef]
- Pillet, S.; Jarlot, C.; Courault, M.; Del Tedesco, E.; Chardon, R.; Saint-Sardos, P.; Presles, E.; Phelip, J.-M.; Berthelot, P.; Pozzetto, B.; et al. Infliximab Does Not Worsen Outcomes During Flare-ups Associated with Cytomegalovirus Infection in Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2015, 21, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, E.; Bracco, C.; Biancheri, P.; Falcetta, A.; Martini, M.B.; Melchio, R.; Fenoglio, L. Fever During Anti-integrin Therapy: New Immunodeficiency. Eur. J. Case Rep. Intern. Med. 2020, 7, 001288. [Google Scholar] [CrossRef] [PubMed]
- Hommel, C.; Pillet, S.; Rahier, J.-F. Comment on: “Resolution of CMV Infection in the Bowel on Vedolizumab Therapy”. J. Crohns Colitis 2019. [Google Scholar] [CrossRef]
- Rawa-Gołębiewska, A.; Lenarcik, M.; Zagórowicz, E. Resolution of CMV Infection in the Bowel on Vedolizumab Therapy. J. Crohns Colitis 2019, 13, 1234–1235. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Panés, J.; D’Haens, G.R.; Sands, B.E.; Su, C.; Moscariello, M.; Jones, T.; Pedersen, R.; Friedman, G.S.; Lawendy, N.; et al. Safety of Tofacitinib for Treatment of Ulcerative Colitis, Based on 4.4 Years of Data From Global Clinical Trials. Clin. Gastroenterol. Hepatol. 2019, 17, 1541–1550. [Google Scholar] [CrossRef]
- Egli, A.; Kumar, D.; Broscheit, C.; O’Shea, D.; Humar, A. Comparison of the Effect of Standard and Novel Immunosuppressive Drugs on CMV-Specific T-Cell Cytokine Profiling. Transplantation 2013, 95, 448–455. [Google Scholar] [CrossRef]
- Weinhold, K.J.; Bukowski, J.F.; Brennan, T.V.; Noveck, R.J.; Staats, J.S.; Lin, L.; Stempora, L.; Hammond, C.; Wouters, A.; Mojcik, C.F.; et al. Reversibility of peripheral blood leukocyte phenotypic and functional changes after exposure to and withdrawal from tofacitinib, a Janus kinase inhibitor, in healthy volunteers. Clin. Immunol. 2018, 191, 10–20. [Google Scholar] [CrossRef]
- Amiot, A.; Filippi, J.; Abitbol, V.; Cadiot, G.; Laharie, D.; Serrero, M.; Altwegg, R.; Bouhnik, Y.; Peyrin-Biroulet, L.; Gillettan, C. Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: A GETAID multicentre real-world cohort study. Aliment Pharmacol Ther. 2020, 51, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Matsui, T.; Matake, H.; Sakurai, T.; Yamamoto, J.; Kikuchi, Y.; Yorioka, M.; Tsuda, S.; Yao, T.; Yao, S.; et al. Intractable ulcerative colitis caused by cytomegalovirus infection: A prospective study on prevalence, diagnosis, and treatment. Dis. Colon Rectum 2003, 46, S59–S65. [Google Scholar] [CrossRef]
- Kim, J.J.; Simpson, N.; Klipfel, N.; Debose, R.; Barr, N.; Laine, L. Cytomegalovirus infection in patients with active inflammatory bowel disease. Dig. Dis. Sci. 2010, 55, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kato, J.; Kuriyama, M.; Hiraoka, S.; Kuwaki, K.; Yamamoto, K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J. Gastroenterol. 2010, 16, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, T.; Kato, J.; Hiraoka, S.; Suzuki, H.; Nakarai, A.; Hirakawa, T.; Akita, M.; Takahashi, S.; Harada, K.; Okada, H.; et al. Long-term follow-up of ulcerative colitis patients treated on the basis of their cytomegalovirus antigen status. World J. Gastroenterol. 2014, 20, 509–517. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.-H.; Kim, J.S.; Jeong, S.Y.; Park, S.J.; Cheon, J.H.; Ye, B.D.; Jung, S.-A.; Park, Y.S.; Choi, C.H.; et al. Long-term outcomes of cytomegalovirus reactivation in patients with moderate to severe ulcerative colitis: A multicenter study. Gut Liver 2014, 8, 643–647. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yoshida, Y. What are the factors that affect hospitalization and surgery for aggravation of ulcerative colitis? Eur. J. Gastroenterol. Hepatol. 2014, 26, 282–287. [Google Scholar] [CrossRef]
- McCurdy, J.D.; Jones, A.; Enders, F.T.; Killian, J.M.; Loftus, E.V.; Smyrk, T.C.; Bruining, D.H. A model for identifying cytomegalovirus in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2015, 13, 131–137. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Racca, F.; Scudeller, L.; Piralla, A.; Formagnana, P.; Pozzi, L.; Betti, E.; Vanoli, A.; Riboni, R.; Kruzliak, P.; et al. Differential cellular localization of Epstein-Barr virus and human cytomegalovirus in the colonic mucosa of patients with active or quiescent inflammatory bowel disease. Immunol. Res. 2016, 64, 191–203. [Google Scholar] [CrossRef]
- Okahara, K.; Nagata, N.; Shimada, T.; Joya, A.; Hayashida, T.; Gatanaga, H.; Oka, S.; Sakurai, T.; Uemura, N.; Akiyama, J. Colonic cytomegalovirus detection by mucosal PCR and antiviral therapy in ulcerative colitis. PLoS ONE 2017, 12, e0183951. [Google Scholar] [CrossRef]
- Henmi, Y.; Kakimoto, K.; Inoue, T.; Nakazawa, K.; Kubota, M.; Hara, A.; Mikami, T.; Naka, Y.; Hirata, Y.; Hirata, Y.; et al. Cytomegalovirus infection in ulcerative colitis assessed by quantitative polymerase chain reaction: Risk factors and effects of immunosuppressants. J. Clin. Biochem. Nutr. 2018, 63, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kishore, J.; Ghoshal, U.; Ghoshal, U.C.; Krishnani, N.; Kumar, S.; Singh, M.; Ayyagari, A. Infection with cytomegalovirus in patients with inflammatory bowel disease: Prevalence, clinical significance and outcome. J. Med. Microbiol. 2004, 53, 1155–1160. [Google Scholar] [CrossRef]
- Maconi, G.; Colombo, E.; Zerbi, P.; Sampietro, G.M.; Fociani, P.; Bosani, M.; Cassinotti, A.; Casini, V.; Russo, A.; Ardizzone, S.; et al. Prevalence, detection rate and outcome of cytomegalovirus infection in ulcerative colitis patients requiring colonic resection. Dig. Liver Dis. 2005, 37, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Watanabe, T.; Hata, K.; Shinozaki, M.; Yokoyama, T.; Nagawa, H. Cytomegalovirus infection in ulcerative colitis. Scand. J. Gastroenterol. 2006, 41, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, A.; Okamoto, H.; Suda, T.; Ajioka, Y.; Hatakeyama, K. Clinicopathologic characteristics of clinically relevant cytomegalovirus infection in inflammatory bowel disease. J. Gastroenterol. 2007, 42, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Nakase, H.; Matsuura, M. Letter: Mucosal PCR for cytomegalovirus in refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2012, 36, 811–812. [Google Scholar] [CrossRef]
- Kopylov, U.; Sasson, G.; Geyshis, B.; Oikawa, M.T.; Barshack, I.; Eliakim, R.; Ben-Horin, S. Cytomegalovirus positive ulcerative colitis: A single center experience and literature review. World J. Gastrointest. Pathophysiol. 2013, 4, 18–23. [Google Scholar] [CrossRef]
- Kim, J.W.; Boo, S.-J.; Ye, B.D.; Kim, C.L.; Yang, S.-K.; Kim, J.; Kim, S.A.; Park, S.H.; Park, S.-K.; Yang, D.-H.; et al. Clinical utility of cytomegalovirus antigenemia assay and blood cytomegalovirus DNA PCR for cytomegaloviral colitis patients with moderate to severe ulcerative colitis. J. Crohns Colitis 2014, 8, 693–701. [Google Scholar] [CrossRef]
- Olaisen, M.; Rydning, A.; Martinsen, T.C.; Nordrum, I.S.; Mjønes, P.; Fossmark, R. Cytomegalovirus infection and postoperative complications in patients with ulcerative colitis undergoing colectomy. Scand. J. Gastroenterol. 2014, 49, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Minami, N.; Yoshino, T.; Matsuura, M.; Koshikawa, Y.; Yamada, S.; Toyonaga, T.; Madian, A.; Honzawa, Y.; Nakase, H. Tacrolimus or infliximab for severe ulcerative colitis: Short-term and long-term data from a retrospective observational study. BMJ Open Gastroenterol. 2015, e000021. [Google Scholar] [CrossRef] [PubMed]
- Schenk, W.; Klugmann, T.; Borkenhagen, A.; Klecker, C.; Dietel, P.; Kirschner, R.; Schneider, E.; Bruns, T.; Stallmach, A.; Teich, N. The detection of the cytomegalovirus DNA in the colonic mucosa of patients with ulcerative colitis is associated with increased long-term risk of proctocolectomy: Results from an outpatient IBD clinic. Int. J. Colorectal Dis. 2019, 34, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.M.; Nassar, M.I. Acute cytomegalovirus infection is a risk factor in refractory and complicated inflammatory bowel disease. Dig. Dis. Sci. 2009, 54, 2456–2462. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.-H.; Kim, J.S.; Cheon, J.H.; Ye, B.D.; Jung, S.-A.; Park, Y.S.; Choi, C.H.; Jang, B.I.; Han, D.S.; et al. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: A prospective multicenter study. J. Clin. Gastroenterol. 2012, 46, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Ikeya, K.; Watanabe, F.; Abe, J.; Maruyama, Y.; Ohata, A.; Teruyuki, S.; Sugimoto, K.; Hanai, H. Looking for endoscopic features of cytomegalovirus colitis: A study of 187 patients with active ulcerative colitis, positive and negative for cytomegalovirus. Inflamm. Bowel Dis. 2013, 19, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, C.; Kwon, J.-E.; Hwang, S.W.; Kim, S.G.; Kim, J.S.; Jung, H.C.; Im, J.P. Usefulness of the cytomegalovirus antigenemia assay in patients with ulcerative colitis. Intest. Res. 2015, 13, 50–59. [Google Scholar] [CrossRef][Green Version]
- Ormeci, A.C.; Akyuz, F.; Baran, B.; Soyer, O.M.; Gokturk, S.; Onel, M.; Onel, D.; Agacfidan, A.; Demirci, M.; Yegen, G.; et al. Steroid-refractory inflammatory bowel disease is a risk factor for CMV infection. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 858–865. [Google Scholar]
- Hissong, E.; Chen, Z.; Yantiss, R.K. Cytomegalovirus reactivation in inflammatory bowel disease: An uncommon occurrence related to corticosteroid dependence. Mod. Pathol. 2019, 32, 1210–1216. [Google Scholar] [CrossRef]
- McCurdy, J.D.; Enders, F.T.; Khanna, S.; Bruining, D.H.; Jones, A.; Killian, J.M.; Tariq, R.; Smyrk, T.C.; Loftus, E.V. Increased Rates of Clostridium difficile Infection and Poor Outcomes in Patients with IBD with Cytomegalovirus. Inflamm. Bowel Dis. 2016, 22, 2688–2693. [Google Scholar] [CrossRef]
- Xu, H.; Tang, H.; Xu, T.; Xiao, M.; Li, J.; Tan, B.; Yang, H.; Lv, H.; Li, Y.; Qian, J. Retrospective analysis of Clostridium difficile infection in patients with ulcerative colitis in a tertiary hospital in China. BMC Gastroenterol. 2019, 19, 3. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Xu, T.; Xiao, M.; Tang, H.; Wu, D.; Tan, B.; Li, J.; Yang, H.; Lv, H.; et al. Case-Control Study of Inflammatory Bowel Disease Patients with and without Clostridium difficile Infection and Poor Outcomes in Patients Coinfected with C. difficile and Cytomegalovirus. Dig. Dis. Sci. 2018, 63, 3074–3083. [Google Scholar] [CrossRef]
- Grossberg, L.B.; Ezaz, G.; Grunwald, D.; Cohen, J.; Falchuk, K.R.; Feuerstein, J.D. A National Survey of the Prevalence and Impact of Cytomegalovirus Infection Among Hospitalized Patients With Ulcerative Colitis. J. Clin. Gastroenterol. 2016. [Google Scholar] [CrossRef]
- Shukla, T.; Singh, S.; Loftus, E.V.; Bruining, D.H.; McCurdy, J.D. Antiviral Therapy in Steroid-refractory Ulcerative Colitis with Cytomegalovirus: Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R. Letter: Cytomegalovirus infection in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 42, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Onodera, K. Targeting cytomegalovirus during ulcerative colitis flare-ups. Expert Rev. Gastroenterol. Hepatol. 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Yaari, S.; Stoff, R.; Caplan, O.; Wolf, D.G.; Israeli, E. Diagnosis of Cytomegalovirus Infection during Exacerbation of Ulcerative Colitis. Digestion 2017, 96, 142–148. [Google Scholar] [CrossRef]
- Beswick, L.; Ye, B.; van Langenberg, D.R. Toward an Algorithm for the Diagnosis and Management of CMV in Patients with Colitis. Inflamm. Bowel Dis. 2016, 22, 2966–2976. [Google Scholar] [CrossRef]
- Ahmed, I.; Kassem, W.; Salam, Y.; Furnari, M.; Mehta, T. Outcome of Cytomegalovirus Colitis in Inflammatory Bowel Disease with Different Regimes of Ganciclovir. Middle East. J. Dig. Dis. 2018, 10, 220–229. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Kopylov, U.; Papamichael, K.; Katsanos, K.; Waterman, M.; Bar-Gil Shitrit, A.; Boysen, T.; Portela, F.; Peixoto, A.; Szilagyi, A.; Silva, M.; et al. Impact of Infliximab and Cyclosporine on the Risk of Colectomy in Hospitalized Patients with Ulcerative Colitis Complicated by Cytomegalovirus-A Multicenter Retrospective Study. Inflamm. Bowel Dis. 2017. [Google Scholar] [CrossRef]
- Hommel, C.; Roblin, X.; Brichet, L.; Bihin, B.; Pillet, S.; Rahier, J.-F. P579 Risk of CMV reactivation in UC patients with previous history of CMV infection following infliximab or vedolizumab treatments. J. Crohns Colitis 2018, 12, S400. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Feagan, B.; Löfberg, R.; Hibi, T.; Wang, T.; Gustofson, L.-M.; Wong, C.J.; Vandervoort, M.K.; Hanauer, S.; et al. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology 2008, 135, 400–409. [Google Scholar] [CrossRef]
- Yoshino, T.; Nakase, H.; Matsuura, M.; Matsumura, K.; Honzawa, Y.; Fukuchi, T.; Watanabe, K.; Murano, M.; Tsujikawa, T.; Fukunaga, K.; et al. Effect and safety of granulocyte-monocyte adsorption apheresis for patients with ulcerative colitis positive for cytomegalovirus in comparison with immunosuppressants. Digestion 2011, 84, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, T.; Nakase, H.; Matsuura, M.; Yoshino, T.; Toyonaga, T.; Ohmori, K.; Ubukata, S.; Ueda, A.; Eguchi, T.; Yamashita, H.; et al. Effect of intensive granulocyte and monocyte adsorptive apheresis in patients with ulcerative colitis positive for cytomegalovirus. J. Crohns Colitis 2013, 7, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Bonnaud, G.; Haennig, A.; Levy, J.; Sigur, N.; Ledit, A.; Cabarrot, P.; Faure, P.; Auzimour, C.; El Atmani, A.; Hebuterne, X.; et al. Implementation of the French national consensus for the management of ulcerative colitis into clinical practice. Dig. Liver Dis. 2016, 48, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Whaley, K.G.; Rosen, M.J. Contemporary Medical Management of Acute Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 25, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, S.; Watanabe, K.; Nishida, Y.; Yamagami, H.; Yukawa, T.; Otani, K.; Nagami, Y.; Tanaka, F.; Taira, K.; Kamata, N.; et al. Combined Infection of Human Herpes Viruses: A Risk Factor for Subsequent Colectomy in Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1307–1315. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef]

| Method | Number of patients | Threshold | Reference |
|---|---|---|---|
| IHC | |||
| 80 UC | 0.01 cells/mm2 | [82] | |
| 114 UC | 1 inclusion body in histology, positive specific IHC staining for CMV in 10–20 fields of colonic biopsies | [55] | |
| 13 CD, 18 UC | semiquantitative ranging from 1 to 3 (1=rare, 2=common and easy to find, and 3=numerous cells positive for CMV) | [83] | |
| 30 UC, 8 CD | semiquantitative ranging from 1 to 3 (1 = rare, 2 = common and easy to find, and 3 = numerous cells positive for CMV) | [84] | |
| 95 UC | ≥ 5 IHC positive cells/biopsy | [85] | |
| 46 UC | colectomy was only associated with > 2 positive cells/biopsy | [86] | |
| 257 UC | semiquantitative low grade (1 to 4 inclusion bodies per section) high grade (at least 5 inclusion bodies per section) | [70] | |
| qPCR | |||
| 30 UC | 10 copies/µg of DNA | [87] | |
| 42 UC | 10 copies/mg of tissue | [44] | |
| 40 IBD | > 1000 copies/100,000 cells | [80] | |
| 149 UC | 5 IU/100,000 cells | [81] | |
| 92 UC, 9 CD | ≥ 316 copies/mg of DNA | [64] | |
| 75 IBD | 10,000 copies/µg of DNA | [88] | |
| 103 UC, 30 CD | > 600 copies/100 000 cells of tissue | [45] | |
| 47 IBD | > 250 copies/mg of tissue | [42] |
| Treatment | Route of Administration | Mechanism of Action | Recommendations |
|---|---|---|---|
| Salicylates | |||
| 5-ASA | Oral | Decrease inflammation by blocking cyclooxygenase and inhibiting the production of prostaglandins. Inhibition of the NFкB pathway (decrease in the production of pro-inflammatory cytokines). | Initial treatment of mild to moderate UC should be treated with oral 5-ASA. Oral 5-ASA should be the standard maintenance medical therapy in ulcerative colitis. |
| Corticosteroid therapy | |||
| Oral IV | Decrease of chemotaxis towards inflammatory sites. Inhibition of microbicidal and phagocytosis functions. Inhibition of T lymphocyte functions | Moderate to severe ulcerative colitis should be treated with oral corticosteroids such as prednisolone weaning over 6–8 weeks. Patients with acute severe UC should be treated with high-dose IV corticosteroids such as methylprednisolone or hydrocortisone. | |
| Thiopurines | |||
| Azathioprine | Oral | Inhibition of proliferation of T and B lymphocytes. Decreased antibody production. Myelosuppression | Ulcerative colitis patients on maintenance therapy with high-dose 5-ASA, who required two or more courses of corticosteroids in the past year, or who become corticosteroid-dependent or refractory, require treatment with thiopurine. |
| Monoclonal antibodies | |||
| Monoclonal antibodies anti-TNF-α | Inhibition of T lymphocytes differentiation. Induction of Treg lymphocytes. Decrease of macrophages activation. Diminution of NF-κβ pathways. Barrier improvement | Ulcerative colitis patients on maintenance therapy with high-dose 5-ASA, who required two or more courses of corticosteroids in the past year, or who become corticosteroid-dependent or refractory, require treatment with anti-TNF-α. | |
| Infliximab | IV | Patients with acute severe UC failing to respond by day 3, as judged by a suitable scoring system, should be treated with rescue therapy in the form of intravenous infliximab for patients who have not previously failed thiopurine therapy | |
| SC SC | ||
| anti-integrin monoclonal antibody | |||
| IV | Inhibition of the adhesion of the T lymphocytes expressing the α4β7 to the molecule-1 of cellular adhesion of mucosal addressin (MAdCAM-1) mainly expressed on the intestinal endothelial cells. Decrease intestinal recruitment of T lymphocytes. | Ulcerative colitis patients on maintenance therapy with high-dose 5-ASA, who required two or more courses of corticosteroids in the past year, or who become corticosteroid-dependent or refractory, require treatment with Vedolizumab. |
| Othertreatments | |||
| Cyclosporin | IV | Inhibitory effects on TH lymphocyte production of IL-2, and IFN-γ. Diminution of cytokines production (IL-3, 4 and 5, TNF-α). Diminution of T lymphocytes and B lymphocytes activation. | Patients with acute severe UC failing to respond by day 3, as judged by a suitable scoring system, should be treated with rescue therapy in the form of ciclosporin for patients who have not previously failed thiopurine therapy |
| Tofacitinib JAK inhibitor | Oral | Diminution of immune and inflammatory responses of JAK/STAT pathway. | Induction and maintenance of remission of ulcerative colitis in patients where anti-TNF treatment has failed. Ulcerative colitis patients on maintenance therapy with high-dose 5-ASA, who required two or more courses of corticosteroids in the past year, or who become corticosteroid-dependent or refractory, require treatment with tofacitinib. |
| Methods Used | Number of Patients Analyzed | Criteria Observed in Case of CMV Colitis when Compared to CMV Negative | Criteria and Publication |
|---|---|---|---|
| Severe disease | |||
| pp65 antigenemia, IHC | 47 UC | Higher endoscopic score | [122] |
| IHC | 122 UC | Hospitalization | [123] |
| pp65 antigenemia | 73 UC | Ulcers | [124] |
| CMV-pp65 antigenemia assay | 118 UC | Delay to clinical remission | [125] |
| Heterogeneous (serology, histology, IHC, PCR) | 72 UC | Higher disease flare-ups rate | [126] |
| Antigenemia, histology | 222 UC | Hospitalization | [127] |
| Histology, pp65 antigenemia, IHC, PCR (in tissue or blood) | 166 UC and 131 CD | Longer hospital stays | [108] |
| Histology, ISH, IHC | 45 UC and 21 CD | Endoscopic ulcers | [128] |
| qPCR | 40 IBD | Refractory disease | [80] |
| Histology, IHC | 149 UC | Higher Mayo score and need for rescue therapy | [90] |
| IHC, qPCR | 50 IBD | Refractory disease | [129] |
| Histology, IHC | 56 UC | Longer hospital stays | [50] |
| Histology, IHC | 257 UC | Deep ulcerations and higher disease activity, global poor outcome | [70] |
| qPCR in tissue | 46 UC | Higher endoscopic score | [130] |
| qPCR in tissue | 86 UC | Higher endoscopic score | [131] |
| PCR, IHC | 239 UC | More severe disease | [63] |
| Increased mortality | |||
| PCR, Serology (IgM), Histology | 61 UC and 2 CD | Surgery, fatal outcome | [132] |
| IHC | 95 UC | Lower hemoglobin and albumin levels, more intense histological inflammation | [85] |
| Not described | 406,118 UC patients | Mortality, longer hospital stays, hospital charges | [1] |
| Surgery | |||
| PCR, Serology (IgM), Histology | 61 UC and 2 CD | Colectomy | [132] |
| Histology, IHC | 77 UC | CMV found in surgical specimens of colectomy | [133] |
| Histology, IHC | 126 UC | Colectomy | [134] |
| IHC | 34 UC and 16 CD | Colectomy | [135] |
| Heterogeneous (serology, histology, IHC, PCR) | 23 UC | Colectomy | [113] |
| Histology, IHC | 26 UC and 17 CD | Colectomy | [72] |
| IHC | 13 CD and 18 UC | Colectomy | [83] |
| qPCR in tissue | 17 UC | Colectomy | [136] |
| IHC | 13 UC | Colectomy | [137] |
| Heterogeneous (serology, histology, IHC, PCR) | 72 UC | Colectomy | [126] |
| IHC, pp65 antigenemia | 229 UC | Colectomy | [138] |
| IHC | 77 UC | Surgical complications | [139] |
| Histology, IHC, qPCR in tissue | 29 UC | Colectomy | [140] |
| IHC | 95 UC | Colectomy | [85] |
| qPCR in tissue | 108 UC | Protocolectomy | [141] |
| Histology, IHC | 149 UC | Colectomy | [90] |
| Histology, IHC | 56 UC | Colectomy | [50] |
| IHC | 46 UC | Colectomy | [86] |
| Not described | 406,118 UC patients | Colectomy | [1] |
| Resistance to immunosuppressive therapy | |||
| pp65 antigenemia, IHC | 47 UC | Steroid resistance | [122] |
| IHC | 80 UC | Steroid resistance | [82] |
| Histology, IHC | 77 UC | Steroid resistance | [133] |
| Histology, IHC | 126 UC | Refractory disease | [134] |
| IHC | 34 UC and 16 CD | Steroid resistance | [135] |
| Histology, IHC | 49 UC and 23 CD | Steroid resistance | [142] |
| qPCR in tissue | 42 UC | Steroid resistance Resistance to successive therapeutic lines | [44] |
| Histology, IHC, PCR | 72 UC | Steroid resistance | [143] |
| pp65 antigenemia | 187 UC | Steroid resistance | [144] |
| pp65 antigenemia | 43 UC | Steroid resistance | [145] |
| qPCR in tissue | 24 UC and 16 CD | Refractory disease | [80] |
| Histology, ISH, IHC | 45 UC and 21 CD | Refractory disease | [128] |
| qPCR | 35 UC | Steroid resistance and resistance to immunosuppressive treatment | [146] |
| qPCR | 149 UC | Steroid resistance Resistance to successive therapeutic lines | [81] |
| Histology and IHC | 56 UC | Steroid resistance | [50] |
| qPCR in tissue | 46 UC | Higher corticosteroid doses | [130] |
| Histology and IHC | 99 CD and 169 UC | Steroid dependence | [147] |
| Tissue PCR | 52 UC patients | Steroid resistance | [43] |
| IHC or qPCR | 80 UC patients | Steroid resistance | [96] |
| Country | Method for CMV detection | Population Studied | Predictive factors identified | OR in Multivariate Analysis | Publication |
|---|---|---|---|---|---|
| Japan | qPCR | 86 patients with UC exacerbation | Age | 1.08 [1.03–1.14] | [131] |
| Endoscopic score | 2.8 [0.65–12] | ||||
| Combined immunosuppressants | 7.44 [1.00–55.30] | ||||
| Germany | PCR in blood or PCR in tissue or IHC in tissue | 239 UC patients | Disease activity (partial Mayo score) | 1.37 [1.09–1.72] | [63] |
| Use of steroids | 2.43 [1.44–4.03] | ||||
| Israel | qPCR in tissue | 28 UC patients | Use of steroids | 14.5 [1.07–198] | [155] |
| Fever > 38 °C | 20 [1.2–330] | ||||
| Korea | H&E and IHC | 149 patients | Use of steroids | 3.30 [1.33–8.19] | [90] |
| High Mayo score | 1.58 [1.05–2.38] | ||||
| Japan | Agpp65 or H&E or IHC | 149 UC | Use of systemic steroid dose at dose > 400 mg for 4 weeks before admission | 26.70 [5.85–121.87] | [91] |
| Punched-out ulcer | 12.67 [4.21–38.14] | ||||
| Germany | IHC or tissue PCR | 297 IBD patients | Age > 30 years | 14.29 [2.89–118.57] | [108] |
| Disease duration > 60 months | 7.69 [1.80–45.41] | ||||
| Blood leucocytes < 11/nl | 4.49 [1.15–21.79] | ||||
| Immunosuppressive therapy at admission | 6.73 [1.67–35.63] | ||||
| United States | H&E and IHC or in situ hybridation | 68 IBD patients | Age > 30 years | 2.26 [1.02–5.03] | [128] |
| Use of immunomodulators | 1.95 [1.05–3.62] | ||||
| Refractory disease | 4.24 [2.21–8.11] | ||||
| Germany | qPCR | 47 IBD patients | Use of steroid | 7.1 [1.7–29.9] | [42] |
| Use of calcineurin inhibitors | 21.3 [2.4–188.7] | ||||
| Use of 2 concurrent lines of immunosuppressive therapy | 13.4 [3.2–56.1] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jentzer, A.; Veyrard, P.; Roblin, X.; Saint-Sardos, P.; Rochereau, N.; Paul, S.; Bourlet, T.; Pozzetto, B.; Pillet, S. Cytomegalovirus and Inflammatory Bowel Diseases (IBD) with a Special Focus on the Link with Ulcerative Colitis (UC). Microorganisms 2020, 8, 1078. https://doi.org/10.3390/microorganisms8071078
Jentzer A, Veyrard P, Roblin X, Saint-Sardos P, Rochereau N, Paul S, Bourlet T, Pozzetto B, Pillet S. Cytomegalovirus and Inflammatory Bowel Diseases (IBD) with a Special Focus on the Link with Ulcerative Colitis (UC). Microorganisms. 2020; 8(7):1078. https://doi.org/10.3390/microorganisms8071078
Chicago/Turabian StyleJentzer, Alexandre, Pauline Veyrard, Xavier Roblin, Pierre Saint-Sardos, Nicolas Rochereau, Stéphane Paul, Thomas Bourlet, Bruno Pozzetto, and Sylvie Pillet. 2020. "Cytomegalovirus and Inflammatory Bowel Diseases (IBD) with a Special Focus on the Link with Ulcerative Colitis (UC)" Microorganisms 8, no. 7: 1078. https://doi.org/10.3390/microorganisms8071078
APA StyleJentzer, A., Veyrard, P., Roblin, X., Saint-Sardos, P., Rochereau, N., Paul, S., Bourlet, T., Pozzetto, B., & Pillet, S. (2020). Cytomegalovirus and Inflammatory Bowel Diseases (IBD) with a Special Focus on the Link with Ulcerative Colitis (UC). Microorganisms, 8(7), 1078. https://doi.org/10.3390/microorganisms8071078







