Abstract
The emergence of biofilm-forming, multi-drug-resistant (MDR) Proteus mirabilis infections is a serious threat that necessitates non-antibiotic therapies. Antibiotic susceptibility and biofilm-forming activity of P. mirabilis isolates from urine samples were assessed by disc diffusion and crystal violet assays, respectively. Antimicrobial activities of probiotic Lactobacilli were evaluated by agar diffusion. Antibiofilm and anti-adherence activities were evaluated by crystal violet assays. While most P. mirabilis isolates were antibiotic-resistant to varying degrees, isolate P14 was MDR (resistant to ceftazidime, cefotaxime, amoxicillin-clavulanic acid, imipenem, ciprofloxacin, and amikacin) and formed strong biofilms. Cultures and cell-free supernatants of Lactobacillus casei and Lactobacillus reuteri exhibited antimicrobial and antibiofilm activities. The 1/16 concentration of untreated supernatants of L. casei and L. reuteri significantly reduced mature biofilm formation and adherence of P14 by 60% and 72%, respectively (for L. casei), and by 73% each (for L. reuteri). The 1/8 concentration of pH-adjusted supernatants of L. casei and L. reuteri significantly reduced mature biofilm formation and adherence of P14 by 39% and 75%, respectively (for L. casei), and by 73% each (for L. reuteri). Scanning electron microscopy (SEM) confirmed eradication of P14’s biofilm by L. casei. L. casei and L. reuteri could be utilized to combat Proteus-associated urinary tract infections.
1. Introduction
Proteus mirabilis is one of the most common pathogens associated with urinary tract infections (UTI) [1,2]. In addition, it can cause surgical wound infections, biliary tract infections, wound infections, and nosocomial infections [3]. It is part of the normal gut flora and can cause opportunistic infections in immunocompromised and elderly patients [3]. The emergence of antibiotic resistance in clinical isolates of P. mirabilis is a major healthcare issue [4,5]. P. mirabilis poses a major challenge in infection management as it produces AmpC β-lactamases and extended spectrum β-lactamases [6]. Furthermore, it can develop complex biofilms with accumulated layers of polysaccharides in which sessile cells are embedded, which adds to the severity of the infection [7]. The severity, chronicity, and dissemination of Proteus infections have been mainly attributed to its ability to form biofilms [7].
Probiotics are living microorganisms that belong to Lactobacillus and Bifidobacterium genera [8,9]. The intestinal probiotic Lactobacillus strains have been recognized for their antimicrobial activities against enteric bacterial pathogens [10]. Different groups showed that Lactobacillus casei produces bacteriocins and anti-adherence biosurfactant proteins against Staphylococcus aureus, Bacillus subtilis, and Micrococcus roseus [11,12]. Human gastrointestinal Lactobacillus reuteri produces a broad-spectrum antimicrobial called reuterin, which possesses activity against Gram-positive and Gram-negative enteric pathogens [13]. Probiotic Lactobacilli can also suppress virulence and dissemination of infectious pathogens [14]. This is typically accomplished through the production of organic acids and antimicrobials, such as bacteriocins, lipopeptides, and surface proteins [11,15,16].
We have previously shown that probiotic lactobacilli inhibited growth, biofilm formation, and gene expression of Streptococcus mutans [17]. The anti-infective and anti-colonization properties of probiotic lacobacilli are of paramount importance in combating various bacterial infections [18,19]. Due to their antimicrobial properties, probiotics may be considered for treatment and prevention of infectious diseases caused by oral, enteric, and urogenital pathogens [20,21]. Lactobacilli have been specifically shown to prevent recurrent urinary tract infections [22]. This indicates their promise as anti-Proteus agents. Given the antibiotic resistance problem, it is important to develop Lactobacillus-based approaches to combat Proteus mirabilis-induced urinary tract infections and catheter-associated infections.
In order to be able to better manage P. mirabilis infections, we investigated the potential inhibitory activities of L. casei and L. reuteri on bacterial growth, mature biofilm formation, and adhesion properties of multi-drug-resistant (MDR) Proetus mirabilis clinical isolates.
We tested pH-adjusted supernatants of Lactobacillus, which is a commonly used approach in Lactobacillus probiotic studies to reduce the acidity of the Lactobacillus supernatants and thus assess if the antimicrobial activity of Lactobacillus products is pH-dependent [23,24,25,26,27]. This is significant given that studies showed that the acidic pH (due to lactic acid secretion) contributed only a small part of the activity [28], or did not contribute any additional activity (activity was pH-independent) [29]. In our study, the pH adjustment was important to reveal how much of the anti-Proteus activity of probiotic Lactobacillus supernatants was related to non-acidic products and whether the antimicrobial activity of the supernatant was pH-dependent.
2. Materials and Methods
2.1. Microorganism and Growth Conditions
P. mirabilis isolates were obtained from urine samples collected from several hospitals in Madinah, KSA (Ohud Hospital, King Fahad Hospital, Al-Ansar Hospital, and Madina Maternity and Children Hospital). P. mirabilis isolates were identified using biochemical assays and confirmed as P. mirabilis using the VITEK compact system. Assays included Gram stain, growth characteristics on MacConkey agar, CLED agar, and triple sugar iron media [30].
Two probiotic strains (L. casei DSM 20011 and L. reuteri DSM 20016) were purchased from MERCEN, Egypt. Lactobacillus isolates were grown on deMan, Rogosa and Sharpe medium (MRS) and were anaerobically (AnaeroGen 2.5 L sachets in 2.5 L AnaeroJar AG25, Oxoid Ltd., Hampshire, UK) incubated at 37 °C [31].
Ethical approvals were obtained from the Institutional Review Boards of the hospitals, ethics committees of the college of pharmacy, Taibah University, KSA and Faculty of Pharmacy, Mansoura University, Egypt. Ethical approval code is TUCODRE/20151025/ALQAIDI (in October 2015).
2.2. Antimicrobial Susceptibility of P. mirabilis Isolates
P. mirabilis isolates were tested for their resistance to different antimicrobials using the disk diffusion method [32]. The antimicrobials used were amoxicillin-clavulanic acid (AMC; 30 mg), imipenem (IMP; 10 mg), cefoxitin (FOX; 30 mg), ceftazidime (CAZ; 30 mg), ciprofloxacin (CIP; 5 mg), cefotaxime; (CTX; 30 mg), and amikacin (AK; 30 mg) (Bioanalyse, Ankara, Turkey).
2.3. Detection of Biofilm Formation by P. mirabilis Isolates
The mature biofilm of P. mirabilis isolates was formed in flat-bottomed 96-well microtiter plates. The overnight culture of each isolate was adjusted to 0.5 McFarland (1.5 × 108 CFU/mL) using Muller Hinton broth. Then, 200 µL of the diluted cultures was distributed in each well and incubated at 37 °C for 48 h. The planktonic cells were removed and the attached cells were gently washed twice with sterile physiological saline. Then, 200 µL of methanol (99%) was added to each well and retained for 15 min to fix the sessile cells. The methanol was discarded, and the plate was left until complete dryness. To stain the adherent cells, 200 µL of 2% w/v crystal violet solution was added to each well and was left for 20 min. The wells were washed gently and left to dry. Glacial acetic acid (200 µL; 33% w/v) was added to release the bound dye and the absorbance was measured at OD540 nm using ELISA microplate reader (MR-960, Perlong Medical Equipment Co. Ltd., Nanjing, China). Depending on the optical density (OD) generated by the bacterial biofilms, the tested P. mirabilis isolates were classified into non-biofilm producers, weak biofilm producers, moderate biofilm producers, and strong biofilm producers [33]. The cut-off OD of the negative control (ODc) was determined by adding the mean of the negative control to three standard deviations of it. The lack of biofilm formation is indicated by ODs ≤ ODc for the tested isolates. Weak biofilm production is indicated by ODc < OD ≤ (2 ODc). Moderate biofilm formation is indicated by (2 ODc) < O.D. ≤ (4 ODc). Strong biofilm formation is indicated by (4 ODc) < O.D. Tests were conducted in triplicates.
2.4. Preparation of Cell-Free Supernatant from Lactobacillus Strains
In order to prepare the Lactobacillus culture, the MRS medium was inoculated with Lactobacillus strains with inoculum size 1% v/v and was incubated anaerobically at 37 °C for 48 h. The grown culture was centrifuged at 6000 rpm for 15 min to separate all cells and the supernatant was filtered through a 0.22 µm membrane filter. The cell-free supernatant was labeled as untreated supernatant (U) and stored at −20 °C. The Lactobacillus supernatant was highly acidic due to lactic acid production. To adjust the pH of the supernatant to pH 6.5–7.0, 1N NaOH was used and this fraction of the supernatant was labeled as treated supernatant (T) and stored at −20 °C [34].
2.5. Antimicrobial Activity of Lactobacillus Supernatants
The antimicrobial activity of Lactobacillus supernatants (treated or untreated) was assessed against the P. mirabilis isolates P2, P4, P14, P15, P23, P24 and P25 using microtiter plate assays. The antibacterial activity was assayed by the agar diffusion method [35]. The P. mirabilis isolates were incubated in Muller Hinton broth at 37 °C for 24 h. The culture inoculum was adjusted to 0.5 McFarland (1.5 × 108 CFU/mL) and was used to inoculate melted Muller Hinton agar at 50 °C. After medium solidification, wells were cut in agar using a cork borer. Wells were filled with 100 μL of the whole cell culture of Lactobacillus or cell-free supernatants (treated or untreated), and the plates were incubated aerobically at 37 °C for 24 h. Inhibition zones were measured to indicate the antimicrobial activity of the corresponding Lactobacillus.
Stock solutions of treated or untreated Lactobacillus supernatants were maintained, and different concentrations 1/2, 1/4, 1/18, 1/16, and 1/32 were prepared.
2.6. Effect of Lactobacillus Supernatant on Mature Biofilms
The mature biofilms of P. mirabilis isolates P2, P4, P14, P15, P23, P24, and P25 were formed in flat-bottomed 96-well microtiter plates. The overnight culture of each isolate was adjusted to 0.5 McFarland using Muller Hinton broth. Then, 100 µL of the diluted Proteus cultures was added to each well and incubated at 37 °C for 48 h. The planktonic cells were removed and the attached cells were gently washed twice with sterile physiological saline. Untreated or treated supernatants of Lactobacillus were then added to the wells and the plates were incubated at 37 °C for 24 h. The biofilm of each Proteus isolate without Lactobacillus supernatants was used as a positive control. The influence of different concentrations of Lactobacillus supernatants (treated or untreated) on the mature biofilm was detected using crystal violet microtiter plate assays. The treated and the mature Proteus biofilms were washed, fixed using methanol, and stained with crystal violet as described before [36].
The effects of different concentrations (1/2, 1/4, 1/18, 1/16, and 1/32) of treated and untreated supernatants of L. casei and L. reuteri on the mature biofilms of the P. mirabilis isolate P14 were also evaluated.
2.7. Anti-Adherence Effect of Lactobacillus Supernatants
In order to study the effect of Lactobacillus on biofilm formation, 100 µL of treated or untreated supernatants of Lactobacillus was mixed at low concentrations (1/8, 1/16 and 1/32) with 100 µL of the P. mirabilis isolate P14 (with inoculum size 1.5 × 108 CFU/mL). The microtiter plate was incubated for 48 h at 37 °C and the biofilm formation was detected using the crystal violet microtiter plate method [17]. Wells containing the Proteus isolate P14 in contact with different concentrations of Lactobacillus supernatant (At) were compared to wells containing Proteus culture without the Lactobacillus supernatant (control, Ac). The percent reduction in biofilm formation was calculated as follows = ((Ac − At)/Ac) ×100.
2.8. Scanning Electron Microscopy (SEM)
The overnight culture of P14 was diluted to 0.5 McFarland using tryptic soy broth (TSB). Similarly, cultures of the tested Lactobacillus strains were diluted in MRS and co-cultured with equal volumes of P14 (1:1) in sterile six well plates (Greiner Bio-One, Kremsmünster, Austria) at 37 °C for 24 h. The control wells containing P14 only with MRS and TSB media were also prepared. A clean sterile cover slide was added to each well. The slides were removed and washed gently with phosphate buffered saline (PBS) to remove the planktonic cells. The biofilm was fixed and prepared for examination by SEM (JSM-7600F, JEOL USA, INC., Peabody, MA, USA) as previously described [37].
2.9. Statistical Analysis
Statistical analysis was performed using one-way ANOVA in order to compare the effect of Lactobacillus, cell-free, and treated supernatants on the mature biofilm and on the adhesion of P. mirabilis isolates. A p value of <0.05 indicated statistical significance.
3. Results
3.1. Antimicrobial Susceptibility and Biofilm Formation of P. mirabilis isolates
Activity of the tested antimicrobial agents against P. mirabilis isolates was evaluated according to CLSI standards [32]. As shown in Table 1, 86% of the tested P. mirabilis isolates were resistant to amoxicillin-clavulanic, 57% were resistant to cefotaxime, 57% were resistant to ceftazidime, 71% were resistant to ciprofloxacin, 57% were resistant to amikacin, 43% were resistant to imipenem, and 0% were resistant to cefoxitin. Furthermore, isolate P14 was resistant to cefotaxime, ceftazidime, amoxicillin-clavulanic acid, ciprofloxacin, and amikacin and intermediately resistant to imipenem (Table 1). In contrast, isolate P2 was sensitive to all the assessed antimicrobials (Table 1).

Table 1.
Antimicrobial susceptibility patterns of P. mirabilis isolates.
P. mirabilis isolates P2, P4, P14, P24 and P25 showed strong biofilm formation. Isolates P15 and P23 showed moderate biofilm formation. Isolate P14 showed the strongest biofilm formation and was resistant to the tested antimicrobials. Isolate P2 showed a strong biofilm formation, but was sensitive to all assessed antimicrobials.
3.2. Antimicrobial Activities of L. casei and L. reuteri against P. mirabilis Isolates
The untreated supernatants of L. casei and L. reuteri had inhibitory effects on the tested P. mirabilis isolates (Table 2). The treated (pH-adjusted) supernatants of L. reuteri were effective against almost all tested P. mirabilis isolates with inhibition zone diameters ranging from 12 to 16 mm. The treated (pH-adjusted) supernatants of L. casei was effective against four isolates (P2, P14, P15 and P24) with inhibition zone diameters ranging from 13 to 16 mm. Notably, the treated supernatants of L. casei and L. reuteri were effective against the multidrug resistant (MDR) Proteus isolate P14 with inhibition zone diameters ranging from 14 to 15 mm (Table 2).

Table 2.
Antimicrobial activities of L. casei and L. reuteri against P. mirabilis isolates.
3.3. Effect of Lactobacillus Supernatants on Mature Biofilm Formation of P. mirabilis Isolates
The effect of treated and untreated supernatants of L. casei and L. reuteri on mature biofilm formation of P. mirabilis isolates P2, P4, P14, P15, P23, P24, and P25 was studied (Figure 1).
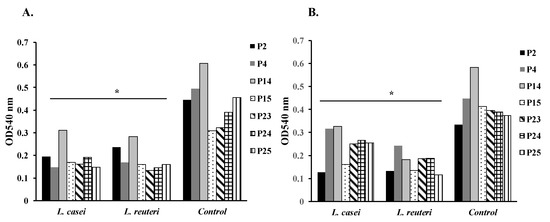
Figure 1.
Effect of supernatants of L. casei and L. reuteri on mature biofilm formation of Proteus mirabilis isolates P2, P4, P14, P15, P23, P24, and P25 (* p < 0.05). (A) Effect of untreated supernatants of L. casei and L. reuteri on mature biofilm formation of Proteus mirabilis isolates P2, P4, P14, P15, P23, P24, and P25 (* p < 0.05). (B) Effect of treated supernatants of L. casei and L. reuteri on mature biofilm formation of Proteus mirabilis isolates P2, P4, P14, P15, P23, P24, and P25 (* p < 0.05).
The untreated supernatants of L. casei significantly reduced biofilm formation of isolates P2, P4, P14, P15, P23, P24, and P25 by 56%, 70%, 48%, 45%, 49%, 50%, and 67%, respectively (p < 0.05) (Figure 1A). Furthermore, the treated supernatants of L. casei significantly reduced biofilm formation of isolates P2, P4, P14, P15, P23, P24, and P25 by 61%, 29%, 40%, 60%, 36%, 32%, and 32%, respectively (p < 0.05) (Figure 1B).
Similarly, the untreated supernatants of L. reuteri significantly reduced biofilm formation of isolates P2, P4, P14, P15, P23, P24, and P25 by 46%, 65%, 52%, 48%, 58%, 62%, and 65%, respectively (p < 0.05) (Figure 1A). Moreover, the treated supernatants of L. reuteri significantly reduced biofilm formation of isolates P2, P4, P14, P15, P23, P24, and P25 by 60%, 45%, 66%, 67%, 52%, 52%, and 69%, respectively (p < 0.05) (Figure 1B).
In brief, the treated and the untreated supernatants of L. casei, and L. reuteri caused significant reductions of mature biofilm formation in the tested P. mirabilis isolates (p < 0.05) (Figure 1).
Next, we tested various concentrations of treated and untreated supernatants of L. casei and L. reuteri on mature biofilm formation of the MDR isolate P14. The diluted untreated supernatants (1/4, 1/8, and 1/16) of L. casei caused a significant reduction of biofilm formation in the P14 isolate by 61%, 61%, and 60%, respectively (p < 0.05) (Figure 2A). Similarly, the diluted treated supernatants (1/2, 1/4, 1/8) of L. casei significantly reduced biofilm formation in the P14 isolate by 55%, 33%, and 39%, respectively (p < 0.05) (Figure 2B.).
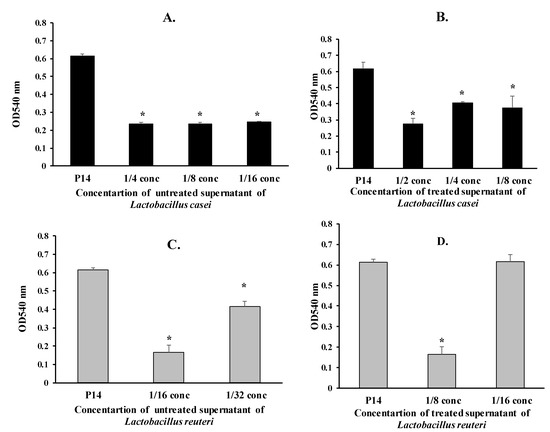
Figure 2.
Effect of supernatants of L. casei and L. reuteri on mature biofilm formation of the P. mirabilis isolate P14 (* p < 0.05). (A) Effect of untreated supernatants of L. casei on mature biofilm formation of the Proteus isolate P14 (* p < 0.05). (B) Effect of treated supernatants of L. casei on mature biofilm formation of the Proteus isolate P14 (* p < 0.05). (C) Effect of untreated supernatants of L. reuteri on mature biofilm formation of the Proteus isolate P14 (* p < 0.05). (D) Effect of treated supernatants of L. reuteri on mature biofilm formation of the Proteus isolate P14 (* p < 0.05).
The 1/16 and 1/32 concentrations of the untreated supernatants of L. reuteri caused a significant reduction in biofilm formation of the P14 isolate by 73% and 32%, respectively (p < 0.05) (Figure 2C). The 1/8 concentration of the treated supernatants of L. reuteri significantly reduced biofilm formation of the P14 isolate by 73% (p < 0.05) (Figure 2D).
3.4. Anti-Adherence Effect of Lactobacillus Supernatants
The anti-adherence effect of diluted Lactobacillus supernatants (treated and untreated) on the P14 isolate was assessed. As in Figure 3, the untreated supernatants of L. casei and L. reuteri significantly reduced the adhesion of the P14 isolate (1/16 concentration) by 72% and 73%, respectively (p < 0.01) (Figure 3A,C). The anti-adherence effect of the untreated supernatants on the P14 isolate decreased by further diluting the supernatant.
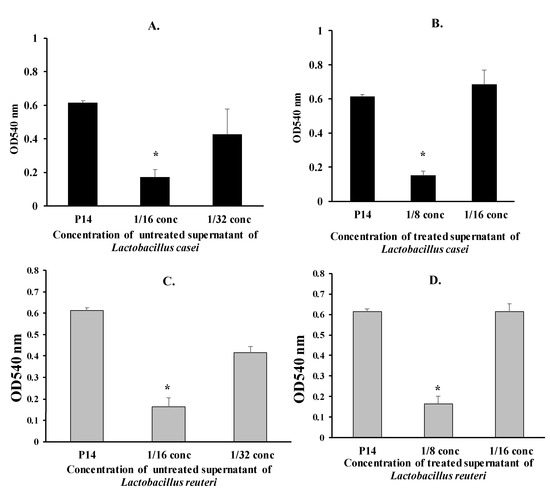
Figure 3.
Adherence of the Proteus isolate P14 in the presence of supernatants of L. casei and L. reuteri (* p < 0.05). (A) Adherence of the Proteus isolate P14 in the presence of untreated supernatants of L. casei (* p < 0.05). (B) Adherence of the Proteus isolate P14 in the presence of treated supernatants of L. casei (* p < 0.05). (C) Adherence of the Proteus isolate P14 in the presence of untreated supernatants of L. reuteri (* p < 0.05). (D) Adherence of the Proteus isolate P14 in the presence of treated supernatants of L. reuteri (* p < 0.05).
3.5. Scanning Electron Micrographs
As in Figure 4A, there is a dense mass of biofilm-forming P. mirabilis P14 (as a positive control). Co-culture of L. casei with P. mirabilis P14 caused the complete elimination of biofilm formation by P14 (Figure 4B). Co-culture of L. reuteri with P. mirabilis P14 showed scattered cells and a loose biofilm architecture but no dense aggregates (Figure 4C).
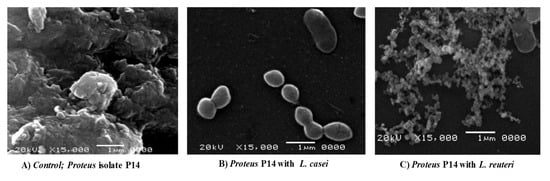
Figure 4.
Scanning Electron micrographs (SEM) (magnification: × 15,000). (A) Biofilm formation of the P14 isolate in the absence of Lactobacillus spp. (control). (B) Biofilm formation of the P14 isolate in the presence of L. casei. (C) Biofilm formation of the P14 isolate in the presence of L. reuteri.
4. Discussion
Bacterial resistance is one of the major public health problems. It includes the emergence of MDR pathogens, such as resistant isolates of Proteus mirabilis, which have been associated with urinary tract infections and nosocomial infections worldwide [4,38]. P. mirabilis isolates have been reported to be resistant to penicillins [39]. They also show a high incidence of resistance to cephalosporins, carbapenems, and quinolones [4,40]. In the current study, a high percentage of the isolates was resistant or intermediately resistant to amoxicillin-clavulanic acid and cefotaxime. There was also significant resistance to ceftazidime, ciprofloxacin, and amikacin (Table 1). It is noteworthy that the isolate P14 was resistant to three different groups of antibiotics with various mechanisms of action (Table 1). Cefotaxime-resistant P. mirabilis has been previously described [41]. Kwiecińska-Piróg et al. reported that P. mirabilis showed resistance against ceftazidime and ciprofloxacin [42]. Amikacin resistance has been shown to be associated with extended spectrum beta-lactamase (ESBL(-producing isolates of P. mirabilis, which were resistant to amikacin (85.1%) [43].
Urinary tract infection with P. mirabilis is associated with biofilm formation and accumulation of the polysaccharide matrix [44]. This is followed by urease production, increase in the pH, attraction of calcium and magnesium ions, and development of crystals [44]. The deposition of crystals within the biofilm can cause catheter blockage and urinary retention. This is because P. mirabilis possesses various virulence factors (lipopolysaccharide, quorum sensing autoinducers, pili, adhesin, and other proteins) that enhance adhesion and crystalline biofilm formation on the abiotic surfaces of urinary catheters [45]. It has been reported that 48% of the isolated proteus species were biofilm producers [42]. The Proteus-biofilm assembly is dangerous, as it interferes with microbial penetration, increases antimicrobial resistance, and renders therapeutic treatments ineffective, which encourages the development and chronicity of infections [46].
Moreover, pathogenicity of P. mirabilis is enhanced by the complex architecture of its biofilm, which is characterized by a high ability for adapting to different environmental conditions, biocides, and antimicrobials [47,48]. Isolates in this study were able to form strong or moderate biofilms. This was true even for the antibiotic-sensitive isolate P2, which was observed to have a strong biofilm-forming ability (Table 1). Typically, the biofilm-producing isolates are more resistant to antibiotics than the non-biofilm producing isolates [49]. The reason is that the development of complex biofilm structures can convey protection to the internal cells from antimicrobials [50]. It can also support the persistence of P. mirabilis in the host cells [50].
Given the above, alternative treatments are required for the management of P. mirabilis infections and the disruption of its biofilm architecture. Probiotics as Lactobacilli, have been used for the treatment of burn infections and have been shown to interfere with activity of Pseudomonas aeruginosa [51,52]. Lactobacilli have also been used for the management of recurrent urinary tract infections caused by E. coli [53]. The antimicrobial activity of L. casei and L. reuteri against various enteropathogenic infections have been previously reported [10]. Their antimicrobial activities against uropathogens have also been reported [20]. L. reuteri has been shown to be effective against Escherichia coli and Listeria monocytogenes [54]. Thus, it was important to examine the antimicrobial and antibiofilm activities of Lactobacillus on P. mirabilis clinical isolates. We examined the cultures and the cell-free supernatants of L. casei, and L. reuteri against P. mirabilis isolates.
In our study, the treated supernatants of L. casei and L. reuteri retained antimicrobial activities against the sensitive P2 isolate and the other resistant Proteus mirabilis isolates (Table 2). Notably, treated supernatants of L. casei and L. reuteri retained significant antibiofilm and anti-adherence activities against P14 at 1/8 concentration (Figure 2 and Figure 3). In addition, L. casei and L. reuteri had significant inhibitory effects on the typical biofilm formed by the MDR isolate P14, as detected by SEM (Figure 4). In another study, the untreated supernatants of fecal Lactobacilli caused an 85 to 95% reduction in biofilm formation of Vibrio cholerae isolates [27]. Similarly, the pH-adjusted supernatants of fecal Lactobacilli significantly reduced biofilm formation of Vibrio cholerae by 50–75% [27]. Additionally, Koohestani and colleagues showed that the untreated supernatants of L. casei significantly reduced biofilm formation of S. aureus [55]. Probiotic lactobacilli have been shown to negatively impact growth and biofilm formation of Streptococcus mutans [34], pathogens in the oral cavity [56], and Salmonella enterica serovar Typhimurium [28]. Moreover, probiotic lactobacilli inhibited cancer cells of the human colonic carcinoma cell line HT-29 [23]. Lastly, Lactobacillus reuteri DPC16 supernatants have been shown to exhibit activity against Escherichia coli, S. aureus, Salmonella derby, and Listeria monocytogenes. This is consistent with results in our study in which the untreated supernatants of L. casei reduced biofilm formation of P. mirabilis isolates by 45–67%.
It is important to mention that the use of probiotic Lactobacillus cell cultures and supernatants was proposed as an alternative to traditional antibiotic therapy against P. mirabilis. In other words, it was proposed as a way to avoid the adverse effects of antibiotic therapy and to circumvent the impact of antibiotic resistance developed by the microbe. Indeed, we saw a significant inhibitory effect of Lactobacillus cell cultures and supernatants on P. mirabilis biofilm formation and adherence. Future studies could examine the effect of Lactobacillus supernatants on the susceptibility of P. mirabilis to the tested antimicrobials. In this case, the focus would be on the possible synergistic effects of combining probiotic Lactobacillus supernatants with anti-Proteus agents.
The antimicrobial, antibiofilm, and anti-adherence activities of probiotic L. casei and L. reuteri used in this study could be attributed to their secreted biosurfactants [15], S-layer proteins [57,58], surface-acting proteins (such as enolase) [59], and peptidoglycan-binding proteins [60].
5. Conclusions
The pathogenicity and virulence of Proteus infections includes a biofilm-forming ability that enables serious urinary tract infection. Isolates in this study showed multidrug resistance (MDR) against more than one antimicrobial agent, highlighting the importance of the development of alternative and adjuvant treatments for the efficient management of P. mirabilis infections. Using cell cultures, cell-free supernatants, and pH-adjusted supernatants, we have shown that L. casei DSM 20011 and L. reuteri DSM 20016 exhibit antimicrobial, anti-adherence, and antibiofilm activities against MDR P. mirabilis. Thus, L. casei and L. reuteri could be utilized to combat Proteus-associated urinary tract infections.
Authors Contributions
M.S., O.A.A.E.-R., B.A.-Q., and H.M.A. contributed to the conception of the study, experimental design, execution, data analysis, writing of the original draft, revision of the manuscript, and final publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors confirm that there are no conflict of interest.
References
- Armbruster, C.E.; Smith, S.N.; Johnson, A.O.; DeOrnellas, V.; Eaton, K.A.; Yep, A.; Mody, L.; Wu, W.; Mobley, H.L.T. The Pathogenic Potential of Proteus mirabilis Is Enhanced by Other Uropathogens during Polymicrobial Urinary Tract Infection. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis Infection. Ecosal. Plus 2018, 8. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, Y.H.; Lu, P.L.; Lin, W.R.; Chen, T.C.; Lin, C.Y. Proteus mirabilis urinary tract infection and bacteremia: Risk factors, clinical presentation, and outcomes. J. Microbiol. Immunol. Infect. 2012, 45, 228–236. [Google Scholar] [CrossRef]
- Pal, N.; Hooja, S.; Sharma, R.; Maheshwari, R.K. Phenotypic Detection and Antibiogram of beta-lactamase-producing Proteus Species in a Tertiary Care Hospital, India. Ann. Med. Health Sci. Res. 2016, 6, 267–273. [Google Scholar] [CrossRef]
- Adamus-Bialek, W.; Zajac, E.; Parniewski, P.; Kaca, W. Comparison of antibiotic resistance patterns in collections of Escherichia coli and Proteus mirabilis uropathogenic strains. Mol. Biol. Rep. 2013, 40, 3429–3435. [Google Scholar] [CrossRef]
- Gajdacs, M.; Urban, E. Comparative Epidemiology and Resistance Trends of Proteae in Urinary Tract Infections of Inpatients and Outpatients: A 10-Year Retrospective Study. Antibiotics (Basel) 2019, 8, 91. [Google Scholar] [CrossRef]
- Nucleo, E.; Fugazza, G.; Migliavacca, R.; Spalla, M.; Comelli, M.; Pagani, L.; Debiaggi, M. Differences in biofilm formation and aggregative adherence between beta-lactam susceptible and beta-lactamases producing P. mirabilis clinical isolates. New Microbiol. 2010, 33, 37–45. [Google Scholar]
- Mercenier, A.; Pavan, S.; Pot, B. Probiotics as biotherapeutic agents: Present knowledge and future prospects. Curr. Pharm. Des. 2003, 9, 175–191. [Google Scholar] [CrossRef]
- Singh, V.P.; Sharma, J.; Babu, S.; Rizwanulla, S.A.; Singla, A. Role of probiotics in health and disease: A review. J. Pak. Med. Assoc. 2013, 63, 253–257. [Google Scholar]
- Lievin-Le Moal, V.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef]
- Sharma, D.; Singh Saharan, B. Simultaneous Production of Biosurfactants and Bacteriocins by Probiotic Lactobacillus casei MRTL3. Int. J. Microbiol. 2014, 2014, 698713. [Google Scholar] [CrossRef] [PubMed]
- Goŀek, P.; Bednarski, W.; Brzozowski, B.; Dziuba, B. The obtaining and properties of biosurfactants synthesized by bacteria of the genusLactobacillus. Ann. Microbiol. 2009, 59, 119–126. [Google Scholar] [CrossRef]
- Spinler, J.K.; Taweechotipatr, M.; Rognerud, C.L.; Ou, C.N.; Tumwasorn, S.; Versalovic, J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 2008, 14, 166–171. [Google Scholar] [CrossRef]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Satish Kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Probiotics and its functionally valuable products-a review. Crit. Rev. Food Sci. Nutr. 2013, 53, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, R.; Abd El-Rahman, O.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Sieo, C.C.; Kalavathy, R.; Liang, J.B.; Alitheen, N.B.; Faseleh Jahromi, M.; Ho, Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed. Res. Int. 2014, 2014, 927268. [Google Scholar] [CrossRef]
- Karska-Wysocki, B.; Bazo, M.; Smoragiewicz, W. Antibacterial activity of Lactobacillus acidophilus and Lactobacillus casei against methicillin-resistant Staphylococcus aureus (MRSA). Microbiol. Res. 2010, 165, 674–686. [Google Scholar] [CrossRef]
- Velraeds, M.M.; van de Belt-Gritter, B.; Busscher, H.J.; Reid, G.; van der Mei, H.C. Inhibition of uropathogenic biofilm growth on silicone rubber in human urine by lactobacilli—A teleologic approach. World J. Urol. 2000, 18, 422–426. [Google Scholar] [CrossRef]
- Pascual, L.M.; Daniele, M.B.; Ruiz, F.; Giordano, W.; Pajaro, C.; Barberis, L. Lactobacillus rhamnosus L60, a potential probiotic isolated from the human vagina. J. Gen. Appl. Microbiol. 2008, 54, 141–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grin, P.M.; Kowalewska, P.M.; Alhazzan, W.; Fox-Robichaud, A.E. Lactobacillus for preventing recurrent urinary tract infections in women: Meta-analysis. Can. J. Urol. 2013, 20, 6607–6614. [Google Scholar] [PubMed]
- Chen, Z.Y.; Hsieh, Y.M.; Huang, C.C.; Tsai, C.C. Inhibitory Effects of Probiotic Lactobacillus on the Growth of Human Colonic Carcinoma Cell Line HT-29. Molecules 2017, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Larsson, C.U.; Sawicki, R.; van Niel, E.W.J.; Roos, S.; Hakansson, S. Impact of the fermentation parameters pH and temperature on stress resilience of Lactobacillus reuteri DSM 17938. AMB Express 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Palmfeldt, J.; Hahn-Hagerdal, B. Influence of culture pH on survival of Lactobacillus reuteri subjected to freeze-drying. Int. J. Food Microbiol. 2000, 55, 235–238. [Google Scholar] [CrossRef]
- Sgouras, D.N.; Panayotopoulou, E.G.; Martinez-Gonzalez, B.; Petraki, K.; Michopoulos, S.; Mentis, A. Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin. Diagn Lab. Immunol. 2005, 12, 1378–1386. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, P.; Kalia, N.; Singh, J.; Kaur, S. Anti-biofilm Properties of the Fecal Probiotic Lactobacilli Against Vibrio spp. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef]
- Fayol-Messaoudi, D.; Berger, C.N.; Coconnier-Polter, M.H.; Lievin-Le Moal, V.; Servin, A.L. pH-, Lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 6008–6013. [Google Scholar] [CrossRef]
- Bian, L.; Molan, A.-L.; Maddox, I.; Shu, Q. Antimicrobial activity of Lactobacillus reuteri DPC16 supernatants against selected food borne pathogens. World J. Microbiol. Biotechnol. 2011, 27, 991–998. [Google Scholar] [CrossRef]
- Cheesbrough, M. Medical Laboratory Manual for Tropical Countries; Cheesbrough, M., Ed.; 14 Bevills Close; PE15 OTT: Doddington/Cambridgeshire, UK, 1981; Volume 1. [Google Scholar]
- Ding, Y.; Wang, W.; Fan, M.; Tong, Z.; Kuang, R.; Jiang, W.; Ni, L. Antimicrobial and anti-biofilm effect of Bac8c on major bacteria associated with dental caries and Streptococcus mutans biofilms. Peptides 2014, 52, 61–67. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019.
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Lin, X.; Chen, X.; Chen, Y.; Jiang, W.; Chen, H. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral. Dis. 2015, 21, e128–e134. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U.; Lucke, F.K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [CrossRef]
- Wasfi, R.; Abd El-Rahman, O.A.; Mansour, L.E.; Hanora, A.S.; Hashem, A.M.; Ashour, M.S. Antimicrobial activities against biofilm formed by Proteus mirabilis isolates from wound and urinary tract infections. Indian J. Med. Microbiol. 2012, 30, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lin, C.T.; Wu, C.Y.; Peng, W.S.; Lee, M.J.; Tsai, Y.C. Inhibitory effect of Lactobacillus salivarius on Streptococcus mutans biofilm formation. Mol. Oral. Microbiol. 2015, 30, 16–26. [Google Scholar] [CrossRef]
- Lin, M.F.; Liou, M.L.; Kuo, C.H.; Lin, Y.Y.; Chen, J.Y.; Kuo, H.Y. Antimicrobial Susceptibility and Molecular Epidemiology of Proteus mirabilis Isolates from Three Hospitals in Northern Taiwan. Microb. Drug Resist. 2019. [Google Scholar] [CrossRef]
- Stock, I. Natural antibiotic susceptibility of Proteus spp., with special reference to P. mirabilis and P. penneri strains. J. Chemother. 2003, 15, 12–26. [Google Scholar] [CrossRef]
- Mathai, D.; Jones, R.N.; Pfaller, M.A.; America, S.P.G.N. Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1510 hospitalized patients: A report from the SENTRY Antimicrobial Surveillance Program (North America). Diagn. Microbiol. Infect. Dis. 2001, 40, 129–136. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Hashikita, G.; Oka, Y.; Takahashi, S.; Yamazaki, T.; Maesaki, S.; Ishii, Y. Study of resistance mechanism on cefotaxime resistant Proteus mirabilis isolated from clinical specimens and its clinical background. Kansenshogaku Zasshi 2004, 78, 1–9. [Google Scholar] [CrossRef][Green Version]
- Kwiecińska-Piróg, J.; Skowron, K.; Zniszczol, K.; Gospodarek, E. The assessment of Proteus mirabilis susceptibility to ceftazidime and ciprofloxacin and the impact of these antibiotics at subinhibitory concentrations on Proteus mirabilis biofilms. Biomed. Res. Int. 2013, 2013, 930876. [Google Scholar] [CrossRef]
- De Champs, C.; Bonnet, R.; Sirot, D.; Chanal, C.; Sirot, J. Clinical relevance of Proteus mirabilis in hospital patients: A two year survey. J. Antimicrob. Chemother. 2000, 45, 537–539. [Google Scholar] [CrossRef][Green Version]
- Wilks, S.A.; Fader, M.J.; Keevil, C.W. Novel insights into the Proteus mirabilis crystalline biofilm using real-time imaging. PLoS ONE 2015, 10, e0141711. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Srivastava, D.; Srivastava, S.; Singh, P.C.; Kumar, A. Mechanisms of Biofilm Development, Antibiotic Resistance and Tolerance and Their Role in Persistent Infections. In Antibacterial Drug Discovery to Combat MDR; Springer: Berlin, Germany, 2019; pp. 115–130. [Google Scholar]
- Jacobsen, S.M.; Shirtliff, M.E. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence 2011, 2, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Pelling, H.; Nzakizwanayo, J.; Milo, S.; Denham, E.L.; MacFarlane, W.M.; Bock, L.J.; Sutton, J.M.; Jones, B.V. Bacterial biofilm formation on indwelling urethral catheters. Lett. Appl. Microbiol. 2019, 68, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Shikh-Bardsiri, H.; Shakibaie, M.R. Antibiotic resistance pattern among biofilm producing and non producing Proteus strains isolated from hospitalized patients; matter of hospital hygiene and antimicrobial stewardship. Pak. J. Biol. Sci. 2013, 16, 1496–1502. [Google Scholar] [CrossRef][Green Version]
- Stickler, D.J. Clinical complications of urinary catheters caused by crystalline biofilms: Something needs to be done. J. Intern. Med. 2014, 276, 120–129. [Google Scholar] [CrossRef]
- Valdez, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigon, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef]
- Shokri, D.; Khorasgani, M.R.; Mohkam, M.; Fatemi, S.M.; Ghasemi, Y.; Taheri-Kafrani, A. The Inhibition Effect of Lactobacilli Against Growth and Biofilm Formation of Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins 2018, 10, 34–42. [Google Scholar] [CrossRef]
- Gupta, V.; Nag, D.; Garg, P. Recurrent urinary tract infections in women: How promising is the use of probiotics? Indian J. Med. Microbiol. 2017, 35, 347–354. [Google Scholar] [CrossRef]
- Eslami, G.; Karimiravesh, R.; Taheri, S.; Azargashb, E. Inhibitory Effect of Lactobacillus reuteri on Some Pathogenic Bacteria Isolated from Women with Bacterial Vaginosis. Avicenna J. Clin. Microbiol. Infect. 2014, 1, 19908. [Google Scholar] [CrossRef]
- Koohestani, M.; Moradi, M.; Tajik, H.; Badali, A. Effects of cell-free supernatant of Lactobacillus acidophilus LA5 and Lactobacillus casei 431 against planktonic form and biofilm of Staphylococcus aureus. Vet. Res. Forum 2018, 9, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Donelli, G. Activity of Probiotics on Biofilm-Growing Pathogens of the Oral Cavity. Microb. Ecol. Health Dis. 2013, 24. [Google Scholar]
- Spurbeck, R.R.; Arvidson, C.G. Lactobacillus jensenii surface-associated proteins inhibit Neisseria gonorrhoeae adherence to epithelial cells. Infect. Immun. 2010, 78, 3103–3111. [Google Scholar] [CrossRef]
- Velraeds, M.; Van der Mei, H.; Reid, G.; Busscher, H.J. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl. Environ. Microbiol. 1996, 62, 1958–1963. [Google Scholar] [CrossRef]
- Ren, D.; Li, C.; Qin, Y.; Yin, R.; Li, X.; Tian, M.; Du, S.; Guo, H.; Liu, C.; Zhu, N. Inhibition of Staphylococcus aureus adherence to Caco-2 cells by lactobacilli and cell surface properties that influence attachment. Anaerobe 2012, 18, 508–515. [Google Scholar] [CrossRef]
- Kang, M.S.; Lim, H.S.; Oh, J.S.; Lim, Y.J.; Wuertz-Kozak, K.; Harro, J.M.; Shirtliff, M.E.; Achermann, Y. Antimicrobial activity of Lactobacillus salivarius and Lactobacillus fermentum against Staphylococcus aureus. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).