The Roles of Bacteria in Soil Organic Carbon Accumulation under Nitrogen Deposition in Stipa baicalensis Steppe
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Field Measurements
2.3. Sampling and Chemical Analyses
2.4. Soil Physicochemical Factors
2.5. DNA Extraction and Illumina Sequencing
2.6. Bioinformatics
2.7. Data Processing and Analysis
3. Results
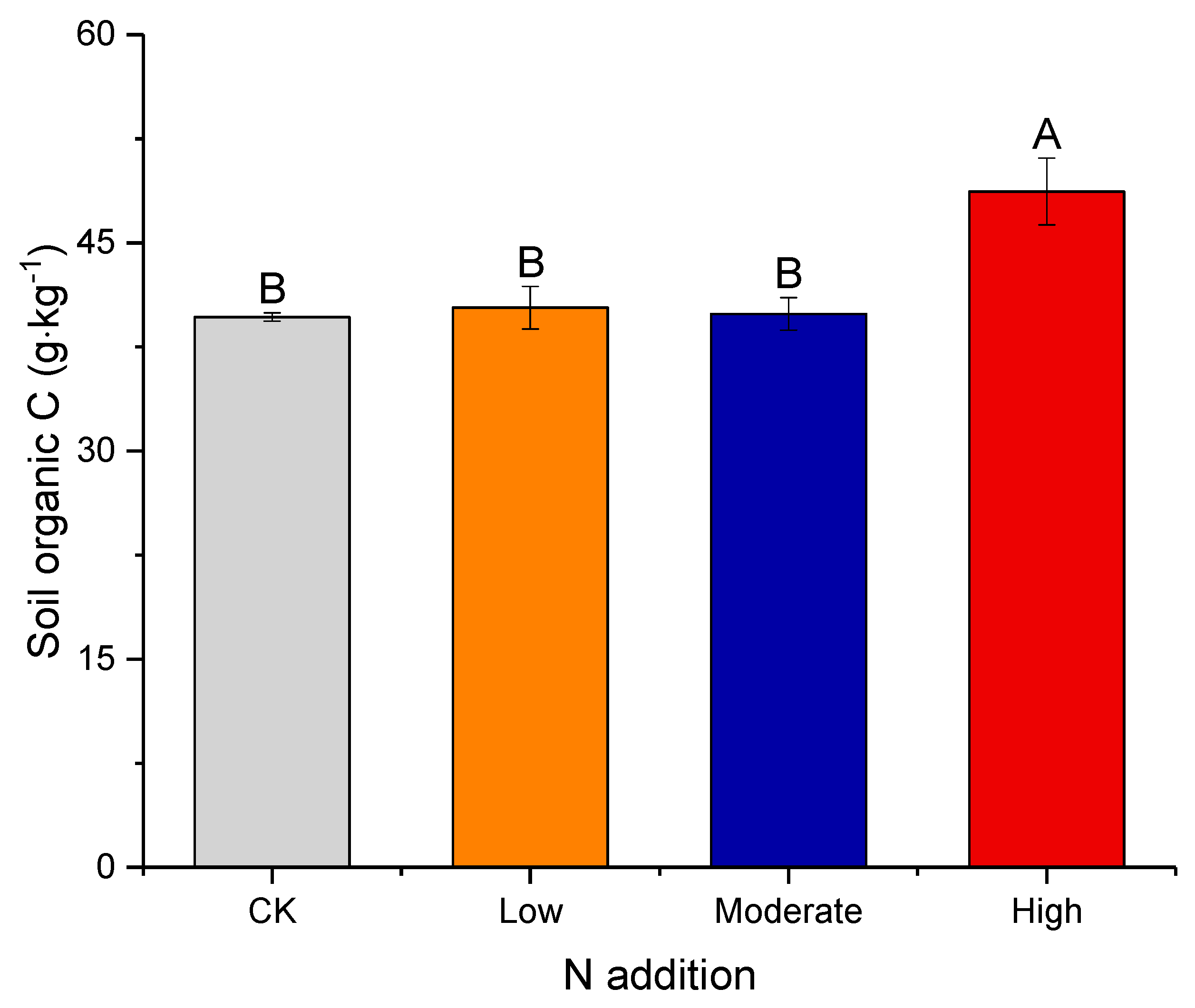
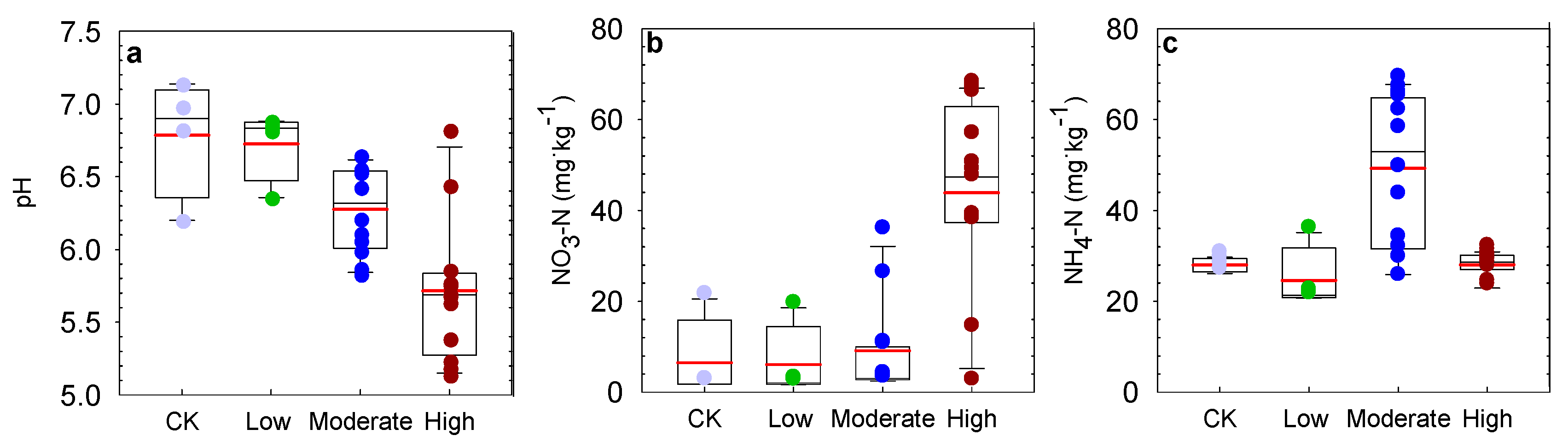
3.1. Effect of Nitrogen Deposition on Soil Properties
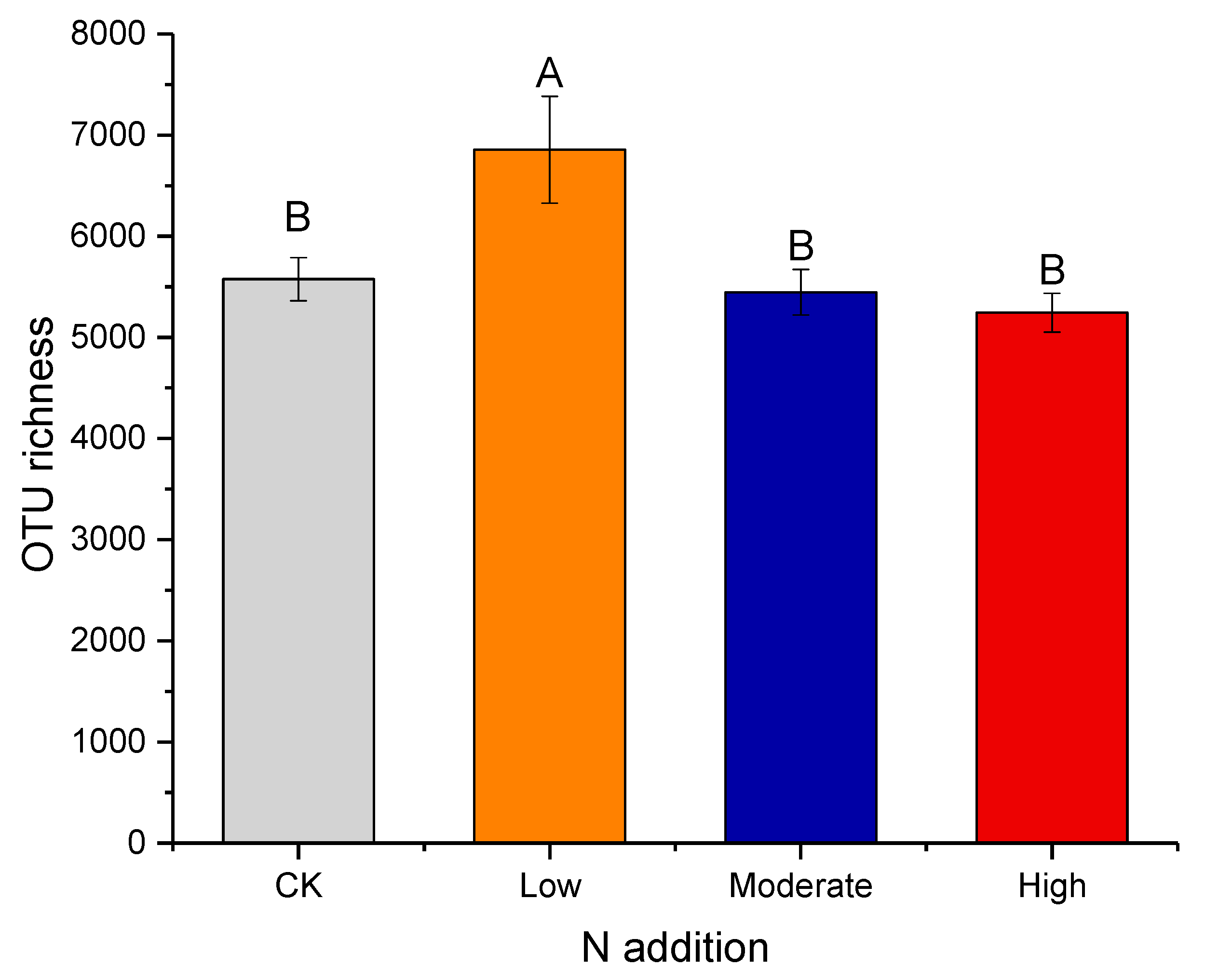
3.2. Effect of Nitrogen Deposition on Bacteria
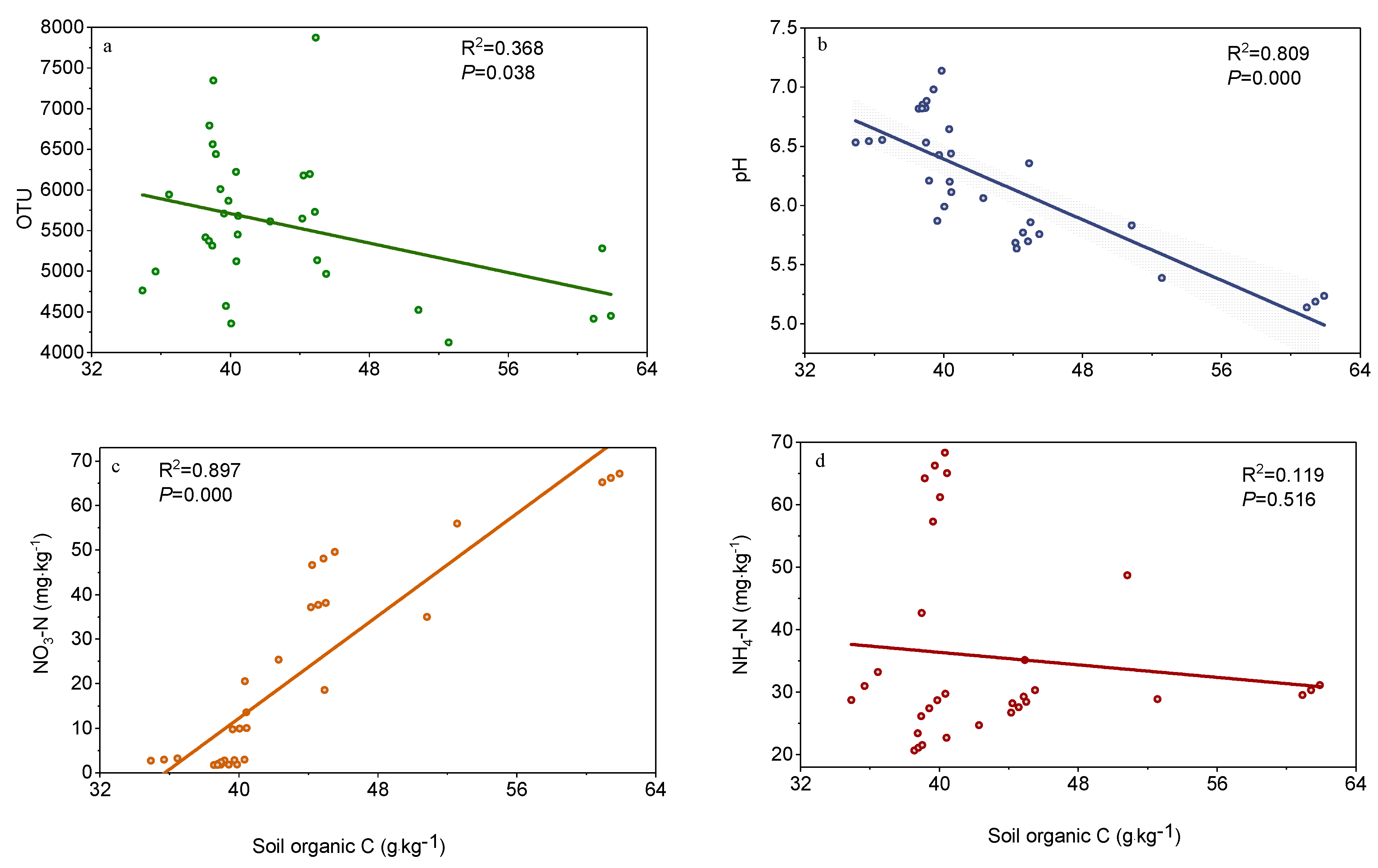
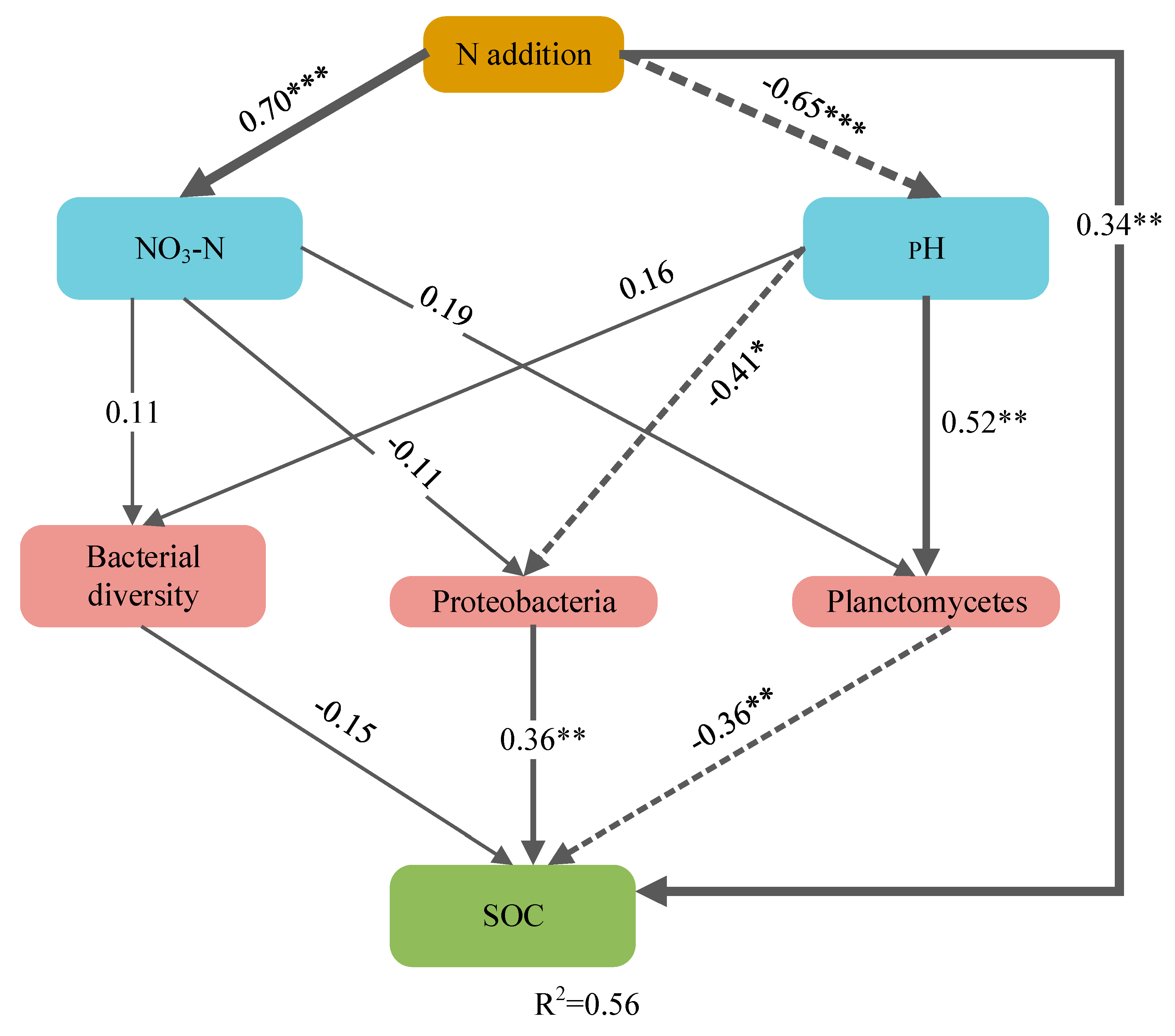
3.3. Factors Affecting Soil Organic Carbon
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Gundersen, P.; Fang, Y.; Li, D.; Wang, H. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Chang. Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Lu, C.Q.; Tian, H.Q. Half-century nitrogen deposition increase across China: A gridded time-series data set for regional environmental assessments. Atmos. Environ. 2014, 97, 68–74. [Google Scholar] [CrossRef]
- Liu, X.J.; Duan, L.; Mo, J.M.; Du, E.Z.; Shen, J.L.; Lu, X.K.; Zhang, Y.; Zhou, X.B.; He, C.N.; Zhang, F.S. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, X.J.; Song, L.; Lin, X.G.; Zhang, H.Y.; Shen, C.C.; Chu, H.Y. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.W.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Hong, S.B.; Gan, P.; Chen, A.P. Environmental controls on soil pH in planted forest and its response to nitrogen deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef]
- Penuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E.; Bach, E.M.; Hofmockel, K.S.; Kazanski, C.E. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 2015, 125, 203–219. [Google Scholar] [CrossRef]
- Fang, H.J.; Cheng, S.L.; Yu, G.R.; Yang, X.M.; Xu, M.J.; Wang, Y.S.; Li, L.S.; Dang, X.S.; Wang, L.; Li, Y.N. Nitrogen deposition impacts on the amount and stability of soil organic matter in an alpine meadow ecosystem depend on the form and rate of applied nitrogen. Eur. J. Soil Sci. 2014, 65, 510–519. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.H.; Luo, Y.Q.; Yang, Y.H.; Fang, C.M.; Chen, J.K.; Li, B. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agric. Ecosyst. Environ. 2011, 140, 234–244. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.J.; Lu, X.; Yang, Y.; Hartley, I.P.; Zhang, W.; Mo, J.; Yu, G.; Zhou, J.; Kuzyakov, Y. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Glob. Chang. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Bowden, R.D.; Lajtha, K.; Washko, S.E.; Wurzbacher, S.J.; Simpson, M.J. Long-term nitrogen addition suppresses microbial degradation, enhances soil carbon storage, and alters the molecular composition of soil organic matter. Biogeochemistry 2019, 142, 299–313. [Google Scholar] [CrossRef]
- Forstner, S.J.; Wechselberger, V.; Muller, S.; Keiblinger, K.M.; Diaz-Pines, E.; Wanek, W.; Schleppi, P.; Hagedorn, F.; Gundersen, P.; Tatzber, M.; et al. Vertical Redistribution of Soil Organic Carbon Pools After Twenty Years of Nitrogen Addition in Two Temperate Coniferous Forests. Ecosystems 2019, 22, 379–400. [Google Scholar] [CrossRef]
- Cheng, S.L.; Fang, H.J.; Yu, G.R. Threshold responses of soil organic carbon concentration and composition to multi-level nitrogen addition in a temperate needle-broadleaved forest. Biogeochemistry 2018, 137, 219–233. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.H.; Zhou, Z.Y.; Zhang, Y.Y. Inorganic Nitrogen Addition Affects Soil Respiration and Belowground Organic Carbon Fraction for a Pinus tabuliformis Forest. Forests 2019, 10, 369. [Google Scholar] [CrossRef]
- Li, J.H.; Li, F.; Li, W.J.; Chen, S.; Abbott, L.K.; Knops, J.M.H. Nitrogen Additions Promote Decomposition of Soil Organic Carbon in a Tibetan Alpine Meadow. Soil Sci. Soc. Am. J. 2018, 82, 614–621. [Google Scholar] [CrossRef]
- Wang, R.Z.; Filley, T.R.; Xu, Z.W.; Wang, X.; Li, M.H.; Zhang, Y.G.; Luo, W.T.; Jiang, Y. Coupled response of soil carbon and nitrogen pools and enzyme activities to nitrogen and water addition in a semi-arid grassland of Inner Mongolia. Plant Soil 2014, 381, 323–336. [Google Scholar] [CrossRef]
- Li, Y.C.; Song, C.C.; Hou, C.C.; Wang, X.W. Effects Of Nitrogen Addition on Carbon Mineralization In Boreal Peatlands Soil In Northeast China: A Laboratory Study. Fresenius Environ. Bull. 2014, 23, 970–975. [Google Scholar]
- Tonitto, C.; Goodale, C.L.; Weiss, M.S.; Frey, S.D.; Ollinger, S.V. The effect of nitrogen addition on soil organic matter dynamics: A model analysis of the Harvard Forest Chronic Nitrogen Amendment Study and soil carbon response to anthropogenic N deposition. Biogeochemistry 2014, 117, 431–454. [Google Scholar] [CrossRef]
- Schleuss, P.M.; Widdig, M.; Heintz-Buschart, A.; Guhr, A.; Martin, S.; Kirkman, K.; Spohn, M. Stoichiometric controls of soil carbon and nitrogen cycling after long-term nitrogen and phosphorus addition in a mesic grassland in South Africa. Soil Biol. Biochem. 2019, 135, 294–303. [Google Scholar] [CrossRef]
- Liu, H.F.; Xue, S.; Wang, G.L.; Liu, G.B. Effects of nitrogen addition on soil oxidisable organic carbon fractions in the rhizospheric and bulk soils of Chinese pines in north-western China. Soil Res. 2018, 56, 192–203. [Google Scholar] [CrossRef]
- Li, Q.R.; Tian, Y.Q.; Zhan, X.Y.; Xu, X.L.; Wang, H.M.; Kuzyakov, Y.K. Labile carbon and nitrogen additions affect soil organic matter decomposition more strongly than temperature. Appl. Soil Ecol. 2017, 114, 152–160. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, Y.; Feng, S.Z.; Ge, Y.H.; Zhang, W.; He, X.Y.; Wang, K.L. Soil organic carbon mineralization with fresh organic substrate and inorganic carbon additions in a red soil is controlled by fungal diversity along a pH gradient. Geoderma 2018, 321, 79–89. [Google Scholar] [CrossRef]
- Fang, X.M.; Zhang, X.L.; Chen, F.S.; Zong, Y.Y.; Bu, W.S.; Wan, S.Z.; Luo, Y.Q.; Wang, H.M. Phosphorus addition alters the response of soil organic carbon decomposition to nitrogen deposition in a subtropical forest. Soil Biol. Biochem. 2019, 133, 119–128. [Google Scholar] [CrossRef]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S.; et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Zhang, T.A.; Chen, H.Y.H.; Ruan, H.H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Stevens, C.J.; Dise, N.B.; Mountford, J.O.; Gowing, D.J. Impact of nitrogen deposition on the species richness of grasslands. Science 2004, 303, 1876–1879. [Google Scholar] [CrossRef]
- Wang, J.; Bao, J.T.; Su, J.Q.; Li, X.R.; Chen, G.X.; Ma, X.F. Impact of inorganic nitrogen additions on microbes in biological soil crusts. Soil Biol. Biochem. 2015, 88, 303–313. [Google Scholar] [CrossRef]
- Guo, Q.X.; Yan, L.J.; Korpelainen, H.; Niinemets, U.; Li, C.Y. Plant-plant interactions and N fertilization shape soil bacterial and fungal communities. Soil Biol. Biochem. 2019, 128, 127–138. [Google Scholar] [CrossRef]
- Mackelprang, R.; Waldrop, M.P.; DeAngelis, K.M.; David, M.M.; Chavarria, K.L.; Blazewicz, S.J.; Rubin, E.M.; Jansson, J.K. Metagenomic analysis of a permafrost microbial community reveals a rapid response to thaw. Nature 2011, 480, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Herath, L.; Moldrup, P.; Arthur, E.; Nicolaisen, M.; Norgaard, T.; Ferre, T.P.A.; de Jonge, L.W. Spatial variability of microbial richness and diversity and relationships with soil organic carbon, texture and structure across an agricultural field. Appl. Soil Ecol. 2016, 103, 44–55. [Google Scholar] [CrossRef]
- Perveen, N.; Barot, S.; Alvarez, G.; Klumpp, K.; Martin, R.; Rapaport, A.; Herfurth, D.; Louault, F.; Fontaine, S. Priming effect and microbial diversity in ecosystem functioning and response to global change: A modeling approach using the SYMPHONY model. Glob. Chang. Biol. 2014, 20, 1174–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Z.; Ren, C.J.; Zhang, L.; Han, X.H.; Yang, G.H.; Wang, J. Changes in soil microbial community are linked to soil carbon fractions after afforestation. Eur. J. Soil Sci. 2018, 69, 370–379. [Google Scholar] [CrossRef]
- Sagova-Mareckova, M.; Zadorova, T.; Penizek, V.; Omelka, M.; Tejnecky, V.; Pruchova, P.; Chuman, T.; Drabek, O.; Buresova, A.; Vanek, A.; et al. The structure of bacterial communities along two vertical profiles of a deep colluvial soil. Soil Biol. Biochem. 2016, 101, 65–73. [Google Scholar] [CrossRef]
- Yang, Y.D.; Wang, P.X.; Zeng, Z.H. Dynamics of Bacterial Communities in a 30-Year Fertilized Paddy Field under Different Organic-Inorganic Fertilization Strategies. Agronomy 2019, 9, 14. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Liu, H.; Zhao, J.; Wang, H.; Zhang, H.; Yang, D.; Zhang, N. The Roles of Bacteria in Soil Organic Carbon Accumulation under Nitrogen Deposition in Stipa baicalensis Steppe. Microorganisms 2020, 8, 326. https://doi.org/10.3390/microorganisms8030326
Qin J, Liu H, Zhao J, Wang H, Zhang H, Yang D, Zhang N. The Roles of Bacteria in Soil Organic Carbon Accumulation under Nitrogen Deposition in Stipa baicalensis Steppe. Microorganisms. 2020; 8(3):326. https://doi.org/10.3390/microorganisms8030326
Chicago/Turabian StyleQin, Jie, Hongmei Liu, Jianning Zhao, Hui Wang, Haifang Zhang, Dianlin Yang, and Naiqin Zhang. 2020. "The Roles of Bacteria in Soil Organic Carbon Accumulation under Nitrogen Deposition in Stipa baicalensis Steppe" Microorganisms 8, no. 3: 326. https://doi.org/10.3390/microorganisms8030326
APA StyleQin, J., Liu, H., Zhao, J., Wang, H., Zhang, H., Yang, D., & Zhang, N. (2020). The Roles of Bacteria in Soil Organic Carbon Accumulation under Nitrogen Deposition in Stipa baicalensis Steppe. Microorganisms, 8(3), 326. https://doi.org/10.3390/microorganisms8030326




