Polyphasic Characterisation of Cedecea colo sp. nov., a New Enteric Bacterium Isolated from the Koala Hindgut
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cell Culture
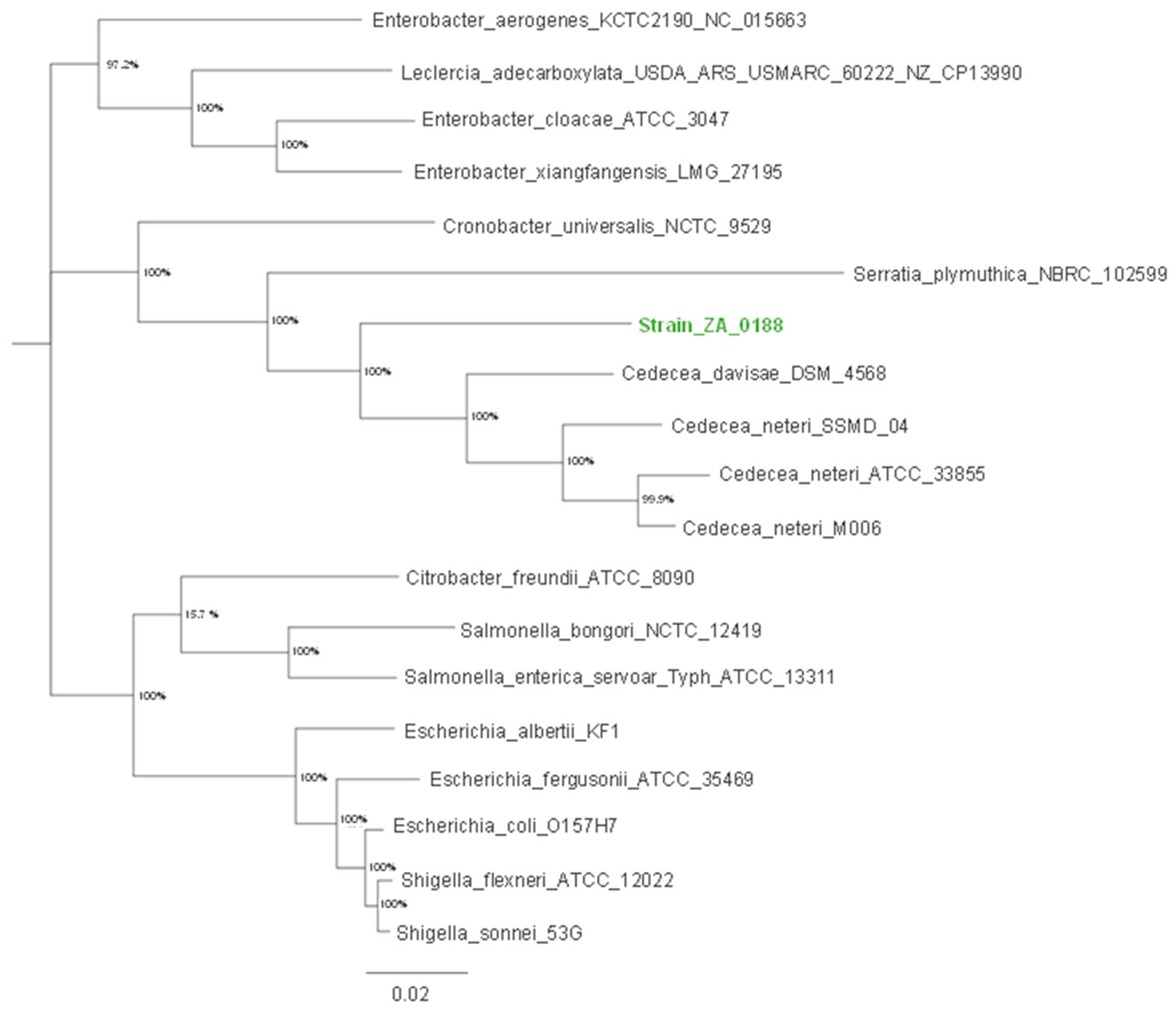
2.2. Single and Multilocus Phylogenetic Sequence Analysis
2.3. Morphological and Biochemical and Nutritional Characterisation
2.4. MALDI-TOF Mass Spectroscopy Analysis
3. Results and Discussion
3.1. Isolation of strain ZA_0188T
3.2. Single-Locus Sequence Analysis of 16S rRNA and GroEL
3.3. Multilocus Sequence Analysis
3.4. Average Nucleotide Identity (ANI) Values and Genomic Guanine–Cytosine Content (DNA G+C Content)
3.5. Morphological Characterisation
3.6. Phenotypic Characterisation
3.7. MALDI-TOF Biotyper Analysis
4. Conclusions
4.1. Description of Cedecea colo Species Novel
4.2. Importance of Cedecea colo sp. nov.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foley, W.J.; Hume, J.D. Nitrogen requirements and urea metabolism in two arboreal marsupials, the greater glider (Petauroides volans) and the brushtail possum (Trichosurus vulpecula), fed eucalyptus foliage. Physiol. Zool. 1987, 60, 241–250. [Google Scholar] [CrossRef]
- Hume, I.D. Marsupial Nutrition; Cambridge Univ. Press: Cambridge, UK; New York, NY, USA, 1999. [Google Scholar]
- Van Soest, P. Plant fiber and its role in herbivore nutrition. Cornell Vet. 1977, 67, 307. [Google Scholar] [PubMed]
- Marschner, C.; Krockenberger, M.B.; Higgins, D.P. Effects of eucalypt plant monoterpenes on koala (Phascolarctos Cinereus) cytokine expression in vitro. Sci. Rep. 2019, 9, 16545–16552. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.J.; Wallis, I.R. Behavioral contributions to the regulated intake of plant secondary metabolites in koalas. Oecologia 2007, 154, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.M.; Wallis, I.R.; Marsh, K.J.; Moore, B.D.; Wiggins, N.L.; Foley, W.J. Four species of arboreal folivore show differential tolerance to a secondary metabolite. Oecologia 2014, 176, 251–258. [Google Scholar] [CrossRef]
- Iason, G. The role of plant secondary metabolites in mammalian herbivory: Ecological perspectives. Proc. Natl. Acad. Sci. USA 2005, 64, 123–131. [Google Scholar] [CrossRef]
- Brice, K.L.; Trivedi, P.; Jeffries, T.C.; Blyton, M.D.J.; Mitchell, C.; Singh, B.K.; Moore, B.D. The koala (Phascolarctos cinereus) faecal microbiome differs with diet in a wild population. PeerJ 2019, 7, e6534. [Google Scholar] [CrossRef]
- Singh, S.; Thavamani, P.; Megharaj, M.; Naidu, R. Multifarious activities of cellulose degrading bacteria from koala (Phascolarctos cinereus) faeces. JAST 2015, 57, 23. [Google Scholar] [CrossRef]
- Denton, M. Enterobacteriaceae. Int. J. Antimicrob. Agents 2007, 29, 9–22. [Google Scholar] [CrossRef]
- Alfano, N.; Courtiol, A.; Vielgrader, H.; Timms, P.; Roca, A.L.; Greenwood, A.D. Variation in koala microbiomes within and between individuals: Effect of body region and captivity status. Sci. Rep. 2015, 5, 10189. [Google Scholar] [CrossRef]
- Vidgen, M.E.; Hanger, J.; Timms, P. Microbiota composition of the koala (Phascolarctos cinereus) ocular and urogenital sites, and their association with Chlamydia infection and disease. Sci. Rep. 2017, 7, 5239. [Google Scholar] [CrossRef]
- Wedrowicz, F.; Karsa, M.; Mosse, J.; Hogan, F.E. Reliable genotyping of the koala (Phascolarctos cinereus) using DNA isolated from a single faecal pellet. Mol. Ecol. Resour. 2013, 13, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-R, L.M.; Konstantinidis, K.T. The enveomics collection: A toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ 2016, 4, e1900v1. [Google Scholar]
- Darling, A.E.; Jospin, G.; Lowe, E.; Matsen, F.A.I.V.; Bik, H.M.; Eisen, J.A. PhyloSift: Phylogenetic analysis of genomes and metagenomes. PeerJ 2014, 2, e243. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning Electron Microscopy. Curr. Protoc. Microbiol. 2012, 25, 2B.2.1–2B.2.47. [Google Scholar]
- Varettas, K.; Mukerjee, C.; Schmidt, M. A comparative study of the BBL Crystal Enteric/Nonfermenter identification system and the BioMerieux API20E and API20NE identification systems after overnight incubation. Pathology 1995, 27, 358–361. [Google Scholar] [CrossRef]
- Cowan, S.T.; Steel, K.J. Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: London, UK, 1965. [Google Scholar]
- Kovacs, N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature 1956, 178, 703. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Karenlampi, R.I.; Tolvanen, T.P.; Hanninen, M.L. Phylogenetic analysis and PCR-restriction fragment length polymorphism identification of Campylobacter species based on partial groEL gene sequences. J. Clin. Microbiol. 2004, 42, 5731–5738. [Google Scholar] [CrossRef]
- Konstantinidis, K.T.; Tiedje, J.M. Genomic insights that advance the species definition for prokaryotes. Proc. Natl. Acad. Sci. USA 2005, 102, 2567–2572. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.H.; Cunningham, S.A.; Dailey, A.L.; Gustafson, D.R.; Patel, R. Identification of anaerobic bacteria by Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry with on-plate formic acid preparation. J. Clin. Microbiol. 2013, 51, 782–786. [Google Scholar] [CrossRef] [PubMed]

| Organism | NCBI/GenBank Accession | ANI Two-Way (%) | 16S rRNA Similarity (%) | GroEL Similarity (%) |
|---|---|---|---|---|
| C. neteri strain M006 | NZ_CP009458.1 | 80.87 | 97.31 | 90.87 |
| C. neteri strain SSMD04 | NZ_CP009451.1 | 81.03 | 97.24 | 90.42 |
| C. neteri ATCC 33855 | NZ_BCTL01000007.1 | 81.65 * | 97.12 | 90.51 |
| C. davisae DSM4568 | NZ_ATDT00000000.1 | 81.10 | 97.17 | 92.17 * |
| C. freundii ATCC 8090 | NZ_ANAV00000000.1 | 79.34 | 96.97 | 90.47 |
| C. universalis NCTC 9529 | NZ_CP012257.1 | 79.65 | 96.07 | 88.91 |
| E. aerogenes KCTC 2190 | NC_015663.1 | 79.61 | 96.82 | 88.85 |
| E. cloacae ATCC 13047 | NC_014121.1 | 79.55 | 96.83 | 89.27 |
| E. xiangfangensis LMG27195 | NZ_CP017183.1 | 79.59 | 97.93 * | 88.96 |
| E. albertii KF1 | NZ_CP007025.1 | 79.17 | 96.56 | 90.53 |
| E. coli strain O157H7 | NC_002695.1 | 79.15 | 97.11 | 90.24 |
| E. fergusonii ATCC35469 | NC_011740.1 | 79.22 | 96.76 | 90.30 |
| K. pneumonia ATCC 13884 | NZ_ACZD00000000.1 | 79.62 | 96.14 | 88.61 |
| K. oxytoca CAV1374 | NZ_CP011636.1 | 79.65 | 97.04 | 90.24 |
| L. adecarboxylata USDA -60222 | NZ_CP13990.1 | 79.39 | 97.31 | 88.77 |
| R. ornithinolytica ATCC 31898 | NZ_BCYR01000001 | 78.58 | 96.11 | 88.97 |
| S. bongori NCTC 12419 | NC_015761.1 | 79.08 | 97.38 | 90.12 |
| S. enterica serovar Typhi strain CT18 | NC_003198.1 | 79.55 | 97.79 | 89.62 |
| S. enterica servoar Typh. ATCC 13311 | CP009102.1 | 79.70 | 97.86 | 89.62 |
| S. plymuthica NBRC 102599 | BCTU00000000.1 | 78.39 | 95.26 | 90.89 |
| S. flexneri ATCC 12022 | NZ_JPPN00000000.1 | 79.15 | 96.53 | 90.06 |
| S. sonnei 53G | NC_016822.1 | 79.30 | 97.38 | 90.06 |
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNase | - | - | - | - | - | - | - | - | - | - | (+) | - | + | + | (+) |
| Oxidase | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Catalase | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Lipase | - | - | + | - | - | - | - | - | - | - | - | - | + | + | + |
| Hydrolysis of: | |||||||||||||||
| ONPG | + | + | + | + | + | + | + | + | + | + | + | - | + | (+) | + |
| Arginine | - | - | + | + | + | - | - | - | - | + | + | (+) | - | (+) | - |
| Decarboxylation of: | |||||||||||||||
| Lysine | - | + | - | - | - | + | + | + | - | + | + | + | - | - | - |
| Ornithine | - | + | - | - | + | + | - | + | - | + | + | - | - | - | + |
| Citrate Utilisation | + | - | + | + | + | + | + | + | - | + | + | - | + | - | - |
| H2S Production | - | - | - | + | - | - | - | - | - | + | + | + | - | - | - |
| Urease | - | - | - | + | + | (+) | + | + | + | - | - | - | - | - | + |
| Indole Test | - | + | - | + | - | - | - | + | + | - | (+) | - | - | + | + |
| Voges-Proskauer Test | - | - | + | - | + | + | + | + | - | - | - | - | + | - | (+) |
| Gelatinase | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - |
| Fermentation of: | |||||||||||||||
| Glucose | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Mannose | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Inositol | - | - | - | - | + | + | + | + | - | - | - | - | + | - | + |
| Sorbitol | - | + | + | + | (+) | + | + | + | - | + | + | + | + | + | + |
| Rhamnose | + | + | - | + | + | + | + | + | + | + | + | - | - | (+) | (+) |
| Sucrose | - | + | + | + | + | + | + | + | + | - | (+) | - | + | (+) | + |
| Melibiose | + | + | - | + | + | + | + | + | + | + | + | + | + | + | (+) |
| Arabinose | + | + | - | + | + | + | + | + | + | + | + | (+) | + | + | + |
| Nitrate reduction | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| DNA G+C (mol %) | 53 | 48–52 | 54–55 | 50–51 | 52–54 | 54–56 | 56–58 | 55–58 | 52–55 | 51–52 | 50–53 | 50–53 | 53–57 | 49–51 | 47–50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boath, J.M.; Dakhal, S.; Van, T.T.H.; Moore, R.J.; Dekiwadia, C.; Macreadie, I.G. Polyphasic Characterisation of Cedecea colo sp. nov., a New Enteric Bacterium Isolated from the Koala Hindgut. Microorganisms 2020, 8, 309. https://doi.org/10.3390/microorganisms8020309
Boath JM, Dakhal S, Van TTH, Moore RJ, Dekiwadia C, Macreadie IG. Polyphasic Characterisation of Cedecea colo sp. nov., a New Enteric Bacterium Isolated from the Koala Hindgut. Microorganisms. 2020; 8(2):309. https://doi.org/10.3390/microorganisms8020309
Chicago/Turabian StyleBoath, Jarryd M., Sudip Dakhal, Thi Thu Hao Van, Robert J. Moore, Chaitali Dekiwadia, and Ian G. Macreadie. 2020. "Polyphasic Characterisation of Cedecea colo sp. nov., a New Enteric Bacterium Isolated from the Koala Hindgut" Microorganisms 8, no. 2: 309. https://doi.org/10.3390/microorganisms8020309
APA StyleBoath, J. M., Dakhal, S., Van, T. T. H., Moore, R. J., Dekiwadia, C., & Macreadie, I. G. (2020). Polyphasic Characterisation of Cedecea colo sp. nov., a New Enteric Bacterium Isolated from the Koala Hindgut. Microorganisms, 8(2), 309. https://doi.org/10.3390/microorganisms8020309






