Staphylococcus sciuri Strain LCHXa is a Free-Living Lithium-Tolerant Bacterium Isolated from Salar de Atacama, Chile
Abstract
1. Introduction
2. Materials and Methods
3. Results
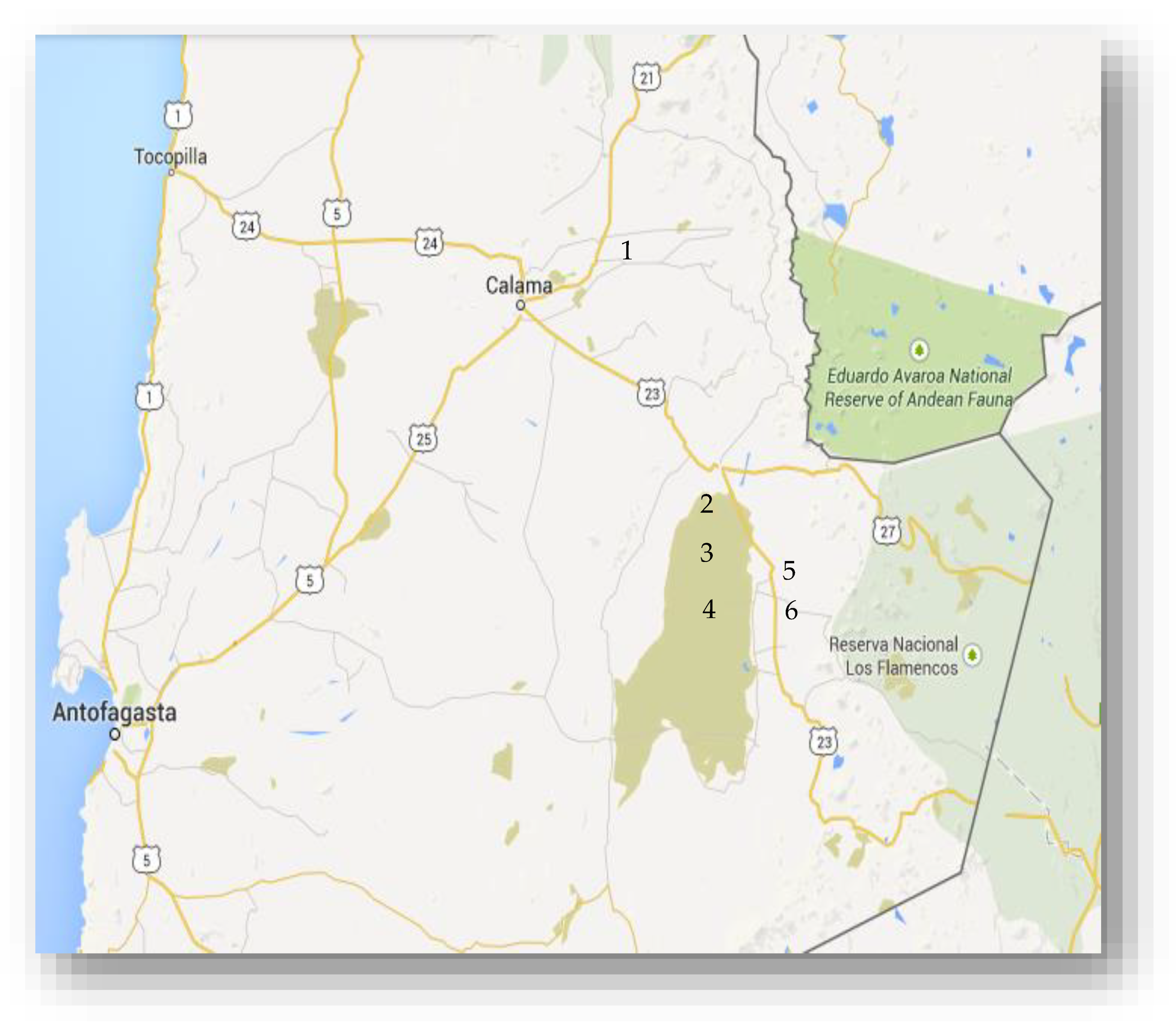
3.1. Sites of Collection
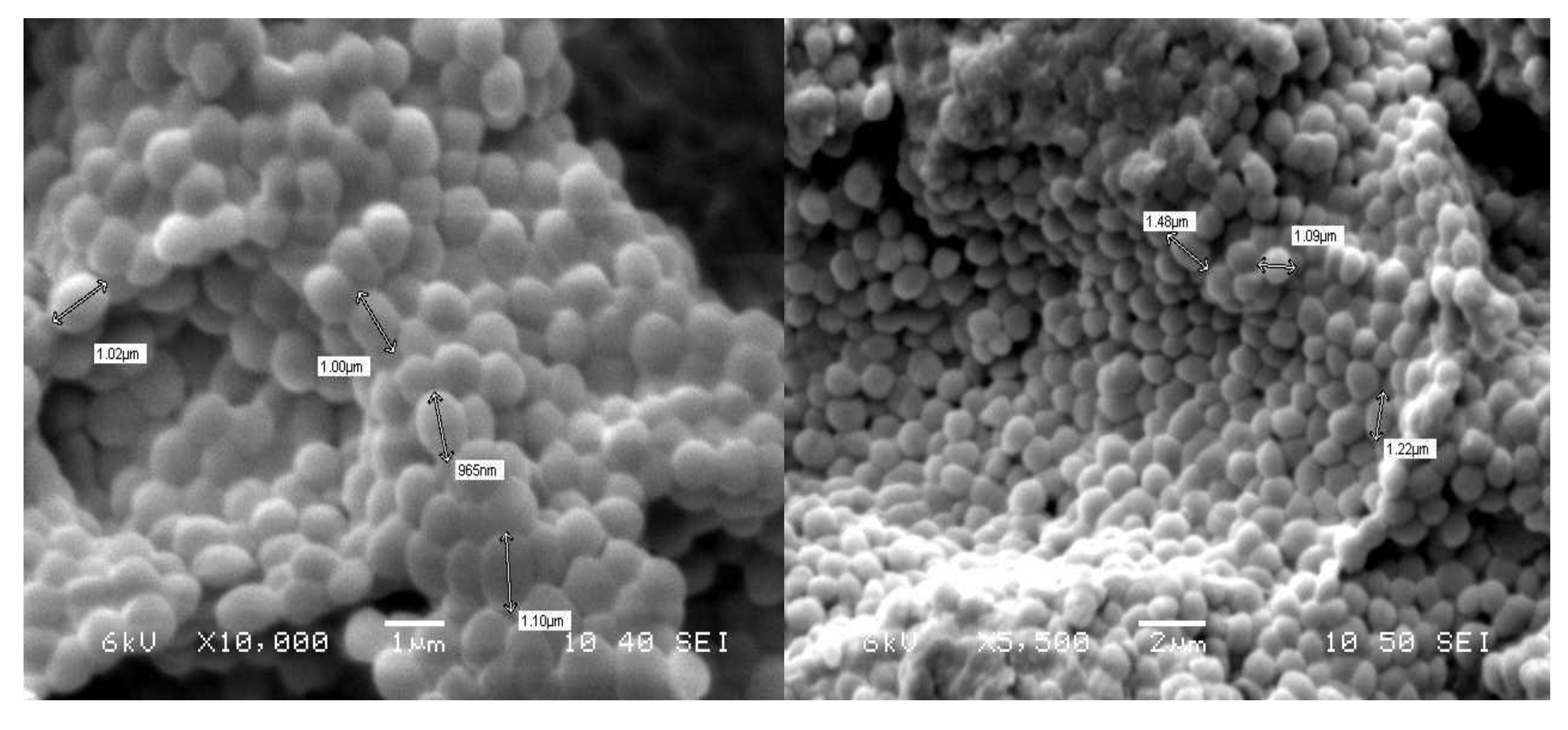
3.2. Morphology of Strain
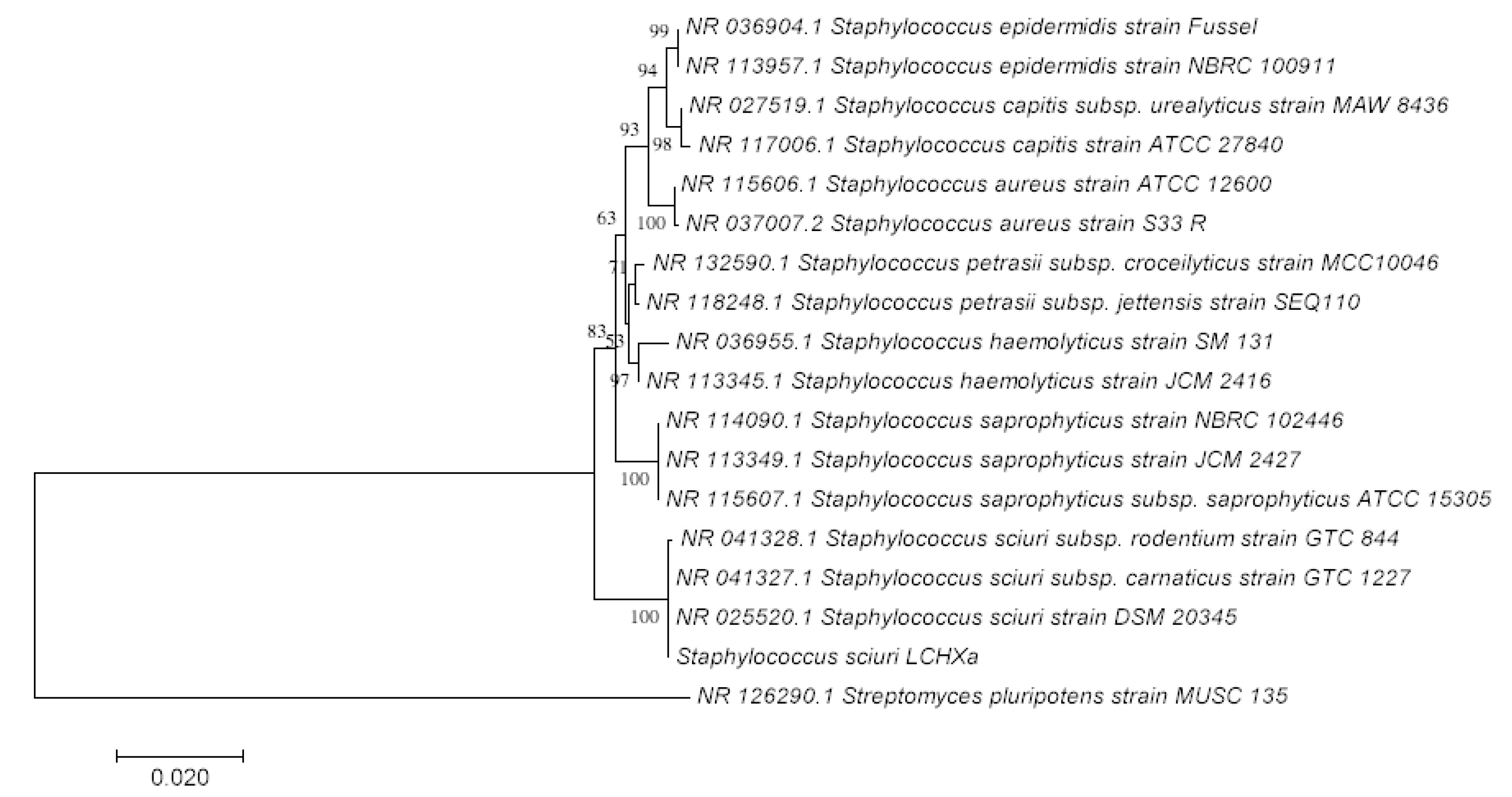
3.3. Phylogeny of Strain LCHXa
3.4. Metabolic Capabilities of Staphylococcus sciuri Strain LCHXa
3.5. Antibiotic Susceptibility of Staphylococcus sciuri Strain LCHXa
3.6. Optimal Temperature and pH for Growth of Staphylococcus sciuri Strain LCHXa
3.7. Effect of Sodium or Lithium Chloride on Staphylococcus sciuri Strain LCHXa Growth
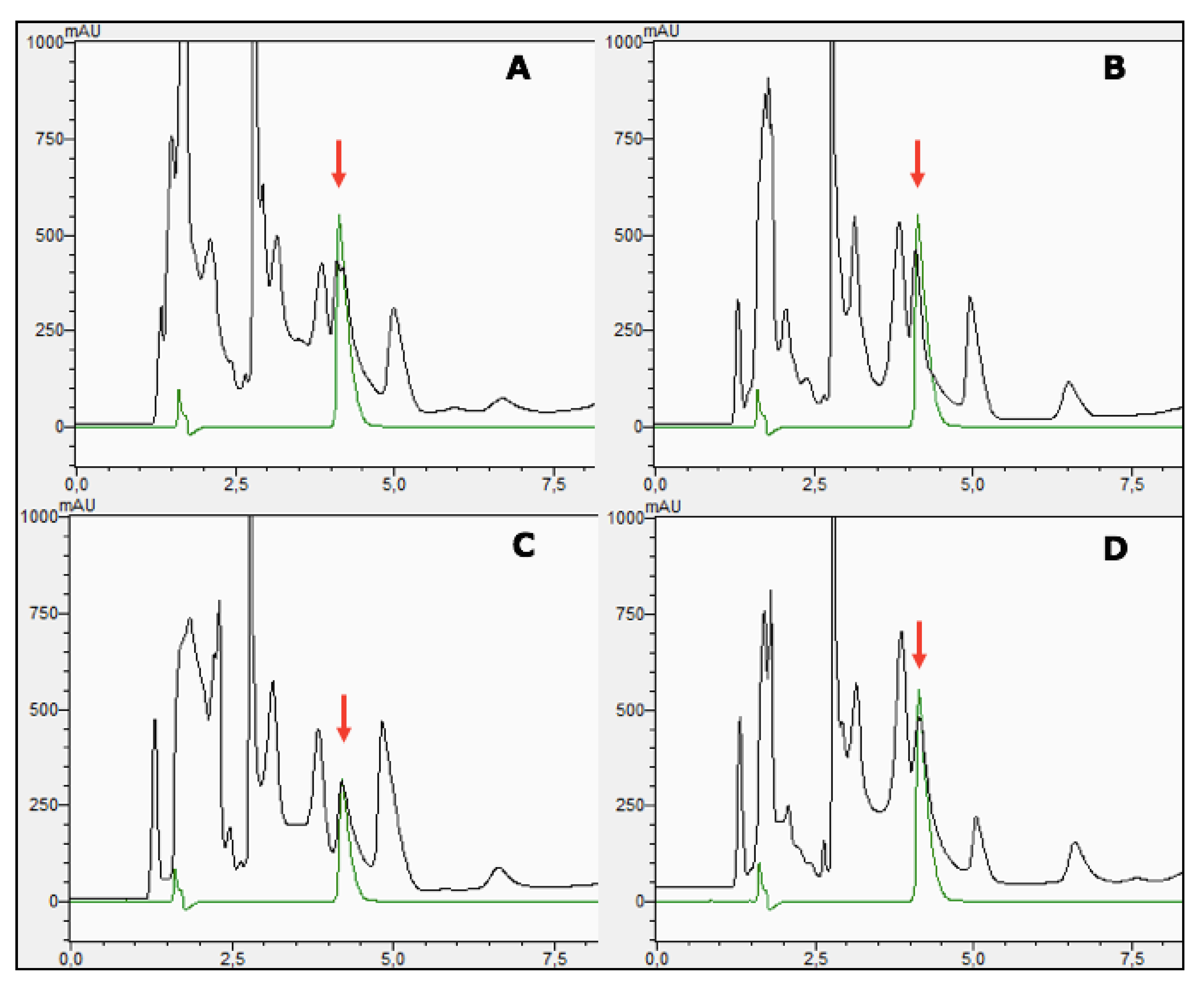
3.8. An Insight on the Osmoregulatory Mechanisms of Staphylococcus sciuri Strain LCHXa
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bull, A.T.; Asenjo, J.A.; Goodfellow, M.; Gómez-Silva, B. The Atacama Desert: Technical Resources and the Growing Importance of Novel Microbial Diversity. Ann. Rev. Microbiol. 2016, 70, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gonzalez, R.; Rainey, F.A.; Molina, P.; Bagaley, D.R.; Hollen, B.J.; de la Rosa, J.; Small, A.M.; Quinn, R.C.; Grunthaner, F.J.; Cáceres, L.; et al. Mars-like soils in the Atacama Desert, Chile, and the dry limit of microbial life. Science 2003, 302, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Silva, B. Lithobiontic life: Atacama rocks are well and alive. Antoine Leeuwenhoek 2018, 111, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Silva, B.; Vilo-Muñoz, C.; Galetovic, A.; Dong, Q.; Castelan-Sanchez, H.; Pérez-Llano, Y.; Sánchez-Carbente, M.; Dávila-Ramos, S.; Cotes-López, N.; Martínez-Ávila, L.; et al. Metagenomics of Atacama lithobiontic extremophile life unveil highlights on fungal communities, biogeochemical cycles and carbohydrate-active enzymes. Microorganism 2019, 7, 619. [Google Scholar] [CrossRef]
- Alonso, H.; Risacher, F. Geoquímica del Salar de Atacama, parte 1: Origen de los componentes y balance salino. Rev. Geol. Chile 1996, 23, 113–122. [Google Scholar]
- El Desarrollo Del Litio en Chile: 1984–2012. Available online: http://www.gustavolagos.cl/uploads/1/2/4/2/12428079/el_desarrollo_del_litio_en_chile_g._lagos_21-8-12.pdf (accessed on 13 October 2019).
- Kavanagh, L.; Keohane, J.; Garcia Cabellos, G.; Lloyd, A.; Cleary, J. Global lithium sources-industrial use and future in the electric vehicle industry: A review. Resources 2018, 7, 57. [Google Scholar] [CrossRef]
- Kamekura, M.; Onishi, H. Cell-associated cations of the moderate halophile Micrococcus varians ssp. halophilus grown in media of high concentrations of LiCl, NaCl, KCl, RbCl, or CsCl. Can. J. Microbiol. 1982, 28, 155–161. [Google Scholar]
- Tsurutu, T. Removal and recovery of lithium using various microorganisms. J. Biosci. Bioeng. 2005, 100, 562–566. [Google Scholar] [CrossRef]
- Yilmaz, F.; Orman, N.; Serim, G.; Kochan, C.; Ergene, A.; Icgen, B. Surface water-borne multidrug and heavy metal-resistant Staphylococcus isolates characterized by 16 rDNA sequencing. Bull. Environ. Contam. Toxicol. 2013, 697–703. [Google Scholar] [CrossRef]
- Belfiore, C.; Curia, M.V.; Farías, M.E. Characterization of Rhodococcus sp. A5wh isolated from a high-altitude Andean lake to unravel the survival strategy under lithium stress. Rev. Argent. Microbiol. 2018, 50, 311–322. [Google Scholar] [CrossRef]
- Cubillos, C.F.; Aguilar, P.; Grágeda, M.; Dorador, C. Microbial communities from the world’s largest lithium reserve, salar de Atacama, Chile: Life at high LiCl concentrations. J. Geophys. Res. Biogeosci. 2018, 123, 3668–3681. [Google Scholar] [CrossRef]
- Cubillos, C.F.; Paredes, A.; Yáñez, C.; Palma, J.; Severino, E.; Vejar, D.; Grágeda, M.; Dorador, C. Insights into the Microbiology of chaotropic brines of salar de Atacama, Chile. Front. Microbiol. 2019, 10, 1611. [Google Scholar] [CrossRef] [PubMed]
- Vilo, C.; Salazar-Ardiles, C.; Caimanque, T.N.; Dong, Q.; Flores, N.; Galetovic, A.; Araya, J.E.; Gómez-Silva, B. Draft genome sequence of Staphylococcus sciuri strain LCHXa, a lithium-tolerant bacterium isolated from Laguna Chaxa, Salar de Atacama, Chile. Microbiol. Resour. Announc. 2019, 8, e0154918. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, T.; Cortecchi, G.; Barbieri, M.; Mussi, M. New and past geochemical data on fresh to brine waters of the Salar de Atacama and Andean Altiplano, northern Chile. Geofluids 2007, 7, 33–50. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Parker, J. Brock’s Biology of Microorganisms, 8th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2004; 1036p, ISBN 978-0135208755. [Google Scholar]
- Gamazo, C.; Lopez-Goñi, I.; Díaz, R. Manual Práctico de Microbiología, 3rd ed.; Elsevier Masson: Barcelona, España, 2005; 264p, ISBN 9788445815199. [Google Scholar]
- Emarin, S.D.A. Antibiotic Provider. Available online: https://www.emarinsda.com (accessed on 3 November 2019).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water-soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Kunte, H.J.; Galinski, E.A.; Trüper, H.G. A modified FMOC-method for the detection of amino acid-type osmolytes and tetrahydropyrimidines (ectoines). J. Microbiol. Meth. 1993, 17, 129–136. [Google Scholar] [CrossRef]
- Dakić, I.; Morrison, D.; Vuković, D.; Savić, B.; Shittu, A.; Ježek, P.; Hauschild, T.; Stepanović, S. Isolation and molecular characterization of Staphylococcus sciuri in the hospital environment. J. Clin. Microbiol. 2005, 43, 2782–2785. [Google Scholar] [CrossRef]
- Boch, J.; Kempf, B.; Bremer, E. Osmoregulation in Bacillus subtilis: Synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 1994, 176, 5364–5371. [Google Scholar] [CrossRef]
- Boch, J.; Kempf, B.; Schmid, R.; Bremer, E. Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: Characterization of the gbsAB genes. J. Bacteriol. 1996, 178, 5121–5129. [Google Scholar] [CrossRef]
- Boch, J.; Nau-Wagner, G.; Kneip, S.; Bremer, E. Glycine betaine aldehyde dehydrogenase from Bacillus subtilis: Characterization of an enzyme required for the synthesis of the osmoprotectant glycine betaine. Arch. Microbiol. 1997, 168, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Kloos, W.E. Natural populations of the genus Staphylococcus. Ann. Rev. Microbiol. 1980, 34, 559–592. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Hauschild, T.; Dakic, I.; Al-Doori, Z.; Svabic-Vlahovic, M.; Ranin, L.; Morrison, D. Evaluation of phenotypic and molecular methods for detection of oxacillin-resistance in members of the Staphylococcus sciuri group. J. Clin. Microbiol. 2006, 44, 934–937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bakan, G.; Böke Özkoc, H.; Tülek, S.; Cüce, H. Integrated environmental quality assessment of Kizilirmak River and its coastal environment. Turk. J. Fish. Aqua. Sci. 2010, 10, 453–462. [Google Scholar] [CrossRef]
- Cochilco, Gobierno de Chile. Compilación de Informes Sobre: Mercado Internacional Del Litio y El Potencial de Litio en Salares Del Norte de Chile. 2013. Available online: https://www.cochilco.cl/Mercado%20de%20Metales/InformeLi.pdf (accessed on 30 October 2019).
- Graham, J.E.; Wilkinson, B.J. Staphylococcus aureus osmoregulation: Roles of choline, glycine betaine, proline and taurine. J. Bacteriol. 1992, 174, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Anyango, L.A.; Alredishi, M.M. Adaptive metabolism in Staphylococci: Survival and persistence in environmental and clinical settings. J. Pathogens. 2018, 2018, 1092632. [Google Scholar] [CrossRef]
- Wood, J.M. Bacterial response to osmotic challenges. J. Gen. Physiol. 2015, 145, 381–388. [Google Scholar] [CrossRef]
- Wie Wu, S.; de Lencastre, H.; Tomasz, A. Recruitment of the mecA gene homologue of Staphylococcus sciuri into a resistance determinant and expression of the resistant phenotype in Staphylococcus aureus. J. Bacteriol. 2001, 183, 2417–2424. [Google Scholar] [CrossRef][Green Version]
- Couto, I.; de Lencastre, H.; Severina, E.; Kloos, W.; Romero, J.A.; Ubner, R.J.; Santos Sanchez, I.; Tomasz, A. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microbl. Drug Resist. 2009, 2. [Google Scholar] [CrossRef]
- Nemeghaire, S.; Argudín, M.A.; Febler, A.T.; Hauschild, T.; Schwarz, S.; Butaye, P. The ecological importance of the Staphylococcus sciuri species group as a reservoir for resistance and virulence genes. Vet. Microbiol. 2014, 171, 342–356. [Google Scholar] [CrossRef]
- Miller, K.J.; Zelt, S.C.; Bae, J. Glycine betaine and proline are the principal compatible solutes of Staphylococcus aureus. Curr. Microbiol. 1991, 23, 131–137. [Google Scholar] [CrossRef]
- Pourkomailian, B.; Booth, I.R. Glycine betaine transport by Staphylococcus aureus: Evidence for two transport systems and for their possible roles in osmoregulation. J. Gen. Microbiol. 1992, 138, 2515–2518. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.M.; Cole, M.B.; Legan, J.D.; Slade, L.; Schaffner, D.W. Solute-specific effects of osmotic stress on Staphylococcus aureus. J. Appl. Microbiol. 2005, 98, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R.; Wetzel, K.J. Osmolyte transport in Staphylococcus aureus and the role in pathogenesis. World J. Clin. Infect. Dis. 2016, 6, 22–27. [Google Scholar] [CrossRef]
| Biochemical Test | Strain LCHXa |
|---|---|
| Simmons citrate | - |
| Mobility | - |
| Indole | - |
| Ornitina | - |
| TSI | A/A |
| LIA | K/K |
| Coagulase | - |
| Catalase | + |
| Oxidase | - |
| Starch hydrolysis | - |
| Casein hydrolysis | + |
| Tween 60 hydrolysis | - |
| Tween 80 hydrolysis | - |
| DNA hydrolysis | + |
| Glucose fermentation | + |
| Lactose fermentation | + |
| Mannitol fermentation | + |
| Xylose fermentation | + |
| Cellobiose fermentation | + |
| N-acetylglucosamine fermentation | - |
| Hemolysis | β |
| +: positive reaction; -: negative reaction;.β: beta hemolysis; A: acid; K: base. | |
| Antibiotic | Potency (μg) | Susceptibility (Inhibition Zone in mm) | ||
|---|---|---|---|---|
| MH | MH-Na | MH-Li | ||
| Ampicillin/Sulbactam | 10/10 | R (0) | R (0) | R (27) |
| Lincomycin | 2 | R (13) | R (16) | R (16) |
| Clindamycin | 2 | R (0) | R (11) | S (23) |
| Penicillin | 10 | R (0) | R (10) | R (21) |
| Ampicillin | 10 | R (0) | R (0) | S (29) |
| Ceftazidime | 30 | R (0) | R (10) | R (0) |
| Fosfomycin | 200 | R (0) | R (0) | S (40) |
| Oxacillin | 1 | R (0) | R (0) | S (14) |
| Amoxicillin | 20/10 | R (14) | R (14) | S (31) |
| Novobiocin | 5 | R (0) | R (16) | R (10) |
| Cefuroxime | 30 | R (16) | S (24) | R (11) |
| Gentamicin | 10 | S (30) | S (25) | S (25) |
| Tetracycline | 30 | S (26) | S (30) | S (22) |
| Vancomycin | 30 | S (20) | S (24) | S (21) |
| Cotrimoxazole | 25 | S (28) | S (32) | nt |
| Erythromycin | 15 | S (22) | S (30) | nt |
| Ciprofloxacin | 5 | S (34) | S (30) | S (31) |
| Chloramphenicol | 30 | S (24) | S (28) | nt |
| Neomycin | 30 | S (21) | S (20) | nt |
| Nitrofurantoin | 300 | S (25) | S (29) | S (22) |
| Cefadroxil | 30 | S (18) | S (15) | S (29) |
| Streptomycin | 300 | S (28) | S (22) | S (26) |
| Levofloxacin | 5 | S (31) | S (30) | S 30) |
| Teicoplanin | 30 | S (17) | S (20) | nt |
| Linezolid | 30 | S (30) | S (32) | nt |
| Amikacin | 30 | S (26) | S (22) | S (23) |
| Concentration (M) | Specific Growth Rate (h−1) | |
|---|---|---|
| NaCl | LiCl | |
| 0.0 | 0.41 ± 0.01 | 0.41 ± 0.01 |
| 0.2 | 0.35 ± 0.01 | 0.39 ± 0.01 |
| 0.4 | 0.35 ± 0.01 | 0.34 ± 0.03 |
| 0.8 | 0.32 ± 0.04 | 0.29 ± 0.01 |
| 1.0 | 0.33 ± 0.02 | 0.31 ± 0.02 |
| 1.5 | 0.19 ± 0.04 | 0.18 ± 0.02 |
| 2.0 | 0.10 ± 0.01 | 0.06 ± 0.01 |
| Microorganism | Specific Growth Rate (h−1) | ||
|---|---|---|---|
| LB | Na-LB | Li-LB | |
| S. sciuri strain LCHXa | 0.58 ± 0.01 | 0.28 ± 0.01 (51) | 0.13 ± 0.01 (78) |
| S. saprophyticus | 0.26 ± 0.01 | 0.13 ± 0.02 (50) | 0.08 ± 0.01 (69) |
| S. aureus | 0.76 ± 0.03 | 0.31 ± 0.01 (59) | 0.18 ± 0.01 (76) |
| S. epidermidis | 0.24 ± 0.01 | 0.32 ± 0.07 (133) | 0.19 ± 0.01 (21) |
| E. coli | 1.42 ± 0.02 | 0.42 ± 0.23 (70) | 0.03 ± 0.00 (98) |
| Function | Subsystem | Gene Copy |
|---|---|---|
| Glycerol uptake facilitator protein | Osmoregulation | 1 |
| Glycine betaine ABC transport system, glycine betaine-binding protein OpuAC | Choline and betaine Uptake and betaine biosynthesis | 2 |
| Osmotically activated L-carnitine/choline ABC transporter, permease protein OpuAC | Choline and betaine Uptake and betaine biosynthesis | 1 |
| Osmotically activated L-carnitine/choline ABC transporter, permease protein OpuCB | Choline and betaine Uptake and betaine biosynthesis | 1 |
| Glycine betaine ABC transport system, ATP binding protein OpuAA (EC 3.6.3.32) | Choline and betaine Uptake and betaine biosynthesis | 2 |
| Betaine aldehyde dehydrogenase (EC 1.2.1.8) | Choline and betaine Uptake and betaine biosynthesis | 1 |
| Glycine betaine transporter OpuD | Choline and betaine Uptake and betaine biosynthesis | 3 |
| Osmotically activated L-carnitine/choline ABC transporter, substrate-binding protein OpuCD | Choline and betaine Uptake and betaine biosynthesis | 1 |
| L-proline glycine betaine binding ABC transporter protein ProX (TC 3.A.1.12.1) | Choline and betaine Uptake and betaine biosynthesis | 1 |
| Osmotically activated L-carnitine/choline ABC transporter, substrate-binding protein OpuCC | Choline and betaine Uptake and betaine biosynthesis | 2 |
| Choline dehydrogenase (EC 1.1.99.1) | Choline and betaine Uptake and betaine biosynthesis | 1 |
| B. Subtilis Gene | Metabolic Function | % Identity | Size (bp) |
|---|---|---|---|
| gbsA (Locus tag: BSU_31060) | Betaine aldehyde dehydrogenase | 66% | 1490 |
| gbsB (Locus tag: BSU_31050) | Choline dehydrogenase | 30% | 1682 |
| gbsR (Locus tag: BSU_31070) | Betaine operon transcriptional regulator | 57% | 554 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Ardiles, C.; Caimanque, T.; Galetović, A.; Vilo, C.; Araya, J.E.; Flores, N.; Gómez-Silva, B. Staphylococcus sciuri Strain LCHXa is a Free-Living Lithium-Tolerant Bacterium Isolated from Salar de Atacama, Chile. Microorganisms 2020, 8, 668. https://doi.org/10.3390/microorganisms8050668
Salazar-Ardiles C, Caimanque T, Galetović A, Vilo C, Araya JE, Flores N, Gómez-Silva B. Staphylococcus sciuri Strain LCHXa is a Free-Living Lithium-Tolerant Bacterium Isolated from Salar de Atacama, Chile. Microorganisms. 2020; 8(5):668. https://doi.org/10.3390/microorganisms8050668
Chicago/Turabian StyleSalazar-Ardiles, Camila, Tamara Caimanque, Alexandra Galetović, Claudia Vilo, Jorge E. Araya, Nataly Flores, and Benito Gómez-Silva. 2020. "Staphylococcus sciuri Strain LCHXa is a Free-Living Lithium-Tolerant Bacterium Isolated from Salar de Atacama, Chile" Microorganisms 8, no. 5: 668. https://doi.org/10.3390/microorganisms8050668
APA StyleSalazar-Ardiles, C., Caimanque, T., Galetović, A., Vilo, C., Araya, J. E., Flores, N., & Gómez-Silva, B. (2020). Staphylococcus sciuri Strain LCHXa is a Free-Living Lithium-Tolerant Bacterium Isolated from Salar de Atacama, Chile. Microorganisms, 8(5), 668. https://doi.org/10.3390/microorganisms8050668





