Application of Recombinase-Based In Vivo Expression Technology to Bifidobacterium longum subsp. longum for Identification of Genes Induced in the Gastrointestinal Tract of Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Animal Experiments
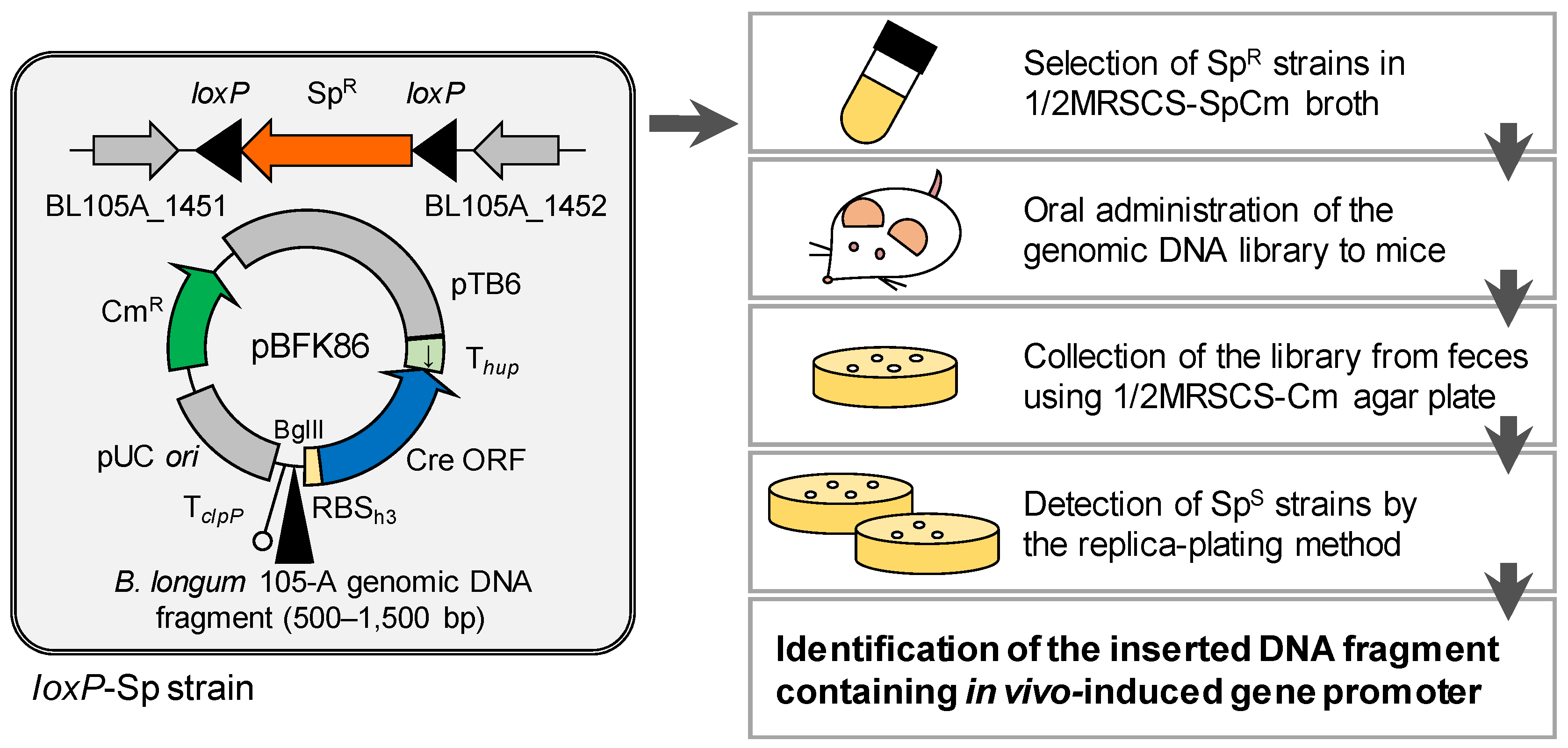
2.3. Generation of the B. longum Strain for the R-IVET System
2.4. Construction of a Plasmid Harboring the Cre Gene for the R-IVET System
2.4.1. Cloning of a Promoterless Cre Gene with an RBS
2.4.2. Insertion of a Transcriptional Terminator
2.4.3. Insertion of a Promoter
2.5. Evaluation of Basal Cre Expression Levels in Promoterless Cre Plasmids
2.6. Construction of the Genomic DNA Library
2.7. Screening for In Vivo-Induced Genes in B. longum
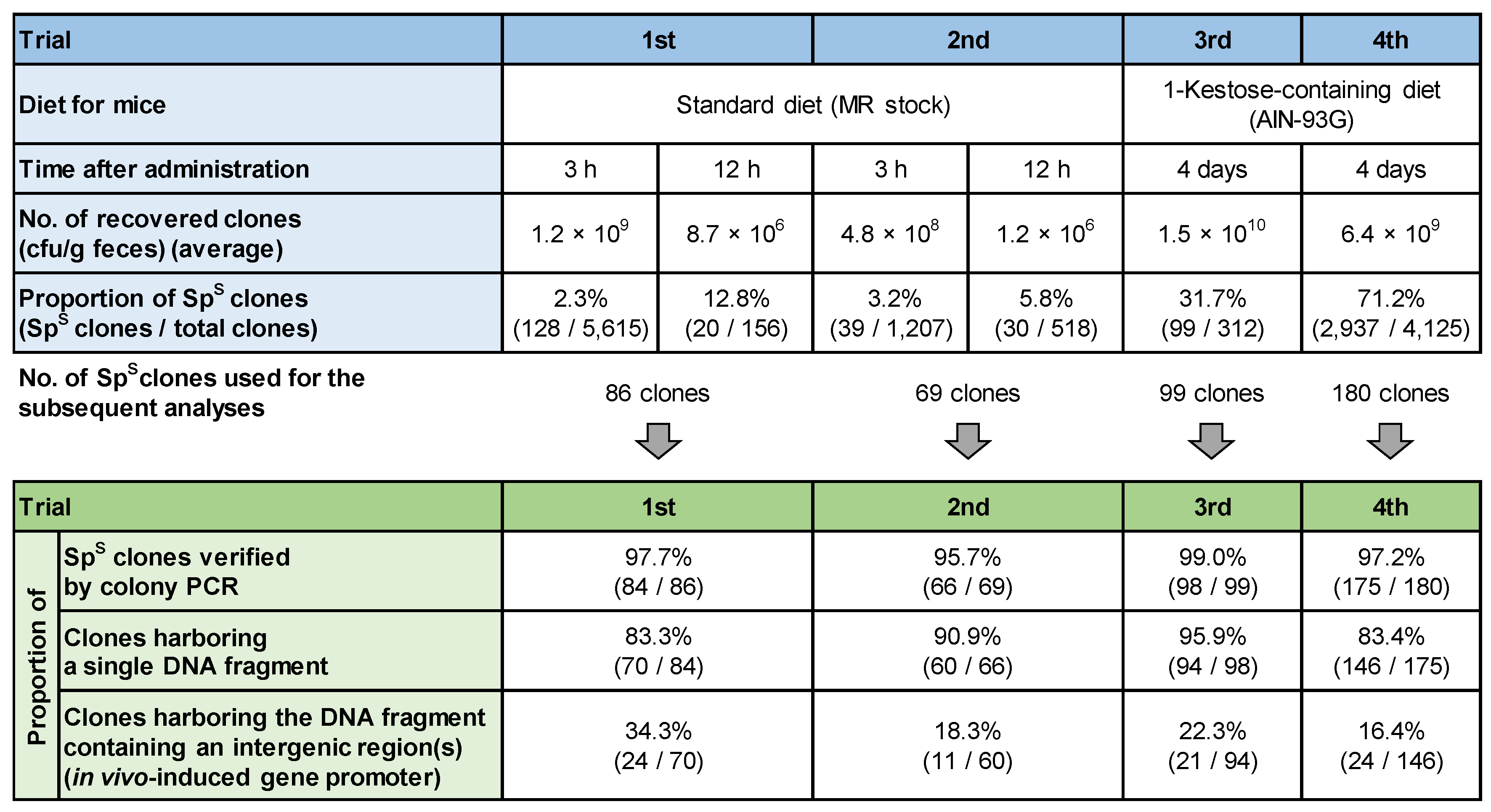
2.7.1. First and Second Trials
2.7.2. Third and Fourth Trials
2.8. RNA Extraction and qRT-PCR Analysis to Verify Specific In Vivo Gene Expression
2.8.1. Administration of B. longum 105-A Harboring pBFS63
2.8.2. RNA Extraction and qRT-PCR Analysis
3. Results
3.1. Development of the R-IVET System for B. longum 105-A
3.2. Construction of the Genomic DNA Library
3.3. Screening of In Vivo-Induced Genes
3.3.1. First and Second Trials
3.3.2. Third and Fourth Trials
3.4. Verification of In Vivo-Induced Gene Expression in the Cecum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, M.; Hansen, M.E.; Gotoh, A.; Katoh, T.; Yoshida, K.; Odamaki, T.; Yachi, H.; Sugiyama, Y.; Kurihara, S.; Hirose, J.; et al. Evolutionary adaptation in fucosyllactose uptake systems supports bifidobacteria-infant symbiosis. Sci. Adv. 2019, 5, eaaw7696. [Google Scholar] [CrossRef]
- Mattarelli, P.; Biavati, B. Species in the genus Bifidobacterium. In The Bifidobacteria and Related Organisms: Biology, Taxonomy, Applications; Mattarelli, P., Biavati, B., Holzapfel, W.H., Wood, B.J.B., Eds.; Academic Press: London, UK, 2018; pp. 9–48. [Google Scholar] [CrossRef]
- Kato, K.; Odamaki, T.; Mitsuyama, E.; Sugahara, H.; Xiao, J.Z.; Osawa, R. Age-related changes in the composition of gut Bifidobacterium species. Curr. Microbiol. 2017, 74, 987–995. [Google Scholar] [CrossRef]
- Odamaki, T.; Bottacini, F.; Kato, K.; Mitsuyama, E.; Yoshida, K.; Horigome, A.; Xiao, J.Z.; van Sinderen, D. Genomic diversity and distribution of Bifidobacterium longum subsp. longum across the human lifespan. Sci. Rep. 2018, 8, 85. [Google Scholar] [CrossRef]
- Milani, C.; Mangifesta, M.; Mancabelli, L.; Lugli, G.A.; James, K.; Duranti, S.; Turroni, F.; Ferrario, C.; Ossiprandi, M.C.; van Sinderen, D.; et al. Unveiling bifidobacterial biogeography across the mammalian branch of the tree of life. ISME J. 2017, 11, 2834–2847. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and their health-promoting effects. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Leahy, S.C.; Higgins, D.G.; Fitzgerald, G.F.; van Sinderen, D. Getting better with bifidobacteria. J. Appl. Microbiol. 2005, 98, 1303–1315. [Google Scholar] [CrossRef]
- Fujita, K.; Sasaki, Y.; Kitahara, K. Degradation of plant arabinogalactan proteins by intestinal bacteria: Characteristics and functions of the enzymes involved. Appl. Microbiol. Biotechnol. 2019, 103, 7451–7457. [Google Scholar] [CrossRef]
- Katayama, T. Host-derived glycans serve as selected nutrients for the gut microbe: Human milk oligosaccharides and bifidobacteria. Biosci. Biotechnol. Biochem. 2016, 80, 621–632. [Google Scholar] [CrossRef]
- Suzuki, K.; Nishiyama, K.; Miyajima, H.; Osawa, R.; Yamamoto, Y.; Mukai, T. Adhesion properties of a putative polymorphic fimbrial subunit protein from Bifidobacterium longum subsp. longum. Biosci. Microbiota Food Health 2016, 35, 19–27. [Google Scholar] [CrossRef]
- McCarville, J.L.; Dong, J.; Caminero, A.; Bermudez-Brito, M.; Jury, J.; Murray, J.A.; Duboux, S.; Steinmann, M.; Delley, M.; Tangyu, M.; et al. A commensal Bifidobacterium longum strain prevents gluten-related immunopathology in mice through expression of a serine protease inhibitor. Appl. Environ. Microbiol. 2017, 83, e01323–e01417. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Chen, C.T.L.; Gordon, J.I. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006, 4, e413. [Google Scholar] [CrossRef]
- Sugahara, H.; Odamaki, T.; Fukuda, S.; Kato, T.; Xiao, J.Z.; Abe, F.; Kikuchi, J.; Ohno, H. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci. Rep. 2015, 5, 13548. [Google Scholar] [CrossRef]
- Ishikawa, E.; Shima, T.; Suda, K.; Shirasawa, Y.; Sato, T.; Umesaki, Y. Comparison of Bifidobacterium breve strain Yakult transcriptomes in germ-free mice with those in fecal cultures. J. Biosci. Bioeng. 2011, 112, 451–457. [Google Scholar] [CrossRef]
- O’Connell-Motherway, M.; Zomer, A.; Leahy, S.C.; Reunanen, J.; Bottacini, F.; Claesson, M.J.; O’Brien, F.; Flynn, K.; Casey, P.G.; Munoz, J.A.M.; et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc. Natl. Acad. Sci. USA 2011, 108, 11217–11222. [Google Scholar] [CrossRef]
- O’Connell-Motherway, M.; O’Brien, F.; O’Driscoll, T.; Casey, P.G.; Shanahan, F.; van Sinderen, D. Carbohydrate syntrophy enhances the establishment of Bifidobacterium breve UCC2003 in the neonatal gut. Sci. Rep. 2018, 8, 10627. [Google Scholar] [CrossRef]
- Bron, P.A.; Grangette, C.; Mercenier, A.; de Vos, W.M.; Kleerebezem, M. Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. J. Bacteriol. 2004, 186, 5721–5729. [Google Scholar] [CrossRef]
- Bachmann, H.; Kleerebezem, M.; van Hylckama Vlieg, J.E.T. High-throughput identification and validation of in situ-expressed genes of Lactococcus lactis. Appl. Environ. Microbiol. 2008, 74, 4727–4736. [Google Scholar] [CrossRef]
- Bachmann, H.; de Wilt, L.; Kleerebezem, M.; van Hylckama Vlieg, J.E.T. Time-resolved genetic responses of Lactococcus lactis to a dairy environment. Environ. Microbiol. 2010, 12, 1260–1270. [Google Scholar] [CrossRef]
- Hanin, A.; Sava, I.; Bao, Y.Y.; Huebner, J.; Hartke, A.; Auffray, Y.; Sauvageot, N. Screening of in vivo activated genes in Enterococcus faecalis during insect and mouse infections and growth in urine. PLoS ONE 2010, 5, e11879. [Google Scholar] [CrossRef]
- Junjua, M.; Galia, W.; Gaci, N.; Uriot, O.; Genay, M.; Bachmann, H.; Kleerebezem, M.; Dary, A.; Roussel, Y. Development of the recombinase-based in vivo expression technology in Streptococcus thermophilus and validation using the lactose operon promoter. J. Appl. Microbiol. 2014, 116, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Uriot, O.; Galia, W.; Awussi, A.A.; Perrin, C.; Denis, S.; Chalancon, S.; Lorson, E.; Poirson, C.; Junjua, M.; Le Roux, Y.; et al. Use of the dynamic gastro-intestinal model TIM to explore the survival of the yogurt bacterium Streptococcus thermophilus and the metabolic activities induced in the simulated human gut. Food Microbiol. 2016, 53, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Takeuchi, A.; Kano, Y. Construction of Escherichia coli–Bifidobacterium longum shuttle vector transforming B. longum 105-A and 108-A. Biosci. Biotechnol. Biochem. 1997, 61, 1211–1212. [Google Scholar] [CrossRef] [PubMed]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Hirayama, Y.; Sakanaka, M.; Fukuma, H.; Murayama, H.; Kano, Y.; Fukiya, S.; Yokota, A. Development of a double-crossover markerless gene deletion system in Bifidobacterium longum: Functional analysis of the α-galactosidase gene for raffinose assimilation. Appl. Environ. Microbiol. 2012, 78, 4984–4994. [Google Scholar] [CrossRef]
- Kanegae, Y.; Lee, G.; Sato, Y.; Tanaka, M.; Nakal, M.; Sakaki, T.; Sugano, S.; Saito, I. Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res. 1995, 23, 3816–3821. [Google Scholar] [CrossRef]
- Sakanaka, M.; Tamai, S.; Hirayama, Y.; Onodera, A.; Koguchi, H.; Kano, Y.; Yokota, A.; Fukiya, S. Functional analysis of bifidobacterial promoters in Bifidobacterium longum and Escherichia coli using the α-galactosidase gene as a reporter. J. Biosci. Bioeng. 2014, 118, 489–495. [Google Scholar] [CrossRef]
- Yasui, K.; Kano, Y.; Tanaka, K.; Watanabe, K.; Shimizu-Kadota, M.; Yoshikawa, H.; Suzuki, T. Improvement of bacterial transformation efficiency using plasmid artificial modification. Nucleic Acids Res. 2009, 37, e3. [Google Scholar] [CrossRef]
- He, J.; Sakaguchi, K.; Suzuki, T. Determination of the ribosome-binding sequence and spacer length between binding site and initiation codon for efficient protein expression in Bifidobacterium longum 105-A. J. Biosci. Bioeng. 2012, 113, 442–444. [Google Scholar] [CrossRef]
- Sternberg, N.; Sauer, B.; Hoess, R.; Abremski, K. Bacteriophage P1 cre gene and its regulatory region. Evidence for multiple promoters and for regulation by DNA methylation. J. Mol. Biol. 1986, 187, 197–212. [Google Scholar] [CrossRef]
- Llanos, R.M.; Harris, C.J.; Hillier, A.J.; Davidson, B.E. Identification of a novel operon in Lactococcus lactis encoding three enzymes for lactic acid synthesis: Phosphofructokinase, pyruvate kinase, and lactate dehydrogenase. J. Bacteriol. 1993, 175, 2541–2551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pátek, M.; Hochmannová, J.; Jelínková, M.; Nešvera, J.; Eggeling, L. Analysis of the leuB gene from Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 1998, 50, 42–47. [Google Scholar] [CrossRef]
- Ruiz, L.; O’Connell-Motherway, M.; Lanigan, N.; van Sinderen, D. Transposon mutagenesis in Bifidobacterium breve: Construction and characterization of a Tn5 transposon mutant library for Bifidobacterium breve UCC2003. PLoS ONE 2013, 8, e64699. [Google Scholar] [CrossRef]
- Kanesaki, Y.; Masutani, H.; Sakanaka, M.; Shiwa, Y.; Fujisawa, T.; Nakamura, Y.; Yokota, A.; Fukiya, S.; Suzuki, T.; Yoshikawa, H. Complete genome sequence of Bifidobacterium longum 105-A, a strain with high transformation efficiency. Genome Announc. 2014, 2, e01311–e01314. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015, 43, D261–D269. [Google Scholar] [CrossRef]
- Sakanaka, M.; Nakakawaji, S.; Nakajima, S.; Fukiya, S.; Abe, A.; Saburi, W.; Mori, H.; Yokota, A. A transposon mutagenesis system for Bifidobacterium longum subsp. longum based on an IS3 family insertion sequence, ISBlo11. Appl. Environ. Microbiol. 2018, 84, e00824–e00918. [Google Scholar] [CrossRef]
- Tanno, H.; Fujii, T.; Ose, R.; Hirano, K.; Tochio, T.; Endo, A. Characterization of fructooligosaccharide-degrading enzymes in human commensal Bifidobacterium longum and Anaerostipes caccae. Biochem. Biophys. Res. Commun. 2019, 518, 294–298. [Google Scholar] [CrossRef]
- Clarke, L.; Carbon, J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell 1976, 9, 91–99. [Google Scholar] [CrossRef]
- Marco, M.L.; Bongers, R.S.; de Vos, W.M.; Kleerebezem, M. Spatial and temporal expression of Lactobacillus plantarum genes in the gastrointestinal tracts of mice. Appl. Environ. Microbiol. 2007, 73, 124–132. [Google Scholar] [CrossRef]
- Bottacini, F.; Zomer, A.; Milani, C.; Ferrario, C.; Lugli, G.A.; Egan, M.; Ventura, M.; van Sinderen, D. Global transcriptional landscape and promoter mapping of the gut commensal Bifidobacterium breve UCC2003. BMC Genom. 2017, 18, 991. [Google Scholar] [CrossRef] [PubMed]


| Strain | Description 1 | Source or Reference |
|---|---|---|
| Escherichia coli | ||
| E. coli DH5α | F−, Φ80d lacZΔM15, Δ(lacZYA-argF) U169, deoR, recA1, endA1, hsdR17(rK− mK+), phoA, supE44, λ−, thi-1, gyrA96, relA1 | National BioResource Project (NIG, Mishima, Japan) |
| Bifidobacterium | ||
| B. longum subsp. longum 105-A (JCM 31944) | Human fecal isolate | [25] |
| loxP-Sp strain | B. longum 105-A derivative strain harboring loxP-SpR-loxP cassette on the chromosome, SpR | This study |
| No. | PCR Product 1 | DNA Template | Cloning Strategy 2 | Primer | Nucleotide Sequence (5′-3′) 3 |
|---|---|---|---|---|---|
| Integration of loxP-SpR-loxP into a chromosome of B. longum 105-A (construction of loxP-Sp strain) | |||||
| 1 | SpR gene | pBS423 [27] | Blunt-end ligation | Pr-Blo0041 | GCATGCCTGCAGGTCGATTTTC |
| Pr-Blo0042 | CAAAAAAATTGAAAAAAGTGTTTCCAC | ||||
| 2 | Homologous region to BL105A_1451 locus (HR1) | B. longum 105-A genomic DNA | Restriction-ligation | Pr-Blo0100 | GCGAATTCATGACGTACGATTTCACGTCG |
| Pr-Blo0101 | TAGAATTCCGCAATCGCGGATGCATGCCGT | ||||
| 3 | Homologous region to BL105A_1452 locus (HR2) | B. longum 105-A genomic DNA | Restriction-ligation | Pr-Blo0098 | AGGGATCCGTGTCCTGGAAAGACGAATGCC |
| Pr-Blo0099 | TGGGATCCATGTCCGTTTCGCAGTCACCGG | ||||
| 4 | HR1-loxP-SpR-loxP-HR2 | pBFH35 (this study) | In-Fusion cloning | Pr-Blo0119 | TATATATGAGTACTGAGGTCGACTCTAGAGGATCC |
| Pr-Blo0120 | AAACGACGGCCAGTTAAACGACGGCCAGTGAATTG | ||||
| 5 | pBS423ΔrepA lacking SpR gene | pBFS423ΔrepA [27] | In-Fusion cloning | Pr-Blo0116 | CAGTACTCATATATACTTTAGATTGATTTA |
| Pr-Blo0117 | AAGCTTGCATGCCTGCAGATAGGCAG | ||||
| Construction of Cre expression plasmid | |||||
| 6 | CmR gene | pBFS38 [29] | In-Fusion cloning | Pr-Blo0239 | AAAGTATATATGAGTACTTGGGCGCGGCGGCCATGAAG |
| Pr-Blo0240 | GCGGCCGCGCCGGCATGCATTATGGAAGCGCTGAACTAGTC | ||||
| 7 | BglII-RBSh4-Cre ORF | Bacteriophage P1 genomic DNA | In-Fusion cloning | Pr-Blo0247 | CTTCCCGGCGAGATCCTAATCGCCATCTTCCAGC |
| Pr-Blo0249 | GATTACTTCGGCGCGAGATCTCCCAAGAAGGATGCTATGTCCAATTTACTGACCGTACAC | ||||
| 8 | BglII-RBSh3-Cre ORF | Bacteriophage P1 genomic DNA | In-Fusion cloning | Pr-Blo0247 | CTTCCCGGCGAGATCCTAATCGCCATCTTCCAGC |
| Pr-Blo0257 | GATTACTTCGGCGCGAGATCTCCCAAGAAGGATGCATGTCCAATTTACTGACCGTACAC | ||||
| 9 | Tlas | L. lactis subsp. cremoris MG1363 genomic DNA | Restriction-ligation | Pr-Blo0258 | ACGTGGATCCGGACAATATGGGGTAAGCG |
| Pr-Blo0259 | AAGAAGATCTCTAAAGCTGACGGGGTAAAC | ||||
| 10 | Trps9 | B. longum 105-A genomic DNA | Restriction-ligation | Pr-Blo0264 | TGACCGAGATCTTGTGGATGATACACCGGACACTC |
| Pr-Blo0265 | TAGGGATCCTCGTGGAGCGCAAGAAGGCTGGTCTG | ||||
| 11 | TleuB | C. glutamicum ATCC 13032 genomic DNA | Restriction-ligation | Pr-Blo0260 | GTATGCAGATCTCCAGCAAGTATTTACACCAAC |
| Pr-Blo0261 | AGTGGATCCTGCGATGCTGCTGCGTCACTTAG | ||||
| 12 | TclpP stem loop-BglII-RBSh3-Cre ORF | pBFK71 (this study) | In-Fusion cloning | Pr-Blo0277 | ATGGCTTCCCGGCGACTAATCGCCATCTTCCAGC |
| Pr-Blo0280 | ATTACTTCGGCGCGAaaaaccctcggtcggtctgaccgggggttttAGATCTCCCAAGAAGGATGCATG | ||||
| 13 | PcscBA | B. longum 105-A genomic DNA | Restriction-ligation | Pr-Blo0292 | ATTAGATCTTTGGTTGGTTATTGGTTATGTAAC |
| Pr-Blo0293 | ATTAGATCTCCGAGTCCCACACGATTTCTC | ||||
| Genotypic analysis of the loxP-Sp strain | |||||
| 14 | SpR gene | Genomic DNA of loxP-Sp strain | NA | Pr-Blo0099 | TGGGATCCATGTCCGTTTCGCAGTCACCGG |
| Pr-Blo0100 | GCGAATTCATGACGTACGATTTCACGTCG | ||||
| Determination of Inserted DNA Fragments in the R-IVET Library | |||||
| 15 | Inserted DNA fragment | pBFK86 derivative carrying a random DNA fragment (this study) | NA | Pr-Blo0277 | ATGGCTTCCCGGCGACTAATCGCCATCTTCCAGC |
| Pr-Blo0318 | GTAAGCGGCAGGGTCGGAACAGGAGAGCG | ||||
| qRT-PCR analysis | |||||
| 16 | BL105A_0130 | B. longum 105-A genomic DNA | NA | Pr-Blo0414 | AGGCGAAAGAACGGCTATGC |
| Pr-Blo0415 | GACTTCAGGATGGCGACCAG | ||||
| 17 | BL105A_0467 | B. longum 105-A genomic DNA | NA | Pr-Blo0416 | CCTTGTTGCCCAGACCCAAC |
| Pr-Blo0417 | CATAAGAGCGACGCAGCGAG | ||||
| 18 | BL105A_0547 | B. longum 105-A genomic DNA | NA | Pr-Blo0432 | TCGGCAACCATGTTGAGCAC |
| Pr-Blo0433 | GCCTACCCCGATCAGCTCTC | ||||
| 19 | BL105A_1291 | B. longum 105-A genomic DNA | NA | Pr-Blo0434 | ATGTTCAAGCCGAAGGCCAC |
| Pr-Blo0435 | GCCATCCACATCGAAGCAGG | ||||
| 20 | BL105A_1293 | B. longum 105-A genomic DNA | NA | Pr-Blo0436 | AAATCGGCAACGCCACCTAC |
| Pr-Blo0437 | CGCAGGAACATCACGGTAGC | ||||
| 21 | BL105A_1294 | B. longum 105-A genomic DNA | NA | Pr-Blo0408 | AAGGTCGACCACCACTACCG |
| Pr-Blo0409 | CTCGTATTCCCAGCGGACCA | ||||
| 22 | BL105A_1798 | B. longum 105-A genomic DNA | NA | Pr-Blo0428 | GCATCGCGGGAAGAACAGAC |
| Pr-Blo0429 | ATACGCAAACGGCTTCACCG | ||||
| 23 | BL105A_1894 | B. longum 105-A genomic DNA | NA | Pr-Blo0430 | CCACCGACGACCCACTTTTG |
| Pr-Blo0431 | AGTCGAACCAGACCATCCCG | ||||
| 24 | BL105A_1946 | B. longum 105-A genomic DNA | NA | Pr-Blo0372 | GCCTTCGCGATCTGCTGATCTAG |
| Pr-Blo0373 | ACCCGTAATACGGTGAAGCGTAG | ||||
| No. | In Vivo Induced Genes 1 | Annotation 1 | Identified Round | COG Category 2, 3 |
|---|---|---|---|---|
| 1 | BL105A_0064 | Hypothetical protein | 2nd | – |
| 2 | BL105A_0075 | Hypothetical protein | 3rd | S |
| 3 | BL105A_0117 | GrpE protein | 1st | O |
| 4 | BL105A_0130 | Presumable pilin subunit for the Tad-pili | 4th | – |
| 5 | BL105A_0136 | Recombination protein RecR | 1st | L |
| 6 | BL105A_0138 | Hypothetical protein | 4th | – |
| 7 | BL105A_0202 | ABC transporter permease component | 4th | G |
| 8 | BL105A_0204 | Glycoside hydrolase family 127 β-l-arabinofuranosidase | 4th | S |
| 9 | BL105A_0248 | Hypothetical protein | 3rd | – |
| 10 | BL105A_0262 | Hypothetical protein | 4th | – |
| 11 | BL105A_0267 | Hypothetical protein | 1st, 2nd, 4th | – |
| 12 | BL105A_0338 | Ribonuclease VapC | 4th | R |
| 13 | BL105A_0374 | Magnesium-translocating P-type ATPase | 4th | – |
| 14 | BL105A_0377 | Hypothetical protein | 1st | – |
| 15 | BL105A_0414 | Oligosaccharide repeat unit polymerase Wzy | 2nd | M |
| 16 | BL105A_0415 | Hypothetical protein | 4th | M |
| 17 | BL105A_0422 | Transposase | 4th | X |
| 18 | BL105A_0423 | Integrase catalytic region | 1st | X |
| 19 | BL105A_0467 | Putative adhesin | 3rd | X, R |
| 20 | BL105A_0490 | Putative ABC transporter ATP-binding component | 3rd | E |
| 21 | BL105A_0507 | Peptides ABC transporter ATP-binding component | 1st | P, E |
| 22 | BL105A_0534 | Hypothetical protein | 3rd | V, M |
| 23 | BL105A_0540 | Hypothetical protein | 3rd | V |
| 24 | BL105A_0547 | ATPase of the ABC transporter | 3rd, 4th | E |
| 25 | BL105A_0662 | Transcriptional regulator | 2nd | M |
| 26 | BL105A_0669 | Putative phosphoribosylpyrophosphate amidotransferase | 3rd | R |
| 27 | BL105A_0776 | Hypothetical protein | 3rd, 4th | – |
| 28 | BL105A_0812 | Shikimate kinase/3-dehydroquinate synthase | 4th | E |
| 29 | BL105A_0835 | NAD(P) transhydrogenase α-2 subunit | 2nd | C |
| 30 | BL105A_0854 | Hypothetical protein | 2nd | V |
| 31 | BL105A_0900 | Hypothetical protein | 3rd | – |
| 32 | BL105A_0929 | Hypothetical protein | 1st | – |
| 33 | BL105A_0934 | Phosphoribosyl-ATP pyrophosphatase | 2nd | E |
| 34 | BL105A_1028 | Hypothetical protein | 3rd | – |
| 35 | BL105A_1049 | Hypothetical protein | 1st | – |
| 36 | BL105A_1053 | Hypothetical protein | 4th | – |
| 37 | BL105A_1079 | tRNA N6-adenosine threonylcarbamoyltransferase | 1st | J |
| 38 | BL105A_1118 | Hypothetical protein | 1st | – |
| 39 | BL105A_1123 | RecX-like protein | 3rd | O |
| 40 | BL105A_1233 | Cell division protein FtsW | 3rd | D |
| 41 | BL105A_1250 | 16S RNA methylase | 1st | J |
| 42 | BL105A_1253 | Transporter | 2nd | G |
| 43 | BL105A_1291 | Serine protease inhibitor | 1st | O |
| 44 | BL105A_1293 | Galactoside transport protein | 1st | P |
| 45 | BL105A_1371 | ABC-type fructose transport system ATPase subunit FruK | 4th | G |
| 46 | BL105A_1419 | Hypothetical protein | 3rd | I |
| 47 | BL105A_1426 | Hypothetical protein | 4th | – |
| 48 | BL105A_1456 | Sugar kinase in PfkB family | 4th | G, F |
| 49 | BL105A_1489 | Endonuclease | 4th | L |
| 50 | BL105A_1517 | Peptide chain release factor 1 | 4th | J |
| 51 | BL105A_1556 | Hypothetical protein | 4th | N |
| 52 | BL105A_1562 | tRNA-Phe | 3rd | – |
| 53 | BL105A_1583 | Hypothetical protein | 3rd | – |
| 54 | BL105A_1603 | Sugar ABC transporter permease component | 2nd | G |
| 55 | BL105A_1605 | Hypothetical protein | 1st | – |
| 56 | BL105A_1637 | DNA-directed RNA polymerase α subunit | 1st | K |
| 57 | BL105A_1680 | Amino acid transporter | 1st | E |
| 58 | BL105A_1696 | Hypothetical protein | 4th | L |
| 59 | BL105A_1707 | Possible extracellular exo-xylanase | 4th | G |
| 60 | BL105A_1708 | endo-1,4-β-Xylanase | 2nd | G |
| 61 | BL105A_1718 | Hypothetical protein | 1st | G |
| 62 | BL105A_1733 | 16S ribosomal RNA | 1st | – |
| 63 | BL105A_1798 | Putative glycosyltransferase | 1st, 3rd | M |
| 64 | BL105A_1810 | Probable potassium uptake protein Kup | 3rd | P |
| 65 | BL105A_1828 | Hypothetical protein | 1st | – |
| 66 | BL105A_1834 | Hypothetical protein | 1st, 1st | L |
| 67 | BL105A_1857 | Hypothetical protein | 4th | R, G |
| 68 | BL105A_1883 | α-Glucosidase | 3rd | G |
| 69 | BL105A_1885 | Glycosidase | 1st | G |
| 70 | BL105A_1886 | Permease protein of ABC transporter system for sugars | 4th | G |
| 71 | BL105A_1894 | Raffinose transport system permease protein | 2nd, 3rd | G |
| 72 | BL105A_1910 | Lipopolysaccharide kinase | 3rd | T |
| 73 | BL105A_1945 | Preprotein translocase subunit YidC | 1st | M |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koguchi, H.; Ishigami, N.; Sakanaka, M.; Yoshida, K.; Hiratou, S.; Shimada, M.; Fukiya, S.; Sonoyama, K.; Yokota, A. Application of Recombinase-Based In Vivo Expression Technology to Bifidobacterium longum subsp. longum for Identification of Genes Induced in the Gastrointestinal Tract of Mice. Microorganisms 2020, 8, 410. https://doi.org/10.3390/microorganisms8030410
Koguchi H, Ishigami N, Sakanaka M, Yoshida K, Hiratou S, Shimada M, Fukiya S, Sonoyama K, Yokota A. Application of Recombinase-Based In Vivo Expression Technology to Bifidobacterium longum subsp. longum for Identification of Genes Induced in the Gastrointestinal Tract of Mice. Microorganisms. 2020; 8(3):410. https://doi.org/10.3390/microorganisms8030410
Chicago/Turabian StyleKoguchi, Hiroka, Natsumi Ishigami, Mikiyasu Sakanaka, Kako Yoshida, Sayaka Hiratou, Mina Shimada, Satoru Fukiya, Kei Sonoyama, and Atsushi Yokota. 2020. "Application of Recombinase-Based In Vivo Expression Technology to Bifidobacterium longum subsp. longum for Identification of Genes Induced in the Gastrointestinal Tract of Mice" Microorganisms 8, no. 3: 410. https://doi.org/10.3390/microorganisms8030410
APA StyleKoguchi, H., Ishigami, N., Sakanaka, M., Yoshida, K., Hiratou, S., Shimada, M., Fukiya, S., Sonoyama, K., & Yokota, A. (2020). Application of Recombinase-Based In Vivo Expression Technology to Bifidobacterium longum subsp. longum for Identification of Genes Induced in the Gastrointestinal Tract of Mice. Microorganisms, 8(3), 410. https://doi.org/10.3390/microorganisms8030410





