Manipulation of Alcohol and Short-Chain Fatty Acids in the Metabolome of Commensal and Virulent Klebsiella pneumoniae by Linolenic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture and Fermentation
2.2. Alcohol Detected by Gas Chromatographic Mass Spectrometry (GC-MS)
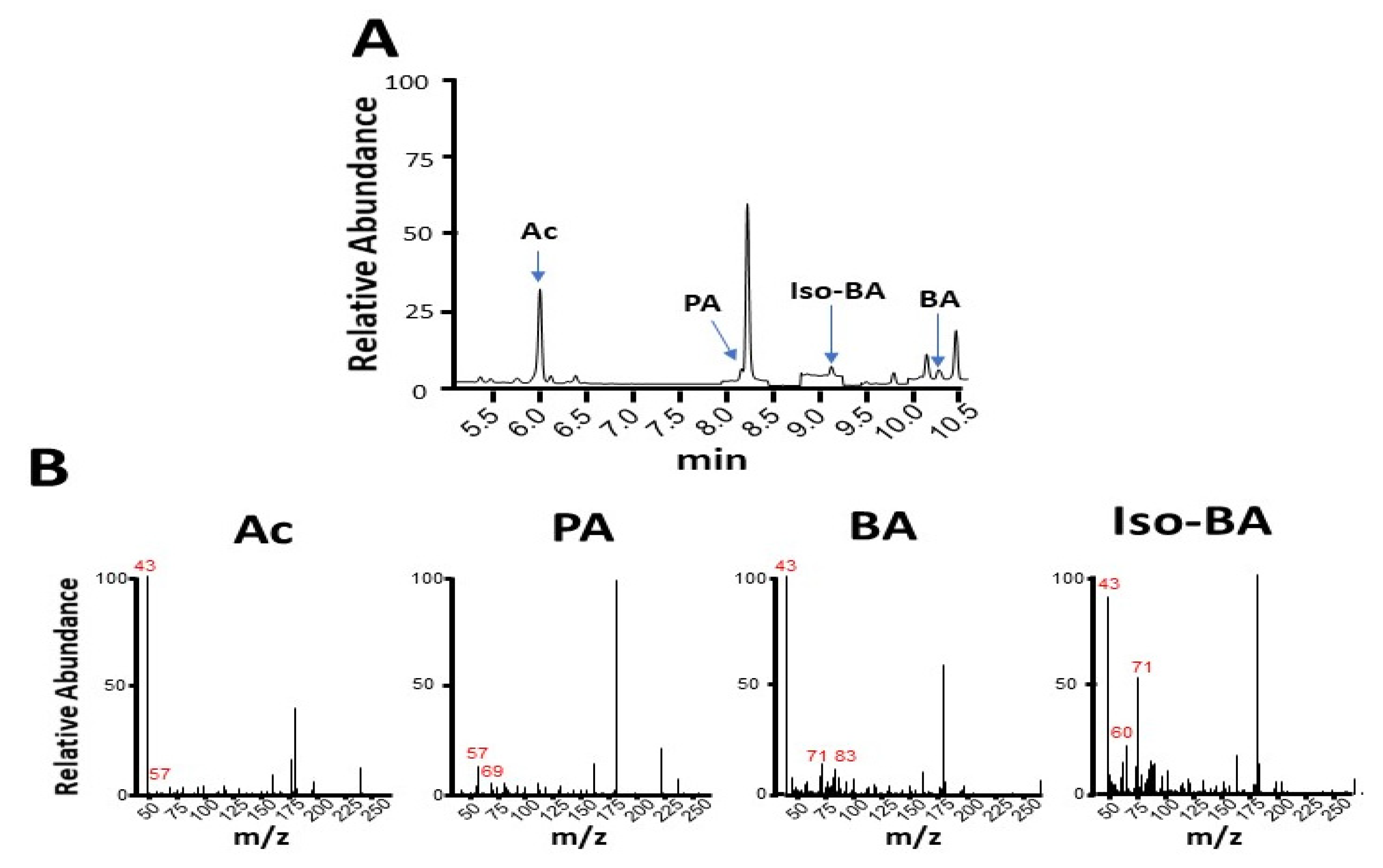
2.3. SCFA Identification by GC-MS
2.4. Statistical Analysis
3. Results
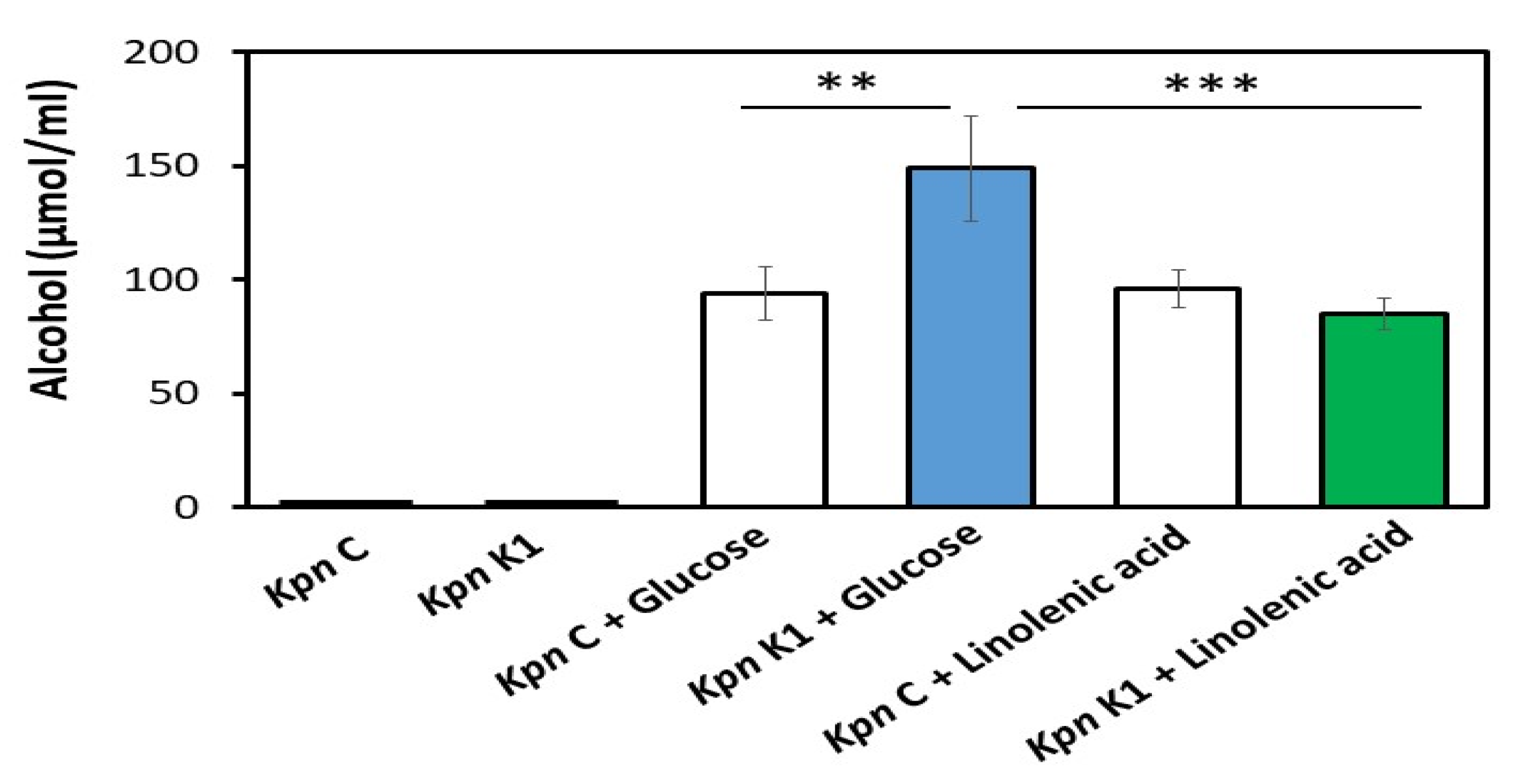
3.1. Alcohol Production in K. pneumoniae
3.2. SCFA Production Enhanced by Linolenic Acid
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwabe, R.F.; Greten, T.F. Gut Microbiome in HCC—Mechanisms, Diagnosis and Therapy. J. Hepatol. 2020, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, C.; Cui, J.; Lu, J.; Yan, C.; Wei, X.; Zhao, X.; Li, N.; Li, S.; Xue, G.; et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019, 30, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.C.; Liao, T.L.; Lin, Y.C.; Lai, Y.C.; Lu, M.C.; Chen, Y.T. Complete genome sequence of Klebsiella pneumoniae 1084, a hypermucoviscosity-negative K1 clinical strain. J. Bacteriol. 2012, 194, 6316. [Google Scholar] [CrossRef]
- Chen, S.S.; Block, B.S.; Chan, P.J. Pentoxifylline attenuates HPV-16 associated necrosis in placental trophoblasts. Arch. Gynecol. Obstet. 2015, 291, 647–652. [Google Scholar] [CrossRef]
- Vargas, J.I.; Arrese, M.; Shah, V.H.; Arab, J.P. Use of Statins in Patients with Chronic Liver Disease and Cirrhosis: Current Views and Prospects. Curr. Gastroenterol. Rep. 2017, 19, 43. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Jayol, A.; Poirel, L.; Villegas, M.V.; Nordmann, P. Modulation of mgrB gene expression as a source of colistin resistance in Klebsiella oxytoca. Int. J. Antimicrob. Agents 2015, 46, 8–10. [Google Scholar] [CrossRef]
- Venugopalan, V.; Shriner, K.A.; Wong-Beringer, A. Regulatory oversight and safety of probiotic use. Emerg. Infect. Dis. 2010, 16, 1661–1665. [Google Scholar] [CrossRef]
- Tong, X.; Yin, L.; Giardina, C. Butyrate suppresses cox-2 activation in colon cancer cells through HDAC inhibition. Biochem. Biophys. Res. Commun. 2004, 317, 463–471. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, L.B.; Olbrich Dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Garau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Engelberth, A.S. Techno-economic analysis for incorporating a liquid-liquid extraction system to remove acetic acid into a proposed commercial scale biorefinery. Biotechnol. Prog. 2016, 32, 971–977. [Google Scholar] [CrossRef]
- Kao, M.S.; Huang, S.; Chang, W.L.; Hsieh, M.F.; Huang, C.J.; Gallo, R.L.; Huang, C.M. Microbiome precision editing: Using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef]
- Sharma, V.; Smolin, J.; Nayak, J.; Ayala, J.E.; Scott, D.A.; Peterson, S.N.; Freeze, H.H. Mannose Alters Gut Microbiome, Prevents Diet-Induced Obesity, and Improves Host Metabolism. Cell Rep. 2018, 24, 3087–3098. [Google Scholar] [CrossRef]
- Meyer, D. Health benefits of prebiotic fibers. Adv. Food Nutr. Res. 2015, 74, 47–91. [Google Scholar] [CrossRef]
- Egert, M.; de Graaf, A.A.; Maathuis, A.; de Waard, P.; Plugge, C.M.; Smidt, H.; Deutz, N.E.; Dijkema, C.; de Vos, W.M.; Venema, K. Identification of glucose-fermenting bacteria present in an in vitro model of the human intestine by RNA-stable isotope probing. FEMS Microbiol. Ecol. 2007, 60, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Shokryazdan, P.; Faseleh Jahromi, M.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maniar, K.; Singh, V.; Moideen, A.; Bhattacharyya, R.; Chakrabarti, A.; Banerjee, D. Inhalational supplementation of metformin butyrate: A strategy for prevention and cure of various pulmonary disorders. Biomed. Pharmacother. 2018, 107, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Chen, W.B.; Wu, X.; Zhang, Y.W.; Jiang, Y.M.; Meng, Q.X.; Zhou, Z.M. Calcium propionate supplementation improves development of rumen epithelium in calves via stimulating G protein-coupled receptors. Animal 2018, 12, 2284–2291. [Google Scholar] [CrossRef]
- Moser, G.; Fournier, C.; Peter, J. Intestinal microbiome-gut-brain axis and irritable bowel syndrome. Wien. Med. Wochenschr. 2018, 168, 62–66. [Google Scholar] [CrossRef]
- Huang, W.; Guo, H.L.; Deng, X.; Zhu, T.T.; Xiong, J.F.; Xu, Y.H.; Xu, Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef]
- Shimizu, H.; Masujima, Y.; Ushiroda, C.; Mizushima, R.; Taira, S.; Ohue-Kitano, R.; Kimura, I. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Sci. Rep. 2019, 9, 16574. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Kankaanpää, P.E.; Salminen, S.J.; Isolauri, E.; Lee, Y.K. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol. Lett. 2001, 194, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Nagatake, T.; Kunisawa, J. Emerging roles of metabolites of ω3 and ω6 essential fatty acids in the control of intestinal inflammation. Int. Immunol. 2019, 31, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Webster, G.F.; Webster, T.G.; Grimes, L.R. Laboratory tests in patients treated with isotretinoin: Occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol. Online J. 2017, 23, 77. [Google Scholar]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar]
- Andreesen, J.R.; Schaupp, A.; Neurauter, C.; Brown, A.; Ljungdahl, L.G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: Effect of metals on growth yield, enzymes, and the synthesis of acetate from CO2. J. Bacteriol. 1973, 114, 743–751. [Google Scholar] [CrossRef]
- Basra, S.; Anand, B.S. Definition, epidemiology and magnitude of alcoholic hepatitis. World J. Hepatol. 2011, 3, 108–113. [Google Scholar] [CrossRef]
- Maddrey, W.C.; Boitnott, J.K.; Bedine, M.S.; Weber, F.L.; Mezey, E.; White, R.I. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978, 75, 193–199. [Google Scholar] [CrossRef]
| SCFAs | Kpn C /Glucose | Kpn C /Linolenic acid | Kpn K1 /Glucose | Kpn K1 / Linolenic acid |
|---|---|---|---|---|
| Acetate (mM) | ||||
| Propionate (µM) | ||||
| Isobutyrate (µM) | ||||
| Butyrate (µM) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.Y.; Raymond Herr, D.; Moochhala, S. Manipulation of Alcohol and Short-Chain Fatty Acids in the Metabolome of Commensal and Virulent Klebsiella pneumoniae by Linolenic Acid. Microorganisms 2020, 8, 773. https://doi.org/10.3390/microorganisms8050773
Huang RY, Raymond Herr D, Moochhala S. Manipulation of Alcohol and Short-Chain Fatty Acids in the Metabolome of Commensal and Virulent Klebsiella pneumoniae by Linolenic Acid. Microorganisms. 2020; 8(5):773. https://doi.org/10.3390/microorganisms8050773
Chicago/Turabian StyleHuang, Ryan Yuki, Deron Raymond Herr, and Shabbir Moochhala. 2020. "Manipulation of Alcohol and Short-Chain Fatty Acids in the Metabolome of Commensal and Virulent Klebsiella pneumoniae by Linolenic Acid" Microorganisms 8, no. 5: 773. https://doi.org/10.3390/microorganisms8050773
APA StyleHuang, R. Y., Raymond Herr, D., & Moochhala, S. (2020). Manipulation of Alcohol and Short-Chain Fatty Acids in the Metabolome of Commensal and Virulent Klebsiella pneumoniae by Linolenic Acid. Microorganisms, 8(5), 773. https://doi.org/10.3390/microorganisms8050773





