New Antimicrobial Phenyl Alkenoic Acids Isolated from an Oil Palm Rhizosphere-Associated Actinomycete, Streptomyces palmae CMU-AB204T
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Material
2.2. Culture Conditions
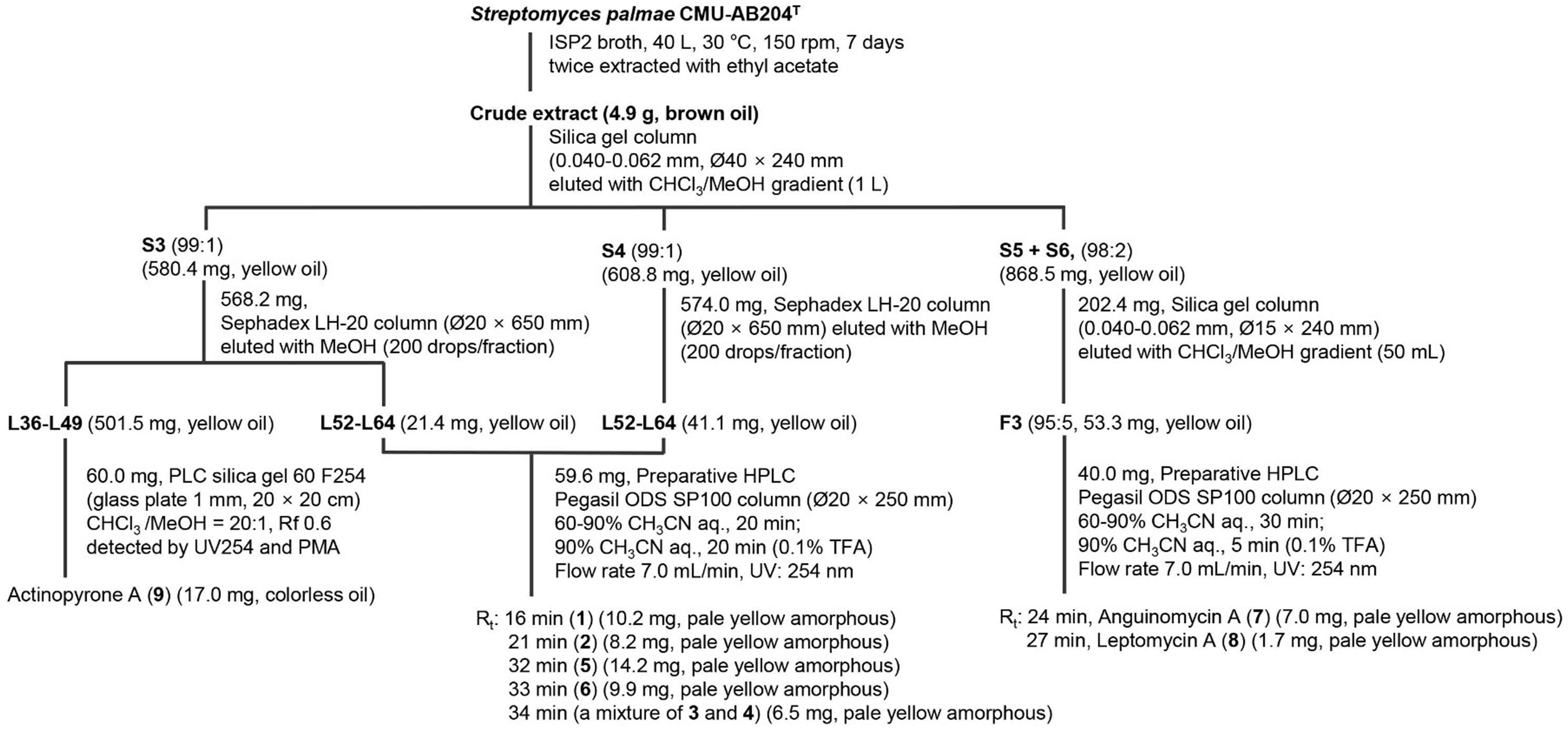
2.3. Compound Extraction and Isolation Procedure
2.4. Analyses of the Chemical Structure and Physicochemical Properties
2.5. Measurement of Antimicrobial Activity
3. Results
3.1. Biological Activity-Guided Purification of Active Components from Culture Broth of S. palmae CMU-AB204T and Structure Determination of Active Components
3.2. Antimicrobial Activities of Isolated Compounds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rees, R.W.; Flood, J.; Hasan, Y.; Cooper, R.M. Effects of inoculum potential, shading and soil temperature on root infection of oil palm seedlings by the basal stem rot pathogen Ganoderma boninense. Plant. Pathol. 2007, 56, 862–870. [Google Scholar] [CrossRef]
- Susanto, A.; Sudharto, P.S.; Purba, R.Y. Enhancing biological control of basal stem rot disease (Ganoderma boninense) in oil palm plantations. Mycopathologia 2005, 159, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Nieto, L. Incidence of oil palm stem rots in Colombia. Palmas 1995, 16, 227–232. [Google Scholar]
- Turner, P.D. Oil Palm Diseases and Disorders; Oxford University Press: Oxford, UK, 1981; pp. 88–110. [Google Scholar]
- Likhitekaraj, S.; Tummakate, A. Basal stem rot of oil palm in Thailand caused by Ganoderma. In Ganoderma Diseases of Perennial Crops; Flood, J., Bridge, P.D., Holderness, M., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 66–70. [Google Scholar]
- Jollands, P. Laboratory investigations on fungicides and biological agents to control three diseases of rubber and oil palm and their potential applications. Trop. Pest. Manag. 1983, 29, 33–38. [Google Scholar] [CrossRef]
- Idris, A.S.; Ismail, S.; Ariffin, D.; Ahmed, D. Control of Ganoderma infected palm-development of pressure injection and field application. MPOB Info. Ser. 2002, 148, 2. [Google Scholar]
- Idris, A.S.; Arifurrahman, R. Determination of 50% effective concentration (EC50) of fungicides against pathogenic Ganoderma. MPOB Info. Ser. 2008, 449, 2. [Google Scholar]
- Naher, L.; Siddiquee, S.; Yusuf, U.K.; Mondal, M.M.A. Issues of Ganoderma spp. and basal stem rot disease management in oil palm. Am. J. Agri. Sci. 2015, 2, 103–107. [Google Scholar]
- Shariffah-Muzaimah, S.; Idris, A.; Madihah, A.; Dzolkhifli, O.; Kamaruzzaman, S.; Cheong, P. Isolation of actinomycetes from rhizosphere of oil palm (Elaeis guineensis Jacq.) for antagonism against Ganoderma boninense. J. Oil Palm Res. 2015, 27, 19–29. [Google Scholar]
- Wightwick, A.; Walters, R.; Allinson, G.; Reichman, S.; Menzies, N. Environmental risks of fungicides used in horticultural production systems. In Fungicides; Carisse, O., Ed.; IntechOpen: London, UK, 2010; pp. 273–304. [Google Scholar]
- Ji, X.Y.; Zhong, Z.J.; Xue, S.T.; Meng, S.; He, W.Y.; Gao, R.M.; Li, Y.H.; Li, Z.R. Synthesis and antiviral activities of synthetic Glutarimide derivatives. Chem. Pharm. Bull. 2010, 58, 1436–1441. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicity of fungicides. In Veterinary Toxicology, Basic and Clinical Principles, 3rd ed.; Gupta, R.C., Ed.; Academic Press: London, UK, 2018; pp. 569–580. [Google Scholar]
- Nur Ain Izzati, M.Z.; Abdullah, F. Disease suppression in Ganoderma-infected oil palm seedlings treated with Trichoderma harzianum. Plant. Protect. Sci. 2008, 44, 101–107. [Google Scholar] [CrossRef]
- Sundram, S.; Abdullah, F.; Ahmad, Z.A.M.; Yusuf, U.K. Efficacy of single and mixed treatments of Trichoderma harzianum as biocontrol agents of Ganoderma basal stem rot in oil palm. J. Oil Palm Res. 2008, 20, 470–483. [Google Scholar]
- Naher, L.; Tan, S.G.; Yusuf, U.K.; Ho, C.-L.; Abdullah, F. Biocontrol agent Trichoderma harzianum strain FA 1132 as an enhancer of oil palm growth. Pertanika J. Trop. Agric. Sci. 2012, 35, 173–182. [Google Scholar]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Naher, L.; Ho, C.-L.; Tan, S.G.; Yusuf, U.K.; Abdullah, F. Cloning of transcripts encoding chitinases from Elaeis guineensis Jacq. and their expression profiles in response to fungal infections. Physiol. Mol. Plant. Pathol. 2011, 76, 96–103. [Google Scholar] [CrossRef]
- Sapak, Z.; Meon, S.; Ahmad, Z.A.M. Effect of endophytic bacteria on growth and suppression of Ganoderma infection in oil palm. Int. J. Agric. Biol. 2008, 10, 127–132. [Google Scholar]
- Bivi, M.R.; Farhana, M.; Khairulmazmi, A.; Idris, A. Control of Ganoderma boninense: A causal agent of basal stem rot disease in oil palm with endophyte bacteria in vitro. Int. J. Agric. Biol. 2010, 12, 833–839. [Google Scholar]
- Sundram, S.; Meon, S.; Seman, I.A.; Othman, R. Symbiotic interaction of endophytic bacteria with arbuscular mycorrhizal fungi and its antagonistic effect on Ganoderma boninense. J. Microbiol. 2011, 49, 551–557. [Google Scholar] [CrossRef]
- Nurrashyeda, R.; Maizatul, S.; Idris, A.; Madihah, A.; Nasyaruddin, M. The potential of endophytic bacteria as a biological control agent for Ganoderma disease in oil palm. Sains Malaysiana 2016, 45, 401–409. [Google Scholar]
- Katz, L.; Baltz, R.H. Natural product discovery: past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Procópio, R.E.; Silva, I.R.; Martins, M.K.; Azevedo, J.L.; Araújo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef]
- Ōmura, S.; Ikeda, H.; Ishikawa, J.; Hanamoto, A.; Takahashi, C.; Shinose, M.; Takahashi, Y.; Horikawa, H.; Nakazawa, H.; Osonoe, T.; et al. Genome sequence of an industrial microorganism Streptomyces avermitilis: Deducing the ability of producing secondary metabolites. PNAS 2001, 98, 12215–12220. [Google Scholar]
- Watve, M.G.; Tickoo, R.; Jog, M.M.; Bhole, B.D. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol. 2001, 176, 386–390. [Google Scholar] [CrossRef]
- Pithakkit, S.; Petcharat, V.; Chuenchit, S.; Pornsuriya, C.; Sunpapao, A. Isolation of antagonistic actinomycetes species from rhizosphere as effective biocontrol against oil palm fungal diseases. Walailak J. Sci. Tech. 2014, 12, 481–490. [Google Scholar]
- Shariffah-Muzaimah, S.; Idris, A.; Madihah, A.; Dzolkhifli, O.; Kamaruzzaman, S.; Maizatul-Suriza, M. Characterization of Streptomyces spp. isolated from the rhizosphere of oil palm and evaluation of their ability to suppress basal stem rot disease in oil palm seedlings when applied as powder formulations in a glasshouse trial. World J. Microbiol. Biotech. 2018, 34, 15. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Hermanto, A.; Peh, K.L. Indigenous actinomycetes from empty fruit bunch compost of oil palm: evaluation on enzymatic and antagonistic properties. Biocat. Agric. Biotech. 2014, 3, 310–315. [Google Scholar] [CrossRef]
- Pal, K.K.; Gardener, B.M. Biological control of plant pathogens. Plant. Health Instructor 2006, 2, 1117–1142. [Google Scholar] [CrossRef]
- Sujarit, K.; Kudo, T.; Ohkuma, M.; Pathom-Aree, W.; Lumyong, S. Streptomyces palmae sp. nov., isolated from oil palm (Elaeis guineensis) rhizosphere soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 3983–3988. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Mukku, V.J.R.V.; Maskey, R.P.; Monecke, P.; Grün-Wollny, I.; Laatsch, H. 5-(2-Methylphenyl)-4-pentenoic acid from a terrestrial Streptomycete. Z. Naturforsch. 2002, 57b, 335–337. [Google Scholar] [CrossRef]
- Shaaban, K.A.; Helmke, E.; Kelter, G.; Fiebig, H.H.; Laatsch, H. Glucopiericidin C: A cytotoxic piericidin glucoside antibiotics produced by a marine Streptomyces isolate. J. Antibiot. 2011, 64, 205–209. [Google Scholar] [CrossRef][Green Version]
- Thong, W.L.; Shin-ya, K.; Nishiyama, M.; Kuzuyama, T. Methylbenzene-containing polyketides from a Streptomyces that spontaneously acquired rifampicin resistance: Structural elucidation and biosynthesis. J. Nat. Prod. 2016, 79, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Mauger, J.B.; Cano, R.J.; Galazzo, J.L.; Lee, M.D. MF-EA-705a & MF-EA-705b, new metabolites from microbial fermentation of a Streptomyces sp. J. Antibiot. 2001, 54, 1100–1103. [Google Scholar] [PubMed]
- Shiomi, K.; Yang, H.; Inokoshi, J.; Van der Pyl, D.; Nakagawa, A.; Takeshima, H.; Ōmura, S. Pepticinnamins, new farnesyl-protein transferase inhibitors produced by an actinomycete. II. Structural elucidation of pepticinnamin E. J. Antibiot. 1993, 46, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, N.; Hayashi, K.; Kayakiri, N.; Takase, S.; Hashimoto, M.; Tanaka, H. Structure of WS9326A, a novel tachykinin antagonist from a Streptomyces. J. Org. Chem. 1993, 58, 170–175. [Google Scholar] [CrossRef]
- Toki, S.; Agatsuma, T.; Ochiai, K.; Saitoh, Y.; Ando, K.; Nakanishi, S.; Lokker, N.; Giese, N.A.; Matsuda, Y. RP-1776, a novel cyclic peptide produced by Streptomyces sp., inhibits the binding of PDGF to the extracellular domain of its receptor. J. Antibiot. 2001, 54, 405–414. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Adachi, K.; Komeshima, N. New antitumor antibiotics, anguinomycins A and B. J. Antibiot. 1987, 40, 1349–1352. [Google Scholar] [CrossRef]
- Hamamoto, T.; Seto, H.; Beppu, T. Leptomycins A and B, new antifungal antibiotics. II. Structure elucidation. J. Antibiot. 1983, 36, 646–650. [Google Scholar] [CrossRef]
- Yano, K.; Yokoi, K.; Sato, J.; Oono, J.; Kouda, T.; Ogawa, Y.; Nakashima, T. Actinopyrones A, B, and C, new physiologically active substances. II. Physicochemical properties and chemical structures. J. Antibiot. 1986, 39, 38–43. [Google Scholar] [CrossRef]
- Angel, L.P.L.; Yusof, M.T.; Ismail, I.S.; Ping, B.T.Y.; Mohamed Azni, I.N.A.; Kamarudin, N.H.; Sundram, S. An in vitro study of the antifungal activity of Trichoderma virens 7b and a profile of its non-polar antifungal components related against Ganoderma boninense. J. Microbiol. 2016, 54, 732–744. [Google Scholar] [CrossRef]
- Lester, G. Inhibition of growth, synthesis, and permeability in Neurospora crassa by phenethyl alcohol. J. Bacteriol. 1965, 90, 29–37. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, Y.; Yang, M.; Liu, Y.; Chen, K.; Long, C.A.; Deng, X. Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol. 2014, 14, 242. [Google Scholar] [CrossRef]
- Staunton, J.; Weissman, K.J. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 2001, 18, 380–416. [Google Scholar] [CrossRef]
- Banga, J.; Praveen, V.; Singh, V.; Tripathi, C.K.M.; Bihari, V. Studies on medium optimization for the production of antifungal and antibacterial antibiotics from a bioactive soil actinomycete. Med. Chem. Res. 2008, 17, 425–436. [Google Scholar] [CrossRef]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Sujarit, K. Selection and Characterization of Actinomycetes for Growth Promotion of Oil Palm and Biological Control of Oil Palm Diseases. Ph.D. Thesis, Chiang Mai University, Chiang Mai, Thailand, 2018. [Google Scholar]
- Bérdy, J. Thoughts and facts about antibiotics: where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef]
- Lucas, X.; Senger, C.; Erxleben, A.; Grüning, B.A.; Döring, K.; Mosch, J.; Flemming, S.; Günther, S. StreptomeDB: A resource for natural compounds isolated from Streptomyces species. Nucleic Acids Res. 2013, 41, D1130–D1136. [Google Scholar] [CrossRef]


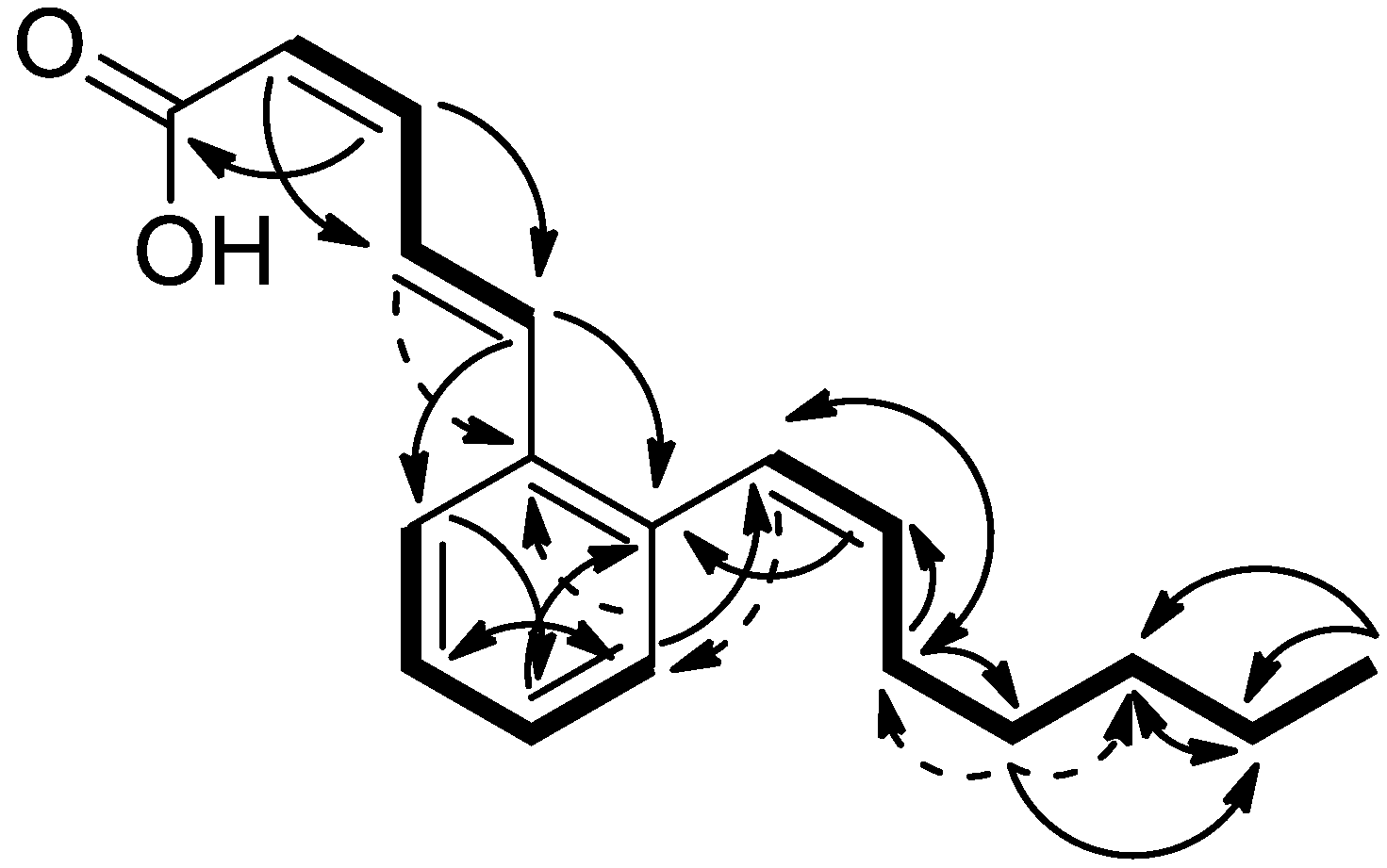

| Carbon No. | 1 (in CDCl3) | 2 (in CDCl3) | ||||
|---|---|---|---|---|---|---|
| δC, Type | δH, Mult (J in Hz) | HMBC | δC, type | δH, Mult (J in Hz) | HMBC | |
| 1 | 177.2, C | 177.6, C | ||||
| 2 | 33.7, CH2 | 2.41–2.44, m | C1, C3, C4 | 33.4, CH2 | 2.30, t (7.5) | C1, C3, C4 |
| 3 | 23.5, CH2 | 2.46–2.51, m | C1, C2, C5 | 24.2, CH2 | 1.63, tt (7.5, 7.5) | C1, C2, C5 |
| 4 | 129.7 or 129.8, CH | 5.70, dt (11.5, 7.0) | C1′ | 29.1, CH2 | 1.45, tt (7.5, 7.5) | C2, C5, C6 |
| 5 | 129.7 or 129.8, CH | 6.51, d (11.5) | C3, C2′, C6′ | 27.8, CH2 | 2.17, dtd (7.5, 7.5, 1.5) | C3, C4, C6, C7 |
| 6 | 132.0, CH | 5.69, dt (11.5, 7.5) | C1′ | |||
| 7 | 128.5, CH | 6.45, br.d (11.5) | C5, C6′ | |||
| 1′ | 136.2, C | 136.7, C | ||||
| 2′ | 136.2, C | 136.2, C | ||||
| 3′ | 129.8, CH | 7.14–7.18 *, m | 129.8, CH | 7.13–7.18 *, m | - | |
| 4′ | 125.4 or 127.1, CH | 7.14–7.18 *, m | 125.3 or 126.8, CH | 7.13–7.18 *, m | C2′ | |
| 5′ | 125.4 or 127.1, CH | 7.14–7.18 *, m | C3′ | 125.3 or 126.8, CH | 7.13–7.18 *, m | C3′ |
| 6′ | 128.8, CH | 7.14–7.18 *, m | 128.9, CH | 7.13–7.18 *, m | C2′ | |
| 2′-Me | 19.8, CH3 | 2.24, s | C1′, C2′, C3′ | 19.9, CH3 | 2.25, s | C1′, C2′, C3′ |
| Carbon No. | 5 (in CD3OD) | 6 (in CDCl3) | ||||
|---|---|---|---|---|---|---|
| δC, Type | δH, Mult (J in Hz) | HMBC | δC, Type | δH, Mult (J in Hz) | HMBC | |
| 1 | 170.5, C | 169.5, C | ||||
| 2 | 122.3, CH | 5.99, d (15.5) | C1, C4 | 115.8, CH | 5.74, d (11.5) | C1, C4 |
| 3 | 146.8, CH | 7.41, dd (15.5, 11.0) | C1, C5 | 147.3, CH | 6.85, t (11.5) | C1, C5 |
| 4 | 128.2, CH | 6.95, dd (15.5, 11.0) | C2, C5, C6 | 125.4, CH | 8.04, dd (15.5, 11.5) | C6 |
| 5 | 140.0, CH | 7.13, d (15.5) | C3, C7, C11 | 140.6, CH | 7.08, d (15.5) | C3, C7, C11 |
| 6 | 135.6, C | 134.3, C | ||||
| 7 | 126.7, CH | 7.67, m | C9, C11 | 126.2, CH | 7.72, m | C9, C11 |
| 8 | 128.4, CH | 7.26 *, m | 127.1, CH | 7.26 *, m | C10 | |
| 9 | 129.5, CH | 7.26 *, m | 128.6, CH | 7.26 *, m | C11 | |
| 10 | 131.0, CH | 7.15, m | C8, C12 | 129.9, CH | 7.17, m | C6, C8, C12 |
| 11 | 138.7, C | 137.5, C | ||||
| 12 | 128.3, CH | 6.54, br.d (11.5) | C6, C10, C14 | 127.0, CH | 6.51, br.d (11.5) | C10, C14 |
| 13 | 135.7, CH | 5.83, dt (11.5, 7.5) | C11 | 134.9, CH | 5.81, dt (11.5, 7.5) | C11 |
| 14 | 29.4, CH2 | 2.02, dtd (7.5, 7.5, 1.5) | C12, C13, C15, C16 | 28.4, CH2 | 2.04, dtd (7.5, 7.5, 1.0) | C12, C13, C15, C16 |
| 15 | 30.2, CH2 | 1.38, m | C13, C14, C16, C17 | 29.2, CH2 | 1.37, tt (7.5, 7.5) | C14, C16, C17 |
| 16 | 32.6, CH2 | 1.22, m | C17 | 31.4, CH2 | 1.23 *, m | C17 |
| 17 | 23.5, CH2 | 1.21, m | C16 | 22.5, CH2 | 1.23 *, m | C16 |
| 18 | 13.3, CH3 | 0.83, t (7.0) | C16, C17 | 14.0, CH3 | 0.84, t (7.0) | C16, C17 |
| Microorganism | Inhibition Zone (mm) of Seven Pure Compounds | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 6 | 7 | 8 | 9 | |
| Gram-positive bacteria | |||||||
| Bacillus subtilis ATCC 6633 | - | - | 35.3 ± 1.4 | 12.3 ± 1.8 | - | - | - |
| Kocuria rhizophila ATCC 9341 | - | - | 41.3 ± 2.0 | 17.5 ± 1.5 | 19.1 ± 1.9 | 30.6 ± 2.3 | - |
| Mycobacterium smegmatis ATCC 607 | - | - | 32.7 ± 1.2 | 14.0 ± 2.2 | - | - | - |
| Staphylococcus aureus ATCC 6538p | - | - | 26.0 ± 1.6 | 13.2 ± 1.9 | - | - | - |
| Gram-negative bacteria | |||||||
| Escherichia coli NIHJ | - | - | - | - | - | - | - |
| Klebsiella pneumonia ATCC 10031 | - | - | - | - | - | - | - |
| Proteus vulgaris NBRC 3167 | - | - | - | - | - | - | - |
| Pseudomonas aeruginosa IFO 3080 | - | - | - | - | - | - | - |
| Xanthomonas campestris pv. oryzae KB 88 | - | - | 10.6 ± 1.3 | 11.0 ± 2.2 | - | - | |
| Yeasts | |||||||
| Candida albicans ATCC 64548 | 13.1 ± 1.6 | 10.4 ± 0.9 | - | - | - | - | 20.8 ± 1.5 |
| Saccharomyces cerevisiae ATCC 9763 | - | - | - | - | - | - | - |
| Fungi | |||||||
| Mucor racemosus IFO 4581 | - | - | - | - | 16.9 ± 1.7 | 49.0 ± 2.3 | 23.1 ± 1.6 |
| Aspergillus niger ATCC 6275 | 11.5 ± 1.1 | 11.1 ± 1.2 | - | - | - | - | 23.9 ± 1.8 |
| Ganoderma boninense BCC 21330 | 11.0 ± 1.4 | 13.2 ± 2.0 | - | - | 19.6 ± 1.6 | 22.1 ± 1.7 | 11.9 ± 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujarit, K.; Mori, M.; Dobashi, K.; Shiomi, K.; Pathom-aree, W.; Lumyong, S. New Antimicrobial Phenyl Alkenoic Acids Isolated from an Oil Palm Rhizosphere-Associated Actinomycete, Streptomyces palmae CMU-AB204T. Microorganisms 2020, 8, 350. https://doi.org/10.3390/microorganisms8030350
Sujarit K, Mori M, Dobashi K, Shiomi K, Pathom-aree W, Lumyong S. New Antimicrobial Phenyl Alkenoic Acids Isolated from an Oil Palm Rhizosphere-Associated Actinomycete, Streptomyces palmae CMU-AB204T. Microorganisms. 2020; 8(3):350. https://doi.org/10.3390/microorganisms8030350
Chicago/Turabian StyleSujarit, Kanaporn, Mihoko Mori, Kazuyuki Dobashi, Kazuro Shiomi, Wasu Pathom-aree, and Saisamorn Lumyong. 2020. "New Antimicrobial Phenyl Alkenoic Acids Isolated from an Oil Palm Rhizosphere-Associated Actinomycete, Streptomyces palmae CMU-AB204T" Microorganisms 8, no. 3: 350. https://doi.org/10.3390/microorganisms8030350
APA StyleSujarit, K., Mori, M., Dobashi, K., Shiomi, K., Pathom-aree, W., & Lumyong, S. (2020). New Antimicrobial Phenyl Alkenoic Acids Isolated from an Oil Palm Rhizosphere-Associated Actinomycete, Streptomyces palmae CMU-AB204T. Microorganisms, 8(3), 350. https://doi.org/10.3390/microorganisms8030350





