Abstract
An eight-week feeding trial was conducted to evaluate the effects of different dietary probiotic supplements in juvenile whiteleg shrimp, Litopenaeus vannamei. A basal control diet without probiotics (CON), and five other diets by supplementing Bacillus subtilis at 107 CFU/g diet (BS7), B. subtilis (BS8), Pediococcus pentosaceus (PP8), and Lactococcus lactis (LL8) at 108 CFU/g diet, and oxytetracycline (OTC) at 4 g/kg diet were used. Whiteleg shrimp with initial body weights of 1.41 ± 0.05 g (mean ± SD) were fed with these diets. Growth of shrimp fed BS8 and LL8 diets was significantly higher than those of shrimp fed the CON diet (p < 0.05). Superoxide dismutase activity in shrimp fed PP8 and LL8 diets was significantly higher than that of shrimp fed the CON diet (p < 0.05). Lysozyme activity of shrimp fed probiotics and OTC diets significantly improved compared to those on the CON diet (p < 0.05). The intestinal histology showed healthier guts for shrimp fed the probiotic diets (p < 0.05). Immune-related gene expression in shrimp fed BS8, PP8 and LL8 diets was recorded as significantly higher than that of shrimp fed CON and OTC diets (p < 0.05). Also, results of the challenge test for 7 days and the digestive enzyme activity of shrimp fed BS8, PP8, and LL8 were significantly improved compared to those on the CON diet (p < 0.05). Therefore, these results indicated that L. lactis at 108 CFU/g could be an ideal probiotic for whiteleg shrimp, and also B. subtilis WB60 and P. pentosaceus at 108 CFU/g could improve the growth, immunity, histology, gene expression, digestive enzyme activity, and disease resistance, while replacing antibiotics.
1. Introduction
The whiteleg shrimp, Litopenaeus vannamei, is a commercially important shrimp species which accounts for 80% of global shrimp production. The global production of whiteleg shrimp has increased rapidly and reached 445 million metric tons in 2017, with an estimated total value of 26.7 billion US dollars [1]. With the high market demand for whiteleg shrimp, it has been cultured intensively, which has led to serious problems such as infectious disease outbreaks by parasites. In particular, Vibrio bacterium is the most frequent pathogen in shrimp farming, which has a serious impact on survival, immune responses, and production losses [2]. The emergence of infectious pathogen diseases in shrimp farming causes the abuse or misuse of antibiotic agents [3,4]. Antibiotics are used as chemotherapeutic agents for shrimp because they can influence an extensive range of gram-negative/positive bacteria. However, the excessive use of antibiotics can result in the occurrence of bacterial resistance [5]. Moreover, the use of antibiotics in shrimp aquaculture has drawn attention from a public health point of view because of the potential risks and issues for human consumers [6]. Consequently, studies on possible replacements for antibiotics in shrimp aquaculture are required.
The benefits of dietary probiotics on growth, immunity, disease resistance, and digestive enzyme activity in shrimp aquaculture have been reported by several authors [7,8]. In addition, according to previous studies [9,10], probiotics are safe approaches used as antibiotic replacers in shrimp aquaculture practices. In fact, probiotics play an important role in preventing and maintaining the microbial balance between necessary and excessive defense mechanisms in terms of innate immune responses [11]. Many bacterial species such as Bacillus subtilis [12,13], Pediococcus pentosaceus [14,15], and Lactococcus lactis [16,17] have been demonstrated as growth, immune response, and disease resistance promoters in shrimp cultures. Some of these bacteria naturally occur in gastrointestinal tract of fish and are able to produce substances with antibacterial activity [18]. As a result, they can increase growth, feed efficiency and immune responses of fish. However, previous studies only evaluated the effects of each of these probiotics and have not compared probiotics with antibiotics.
Therefore, the present study aimed at comparing dietary B. subtilis WB60 that was previously extracted and isolated form Japanese eel Anguilla japonica intestine, with P. pentosaceus, and L. lactis that had been obtained from the whiteleg shrimp digestive tract. Also, comparisons were made with a commonly used antibiotic (oxytetracycline) in shrimp aquaculture.
2. Materials and Methods
The study was conducted under the guidelines of the Animal Ethics Committee Regulations, No.18–0145 issued by the Pukyong National University, Busan, Rep. Korea.
2.1. Probiotic Selection
The selected probiotic strains evaluated in this study were isolated from the intestine of Japanese eel [18] and shrimp [15,17]. Among the probiotics, B. subtilis was isolated from the intestine of juvenile Japanese eel and was identified as B. subtilis WB60 according to [17]. This probiotic was incubated at 30 °C for 72 h in LB broth (Sigma-Aldrich, St. Louis, MO, USA) and measured at 600 nm optical density (OD600) using spectrophotometer. P. pentosaceus and L. lactis were isolated from the intestine of juvenile whiteleg shrimp [15,17]. The strain P. pentosaceus was cultured and incubated at 25 °C for 48 h in TSA. While L. lactis was grown in MRS (De Man Rogosa & Sharp broth) medium and incubated at 30 °C for 48 h. Three probiotics were washed in sterile saline and the concentration of the final suspension was calculated 1 × 107 and 108 CFU/g in the diets [15,17,18]. The final concentration of bacteria was determined using the serial dilution method in each agar broth [19].
2.2. Experimental Diets
Feed formulation and proximate composition of the basal diet is shown in Table 1. A basal control diet without supplementation of probiotics (CON), and five other diets including supplementation of Bacillus subtilis at 1 × 107 CFU/g diet (BS7), B. subtilis (BS8), Pediococcus pentosaceus (PP8) and Lactococcus lactis (LL8) at 1 × 108 CFU/g diet, and oxytetracycline (OTC) at 4 g/kg diet were used in this study. Fishmeal, soybean meal, wheat gluten meal, and squid liver powder were used as the protein sources. Fish oil served as the lipid source, wheat flour and corn starch as the carbohydrate sources. The preparation and storage of experimental diets were conducted following Bai & Kim [20]. Briefly, all the dry ingredients were weighted and mixed in a mixing machine, followed by the addition of fish oil and water, until a dough was formed. Experimental diets were pelleted using a laboratory pelleting machine with a 1 to 2-mm diameter module (Baokyong Commercial Co., Busan, Republic of Korea). The pellets were air-dried for 72 h and then stored at −20 °C in the refrigerator until use.

Table 1.
Formulation and composition (% dry matter) of the basal diet for whiteleg shrimp.
2.3. Experimental Shrimp and Feeding Trial
The feeding trial was carried out at Pukyong National University and Feeds and Foods Nutrition Research Center (FFNRC) in Busan, Republic of Korea. Juvenile whiteleg shrimp were purchased from Palddak shrimp farm (Goseong, Republic of Korea) and were transported to the laboratory. Shrimp were acclimatized to the FFNRC for two weeks and fed a basal diet. Prior to the feeding trial, shrimp were examined for external abnormalities and withheld for 24 h. At the beginning of the experiment, shrimp with the initial body weight of 1.41 ± 0.05 g (mean ± SD) were distributed into 18 (45 L) capacity rectangular tanks (20 shrimp/tank) at a constant flow (1.8 L/min) of filtered seawater. Each tank was randomly distributed into one of the three replicates of the eight dietary treatments. Shrimp were fed four times a day (09:00, 13:00, 17:00 and 21:00 h) at 5%–6 % of wet body weight/day for 8 weeks. Supplemental aeration was provided to stabilize the dissolved oxygen. The temperature of the aquarium was maintained at 27.0 ± 1.0 °C and pH remained at 7.68 ± 0.05. The condition of the tanks was maintained by siphoning off uneaten feeds 2 h after feeding and the walls and bottom of the tanks were scrubbed once a week.
2.4. Sample Collection and Analysis
At the end of the feeding trial, all of the shrimp were withheld for 24 h. The count and weight of total shrimp in each tank was measured to calculate final body weight, weight gain, feed efficiency, specific growth rate, protein efficiency ratio, and survival. Four shrimp from each treatment group were sacrificed to collect the blood samples. Serum samples were separated by centrifugation at 8000× g for 15 min and stored at −70 °C for the analysis of non-specific immune responses, including superoxide dismutase, myeloperoxidase, and lysozyme activity, as well as biochemical parameters such as aspartate aminotransferase activity, aminotransferase activity, total protein, and glucose. In addition, the anterior intestine of shrimp samples were collected for histological sections and digestive enzyme activity measurements.
The proximate composition of experimental diets and whole-body samples were analyzed following the standard methods of AOAC [21]. Moisture content was measured after oven-drying the samples at 105 °C to constant weight, while crude ash was estimated after incineration at 550 °C for 3 h. Crude protein was measured using the Kjeldahl method (N × 6.25) after acid digestion, and crude lipid was determined by Soxhlet extraction using the soxhlet system 1046 (Tacator AB, Hoganas, Sweden) after freeze-drying the samples for 20 h.
2.5. Non-Specific Immune Responses Analysis
Lysozyme activity was measured by supplementing 0.1 mL serum sample to Micrococcus lysodeikticus (0.2 mg/mL, Sigma) in a 0.02 M sodium phosphate buffer (pH 5.52). The reactions were performed at room temperature (20 °C) and the absorbance of the sample at a wavelength of 450 nm was measured between 0.5 min and 4.5 min with a spectrophotometer. The sample unit was defined as the amount of enzyme yielding a decrease in absorbance of 0.001/min. Superoxide dismutase activity was obtained by the superoxide radical dependent reaction inhibition rate of enzyme with Water Soluble Tetrazolium dye substrate and xanthine oxidase with the Superoxide dismutase Assay Kit (Sigma-Aldrich, 19160, St. Lousis, MO, USA), according to the manufacturer’s instructions. Each endpoint assay was estimated at 450 nm absorbance after incubating for 37 °C at 20 min. The percentage of inhibition was normalized by mg protein and expressed as Superoxide dismutase unit/mg. Additionally, myeloperoxidase activity was determined as described by Quade & Roth [22]. Briefly, 20 µL of serum was diluted with Hanks balanced salt solution (HBSS) without Ca2+ or Mg2+ (Sigma-Aldrich, USA) in separated 96 well plates to which 35 µL of 3, 3′, 5, 5′ tetramethylbenzidine hydrochloride (TMB; 20 mM; Sigma-Aldrich) and H2O2 (5 mM) were added afterwards. The color change reaction after 2 min was completed by adding 35 µL of 4 M sulphuric acid. The optical density was measured using a spectrophotometer at 450 nm.
2.6. Real-Time PCR
Five shrimp per experimental diet were used for sample analysis after anesthesia. Total RNA was extracted from mid intestine (50 mg) of shrimp using RiboEx™ (GeneALL, Seoul, Rep. Korea) following the standard procedures (Riboclear plus, GeneAll, South Korea). RNA concentration (ng/μL) and purity (OD 260:280) was determined with a nanodrop (Thremo Fisher Scientific, Waltham, MA, USA) and the 260/280 ratio was >1.8. The cDNA was synthesized from 1 μg of RNA using to the manufacturers’ instructions of cDNA synthesis Kit (Takara, Japan). RNA isolation and preparation of cDNA by 1 μg of RNA was performed following the manufacturer’s instructions (GeneAll, Korea). Then, the primer and target genes were prepared by the Bionics company (Seoul, Rep. Korea) (Table 2). Relative RNA level of the target genes was evaluated and calculated using endogenous β-actin RNA level.

Table 2.
Primers used to quantify relative gene expression.
2.7. Intestinal Histology
The mid-intestines of the shrimp (n = 3) were sampled from each experimental tank and were preserved in 10 % buffered formaldehyde for 24 h, then dehydrated in a graded ethanol series and embedded in paraffin. Tissue blocks were sectioned (5 μm) and stained with hematoxylin and eosin (H&E). The evaluation of villi height and muscular thickness was measured using a light microscope (AX70 Olympus, Tokyo, Japan) equipped with a scientific digital camera for microscopy (DIXI Optics, Daejeon, Rep.of Korea). The image was analyzed using the Image J 1.32j software (National Institute of Health, Bathesda, MD, USA).
2.8. Challenge Test
Vibrio parahaemolyticus is a pathogen that commonly occurs in shrimp environments. The pathogenic bacterium, V. parahaemolyticus KCCM 11965, was obtained from the Department of Biotechnology, Pukyong National University, Busan, Republic of Korea. At first, bacteria was grown in 10 mL of brain heart infusion (BHI; Becton, Dickinson and Company, MD, USA) broth and incubated at 37 °C for 24 h with a shaking incubator. Growth of V. parahaemolyticus was observed by optical density of 600 (OD600 nm) using a spectrophotometer (Mecasys, Optizen, Republic of Korea), harvested by centrifugation and washed two times with 0.1 M PBS for further use. At the end of the experiment, eight shrimp from each tank were randomly collected and distributed based on their previous dietary treatment groups in 12 L tanks. Shrimp were injected intraperitoneally with 0.1 mL per shrimp of V. parahaemolyticus KCCM 11965 at 2 × 107 CFU/mL (2 × LD50). Shrimp mortality was recorded daily up to 7 days and water temperature was maintained at 27 ± 1.0 °C (mean ± SD).
2.9. Digestive Enzyme Activities
The enzyme activities of trypsin, lipase, and amylase were analyzed, following the manufacturer’s instructions, with enzyme assay kits (Biovision, Milpitas, CA, USA) and a spectrophotometer with the linear range. The pre-treatment of each specific enzyme assay kit was carried out with substrate and assay buffer. Trypsin activity was prepared with a mixed solution and measured by spectrophotometer at a wavelength of 405 nm for 40 min. Lipase activity was measured with a spectrophotometer at a wavelength of 412 nm for 20 min after mixing lipase substrate and assay buffer. Amylase activity was reacted with assay buffer and substrate mix, and was measured by absorbance of shrimp samples at a wavelength of 402 nm for 40 min. Specific enzyme activities were defined as the amount of enzyme that catalyzed the conversion of 1 μmol of substrate per minute per mg of protein (i.e., U mg soluble protein− 1) at the respective temperature.
2.10. Statistical Analysis
The values from this study were statistically analyzed using one-way ANOVA (SAS Version 9.1, SAS Institute Inc., Cary, NC, USA) and in order to test the effects of dietary probiotic treatments. When a significant treatment effect was observed, an LSD post hoc test was used to compare means. Treatment effects were considered to be significant at p < 0.05.
3. Results
3.1. Growth Performance and Whole Body Proximate Composition
The growth performances and survival of juvenile whiteleg shrimp fed different probiotics diets are summarized in Table 3. Weight gain, specific growth rate, feed efficiency, and protein efficiency ratio of shrimp fed BS8 and LL8 diets were significantly higher than those of shrimp fed the CON diet (p < 0.05). However, there were no significant differences among shrimp fed BS8, PP8, LL8, BS7, and OTC diets (p > 0.05). Shrimp survival on the LL8 diet was significantly higher than for those shrimp on the CON and OTC diets (p < 0.05). However, there were no significant differences among shrimp fed BS8, PP8, LL8, and BS7 diets (p > 0.05). On the other hands, no significant differences were observed in terms of whole body protein, lipid, moisture, and ash content among all diets (p > 0.05; Table 4).

Table 3.
Growth performance and feed utilization of juvenile whiteleg shrimp fed the experimental diets for 8 weeks1.

Table 4.
Whole-body proximate composition (% dry matter) of juvenile whiteleg shrimp fed the different probiotics diets for 8 weeks 1.
3.2. Non-Specific Immune Responses
The non-specific immune responses are shown in Table 5. Lysozyme activity in shrimp fed probiotics and OTC diets significantly improved compare to in shrimp on the CON diet (p < 0.05). Superoxide dismutase activity in shrimp fed BS8 and PP8 diets was significantly higher than that in shrimp fed the CON diet (p < 0.05). However, there were no significant differences among probiotics and OTC diets (p > 0.05). Meanwhile, myeloperoxidase did not show any significant differences among treatment diets (p > 0.05).

Table 5.
Non-specific immune responses of juvenile whiteleg shrimp fed the experimental diets for 8 weeks 1.
3.3. Intestinal Histology
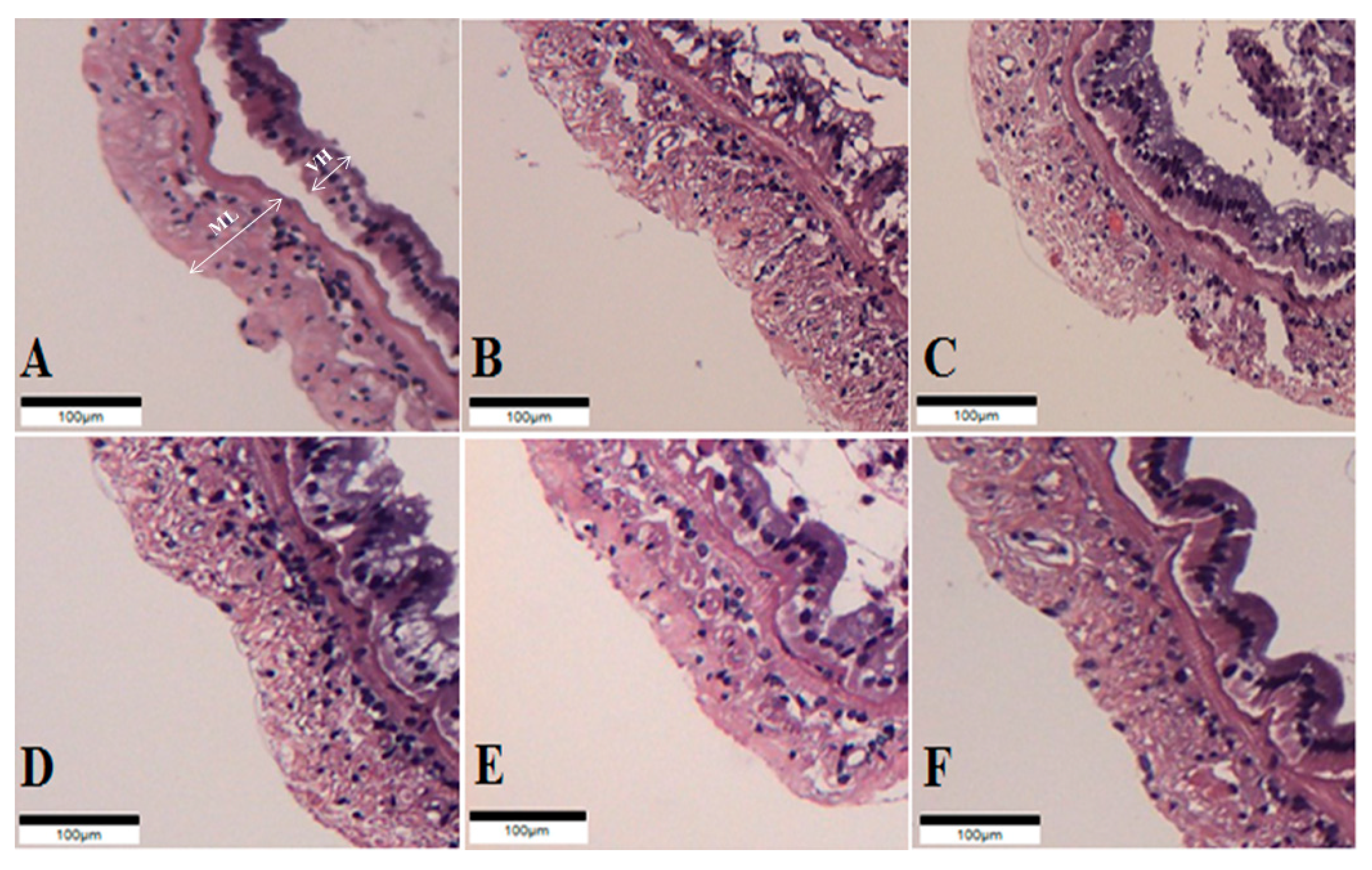
The intestinal histology of whiteleg shrimp fed experimental diets for 8 weeks was described (Figure 1, Table 6). The muscular layer thickness of shrimp fed the probiotic-supplemented diets was longer than those of shrimp fed the CON and OTC diets (p < 0.05). Correspondingly, the villi height of shrimp fed the PP8 and LL8 diets significantly improved compared to shrimp on the CON diet (p < 0.05). However, there were no significant differences among shrimp fed the BS7, BS8, PP8, LL8, and OTC diets (p > 0.05).
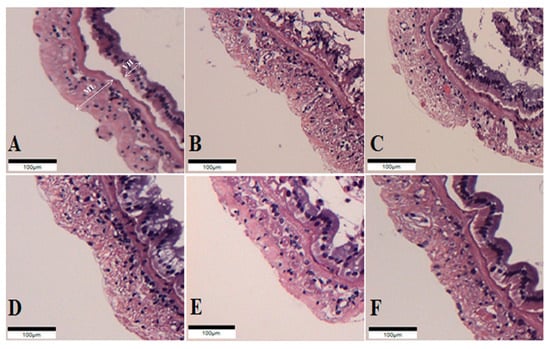
Figure 1.
Histological sections of juvenile whiteleg shrimp intestine fed the experimental diets for 8 weeks. A-CON, basal diet; B-BS7, Bacillus subtilis at 1 × 107 CFU/g; C-BS8, Bacillus subtilis at 1 × 108 CFU/g; D-PP8, Pediococcus pentosaceus at 1 × 108 CFU/g; E-LL7, Lactococcus lactis at 1 × 108 CFU/g; F-OTC, oxytetracycline at 4 g/kg. (Scale bar = 100 µm; Original magnification × 4). ML = muscular layer thickness VH = villi height.

Table 6.
Intestinal histology of juvenile whiteleg shrimp fed the different probiotics diets for 8 weeks 1.
3.4. Haematological Analysis
As shown in Table 7, there were no significant differences among treatment groups in terms of aspartate aminotransferase activity, aminotransferase activity, glucose and total protein (p > 0.05).

Table 7.
Haematological analysis of juvenile whiteleg shrimp fed the different probiotics diets for 8 weeks 1.
3.5. Immune-Related Gene Expressions
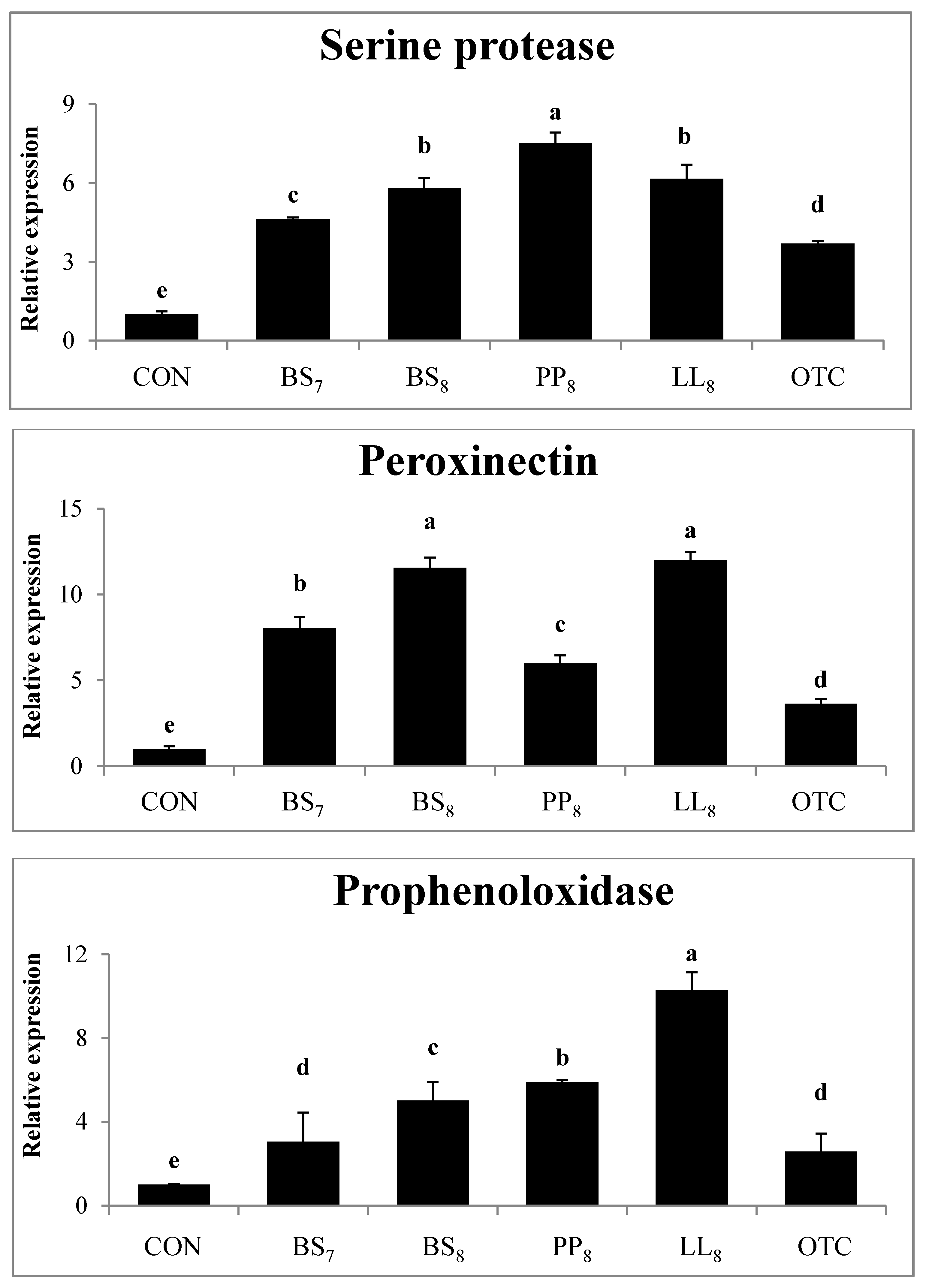
The immunological gene expressions in the intestines of whiteleg shrimp fed experimental diets are demonstrated in Figure 2. The expression levels of serine protease from shrimp fed probiotic diets were significantly higher than those of shrimp fed the CON and OTC diets (p < 0.05). Also, the peroxinectin expression in shrimp fed probiotic diets was higher compared to in shrimp on the CON and OTC diets (p < 0.05). Meanwhile, prophenoloxidase expression in shrimp fed the BS8, PP8, and LL8 diets were significantly improved compare to those on the CON, BS7, and OTC diets. However, there were no significant differences between BS7 and OTC diets (p > 0.05).
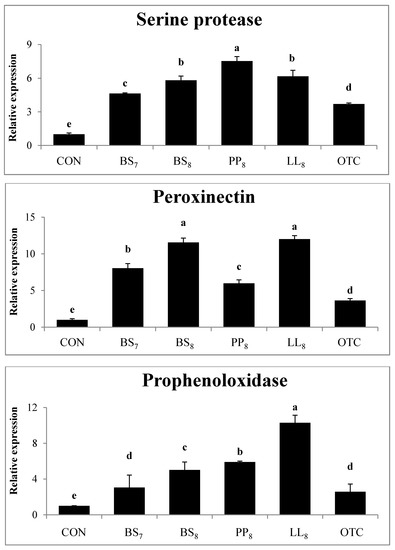
Figure 2.
Intestinal gene expression levels of serine protease, peroxinectin and prophenoloxidase were evaluated in juvenile whiteleg shrimp fed the experimental diets for 8 weeks. Bars with range represent mean ± SD of triplicate samples, and diets refer to Figure 1.
3.6. Challenge Test
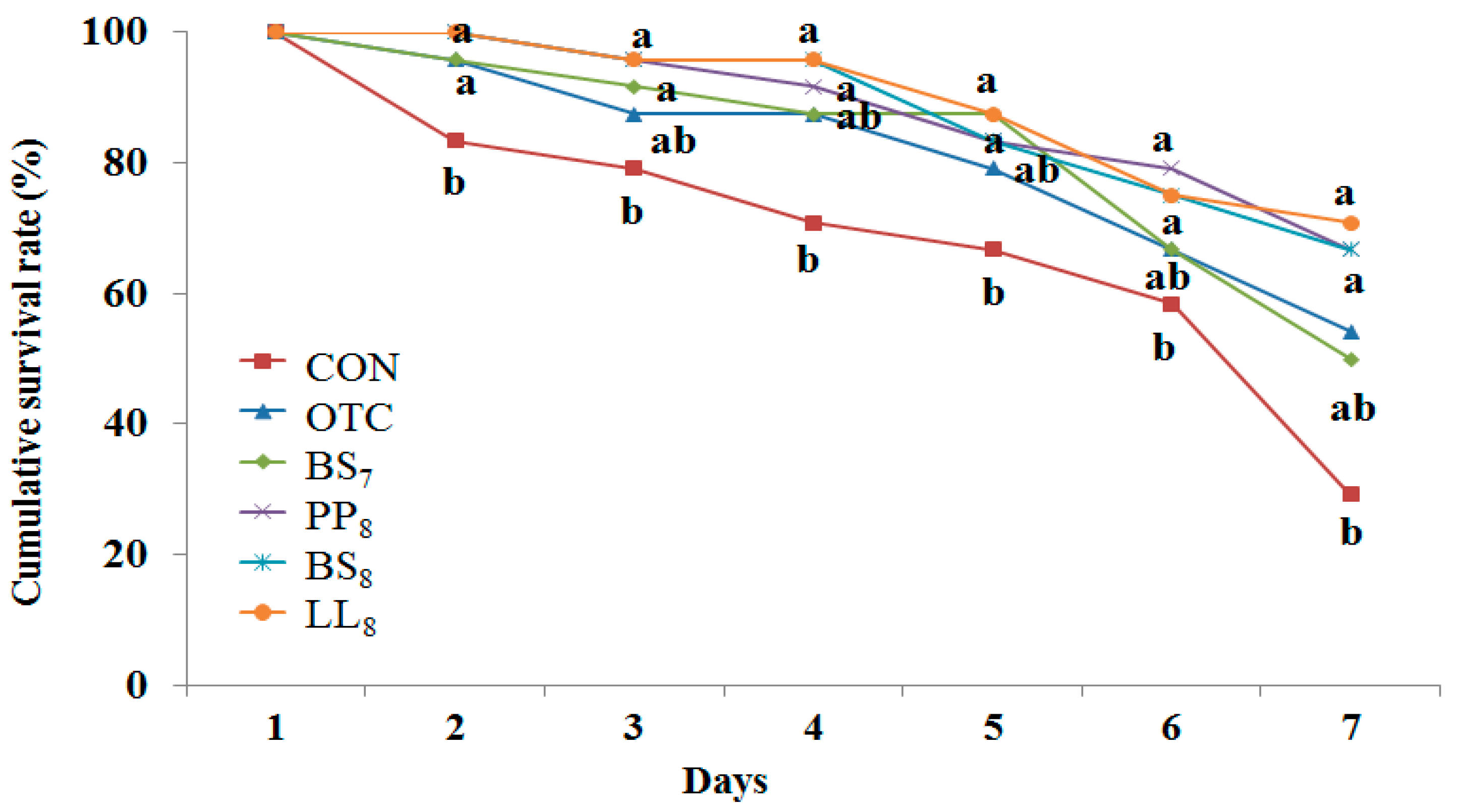
The cumulative survival rate of juvenile whiteleg shrimp challenged with Vibrio parahaemolyticus for 7 days is presented Figure 3. During the challenge test, the first shrimp mortalities occurred on the first day post-injection. Statistical analysis at the end of 7 days of the V. parahaemolyticus challenge showed that shrimp fed the BS8, LL8, and PP8 diets had significantly higher cumulative survival rates than those of shrimp fed the CON diet (p < 0.05). However, there were no significant differences among probiotic and OTC diets (p > 0.05).
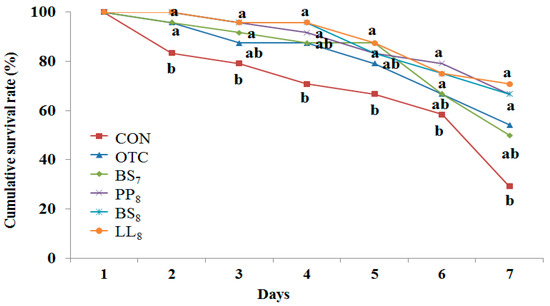
Figure 3.
Cumulative survival rate of juvenile whiteleg shrimp fed the experimental diets with different probiotics for 8 weeks and experimentally challenged with V. parahaemolyticus for 7 days. Each value represents mean of triplicate groups. Significant differences among means are indicated by different superscripts (p < 0.05), and diets refer to Figure 1.
3.7. Digestive Enzyme Activities
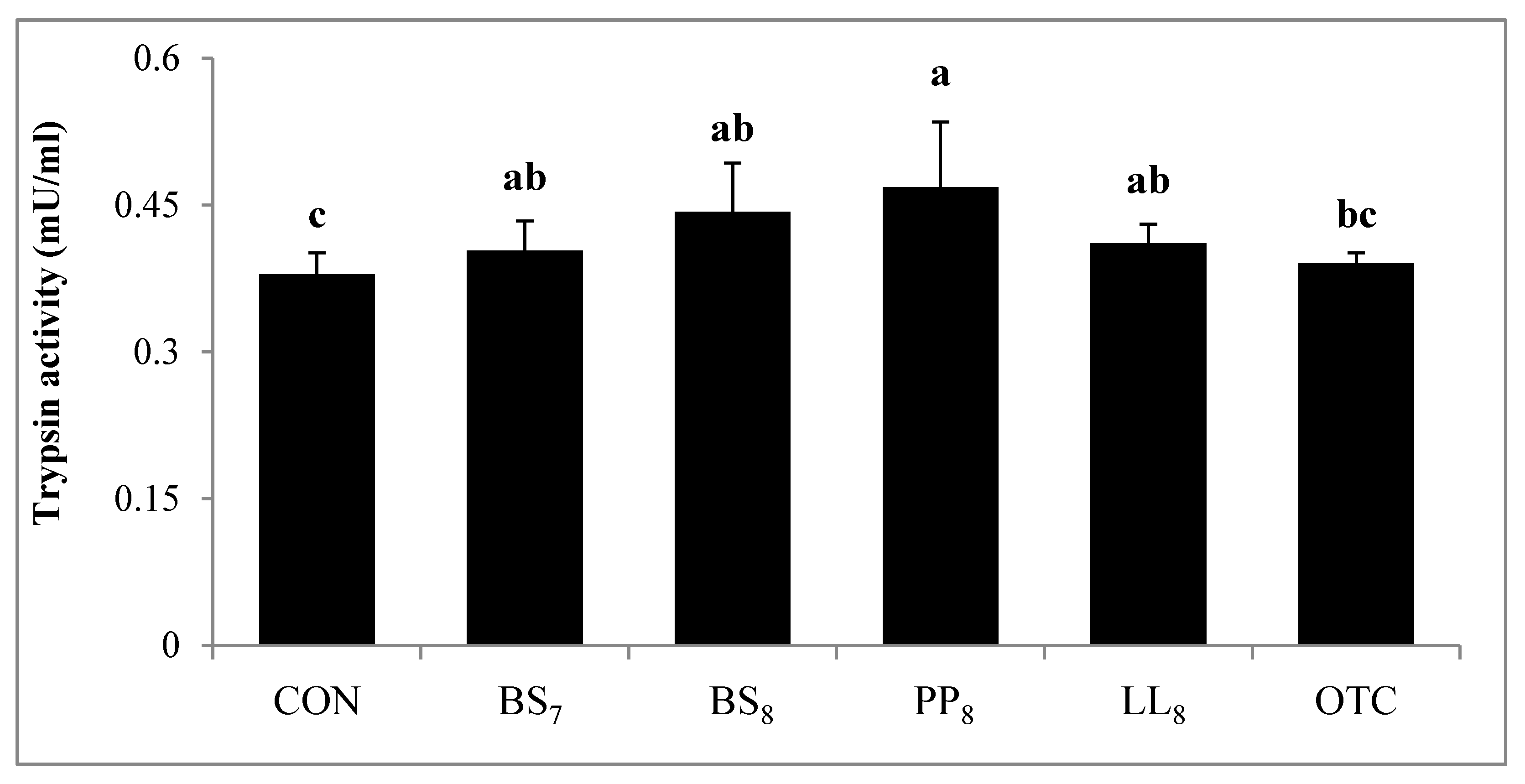
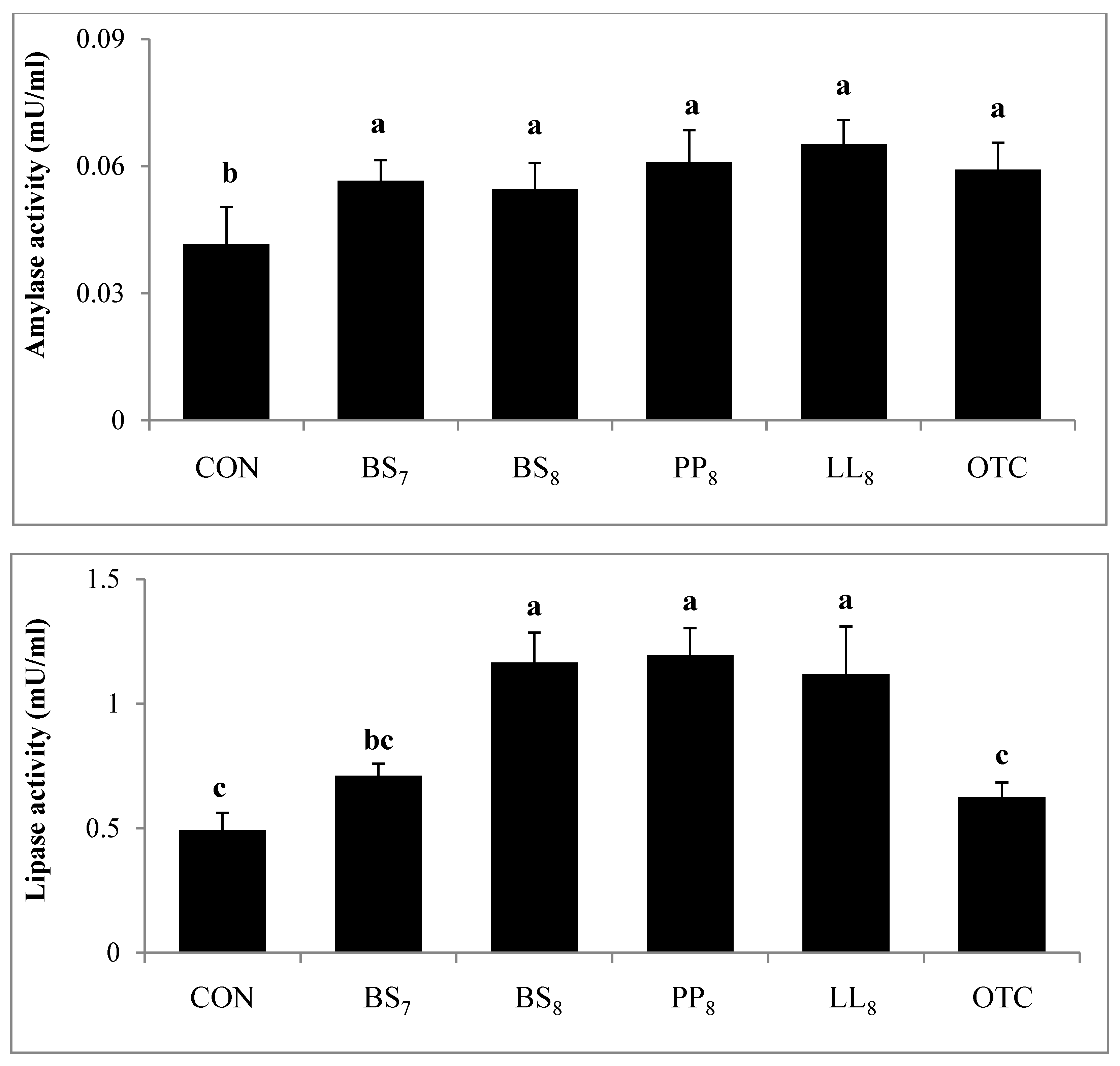
Digestive activity of juvenile whiteleg shrimp is shown in Figure 4. Trypsin activity in shrimp fed the BS8 and PP8 diets were significantly higher than for those on the CON diet (p < 0.05). However, there were no significant differences among shrimp on the BS8, PP8, LL8, BS7, and OTC diets (p > 0.05). Lipase activity of shrimp fed the BS8, PP8, and LL8 diets were significantly improved compared to those on the CON, BS7, and OTC diets (p < 0.05). Moreover, amylase activity in probiotic and OTC groups were significantly higher than the CON group (p > 0.05).

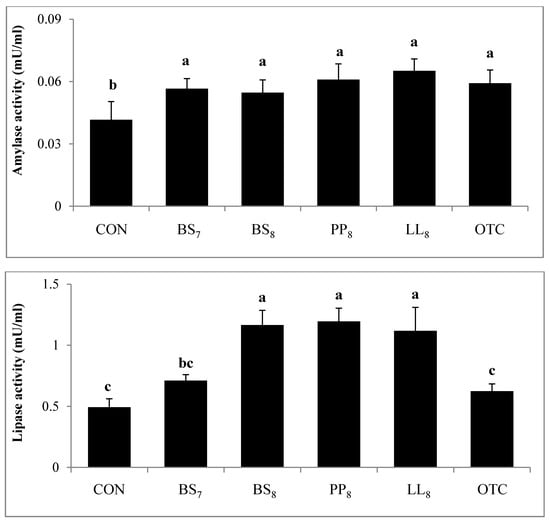
Figure 4.
Specific enzyme activities of 1. Trypsin, 2. Amylase and 3. Lipase measured in the intestines of juvenile whiteleg shrimp fed the experimental diets with different probiotics for 8 weeks, and diets refer to Figure 1.
4. Discussion
The application of probiotics in shrimp diets have shown beneficial effects on the growth [23], immune response [24], and disease resistance [25]. Among the probiotics used in the present study, B. subtilis WB60, which was isolated from the intestine of juvenile Japanese eel Anguilla japonica, was reported as a potential probiotic, as well as an antibiotic replacement in the diet [18]. In this study, the effects of three different dietary isolated probiotic supplementations on growth, immune responses, histology, and gene expression in whiteleg shrimp were investigated.
The results of our study indicated that dietary B. subtilis and L. lactis at 108 CFU/g had a significant influence on the growth and feed utilization of whiteleg shrimp. Similar results were seen in previous studies on improved growth and feed utilization by probiotic supplementation such as B. subtilis [13], L. lactis [17] and P. pentosaceus [15], which were improved compared to the CON diet. The reasons behind the improved growth performance through probiotic supplementation could be explained through two hypotheses. The increased growth performance and feed-utilization of whiteleg shrimp fed probiotics may be due to enhanced: (1) gastrointestinal performance [26,27]; and (2) immune response [24,25].
The digestive enzyme activities of shrimp are important indicators for estimating the organism’s ability to metabolize given nutrients [28,29]. The results of this study indicated that BS8, PP8, and LL8 diets significantly improved trypsin, amylase, and lipase activity of the shrimp intestine compared to those on the CON diet (p < 0.05). A few studies conducted on shrimp with probiotic supplementation with Bacillus sp. [30,31], L. lactis [17], Lactobacillus plantarum [32], and P. acidilactici [33] demonstrated the response similar to our results. Furthermore, our results are similar to the observations of Javahery et al. [34], who demonstrated that the administration of probiotics leads to enhanced digestion and nutrient absorption in the shrimp intestine, which was stimulated by endogenous enzymes produced by probiotics. Also, probiotic supplementation can improve the digestion of protein, starch, and fat in whiteleg shrimp compared to the CON diet, as a result of the increased value of enzyme activity [15]. According to Zheng et al. [35], probiotic supplementation could improve the height and density of intestinal enterocytes. Moreover, the microvilli of the intestine extensively contributes to the absorptive ability of nutrients and expansion of surface area [36]. The intestinal histology has been determined to estimate the gut condition [37]. The muscular layer thickness of whiteleg shrimp fed probiotic diets was significantly higher in the mid-intestine than those of shrimp fed CON and OTC diets (p < 0.05). On the other hands, villi height was significantly higher in the mid-intestine of PP8 and LL8 diets compared to the CON diet (p < 0.05). However, there were no significant differences between supplemented-probiotic and OTC diets (p > 0.05). Our findings are in agreement with [35], who reported that enhanced villi height and muscular layer thickness have been associated with probiotic administration, which could improve its nutrient absorptive ability. As it was mentioned before, the improved growth performance, feed utilization, and survival of shrimp may be due to enhancing digestive enzyme activity and histological value induced by the probiotics. Meanwhile, the improved growth can be explained in view of enhanced immune response. The invertebrates rely on a non-specific immune system against pathogenic organisms, and it has been shown that it can be reacted by probiotics which generate transduction signaling molecules that have the ability to alert the immune responses by pathogenic bacteria [38]. Modulation and disease prevention of the immune system have been shown to be among the beneficial effects of probiotics for aquatic animals [31,39]. Various studies have revealed that probiotic supplementation can improve immune responses in the shrimp diet [25,40,41]. In the current study, the dietary probiotic groups improved in lysozyme activity compared to the CON diet (p < 0.05), whereas there were no significant differences with the OTC diet (p > 0.05). Moreover, Superoxide dismutase activity of shrimp fed BS8 and PP8 diets were significantly higher than those of shrimp fed the CON diet (p < 0.05), and this did not differ between the probiotic and OTC diets (p > 0.05). Previous studies have observed that the administration of probiotics that complement each other and occupy different niches within the gastrointestinal tract could result in improvement or prolongation of the desirable effects on the host immune system and health [42,43].
The prophenoloxidase, serine protease, and peroxinectin serve important roles in immune responses for crustaceans. The proposed mechanism of prophenoloxidase is that active phenoloxidase induces oxidation of phenols to quinones, and results in the production of melanin, which can hold and barricade infectious pathogens [44]. Consequently, this system leads to induced phagocytosis, cytotoxic reactant production, and anti-oxidant defense enzymes. In the current experiment, the BS8, PP8, and LL8 diets significantly improved prophenoloxidase, serine protease and peroxinectin production compared to the CON and OTC diets (p < 0.05). The immune gene expression of shrimp increased with probiotic supplementation, as shown in previous studies [12,25]. Further, Chiu et al. [25] reported that the expression of the prophenoloxidase system can elevate the molecular activity of cell adhesion [45], opsonin [46], degranulation [47], and peroxidase [48] of shrimp. These biological activities were achieved in the present study with the probiotic treatments and gene up-regulation was enhanced in treated shrimp compared to CON diet (p < 0.05).
Disease resistance against V. parahaemolyticus, which generates the common disease of whiteleg shrimp [49], was significantly enhanced for 7 days by administration of the B. subtilis, WB60, P. pentosaceus and L. lactis at 1 × 108 CFU/g diet. This result is in agreement with previous experiments affected by pathogens [12,24,41]. Generally, administration of probiotics in the shrimp diet was shown to decrease mortality rates compared to the CON diet [40,50,51]. Previous studies demonstrated that probiotic supplementation can be used for modulating fish health and disease resistance [52,53]. Indeed, probiotics can beneficially influence the disease resistance of fish to pathogen bacteria by producing antimicrobial substances and competing with pathogens for physical occupation of space [54]. As a result, the enhanced survival and cumulative survival rates could be due to probiotic supplementation.
Basically, the selection of endogenous or exogenous probiotics should be done after their evaluation to colonize, establish, and multiply in the fish gut. Endogenous probiotics are already accustomed to the environmental conditions of the host, while inappropriate exogenous probiotics can cause undesirable effects in the host [55]. In this study, endogenous L. lactis and P. pentosaceus, and exogenous B. subtilis WB60, were evaluated, and it was shown that the exogenous bacteria can also be effective in whiteleg shrimp. Further, the studies on dietary endogenous or exogenous Bacillus strains have demonstrated beneficial effects on growth, immune response, and disease resistance in shrimp [12,56,57].
In conclusion, the present study demonstrated that L. lactis at 1 × 108 CFU/g could be an ideal probiotic in terms of growth performance, immune response, histology, immune-related gene expression, digestive enzyme activity, and disease resistance for whiteleg shrimp. Besides, potential probiotic B. subtilis WB60 and P. pentosaceus at 1 × 108 CFU/g could also be beneficial probiotics and replace antibiotics in whiteleg shrimp. Further, the results of this study could suggest that these probiotics have the potential for biofloc application to improve growth and immune responses in shrimp farms.
Author Contributions
S.W. conducted the experiment, and drafted the manuscript. S.C.B. supervisors and conception. A.H. organized the manuscript. W.C. analysis and statistical analysis. J.B. Feeding trial. S.L. and W.J.J. Data collection and Gene expression analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (Grant No. NRF-2018R1D1A3A03001450).
Acknowledgments
The author appreciates the Feeds & Foods Nutrition Research Center (FFNRC), Pukyong National University (PKNU), Busan, Rep. of Korea.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Food and Agriculture Organization (FAO). Fisheries and Aquaculture Software. FishStatJ-Software for Fishery Statistical Time Series. In FAO Fisheries and Aquaculture Department (online). Rome. Available online: http://www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 20 February 2019).
- Balcázar, J.L.; Blas, I.D.; Ruiz-Zarzuela, I.; Cunningham, D.; Vendrell, D.; Muzquiz, J.L. The role of probiotics in aquaculture. Vet. Microbiol. 2016, 114, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Austin, B. Vibrios as causal agents of zoonoses. Vet. Microbiol. 2010, 140, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zokaeifar, H.; Balcázar, J.L.; Saad, C.R.; Kamarudin, M.S.; Sijam, K.; Arshad, A.; Nejat, N. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2012, 33, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, M.; Brooker, J.; Chung, K.; Kelly, M.; Martinez, J.; Moore, J.; Thomas, M. Exposure of the grass shrimp, Palaemonetes pugio, to antimicrobial compounds affects associated Vibrio bacterial density and development of antibiotic resistance. Environ. Toxicol. 2016, 31, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Amoah, K.; Huang, Q.C.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Dong, X.H. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 796–808. [Google Scholar] [CrossRef]
- Xie, J.J.; Liu, Q.Q.; Liao, S.; Fang, H.H.; Yin, P.; Xie, S.W.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary mixed probiotics on growth, non-specific immunity, intestinal morphology and microbiota of juvenile pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 90, 456–465. [Google Scholar] [CrossRef]
- Moriarty, D.J.W. Disease control in shrimp aquaculture with probiotic bacteria. In Proceedings of the 8th International Symposium on Microbial Ecology; Atlantic Canada Society for Microbial Ecology: Halifax, NS, Canada, 1999; pp. 237–243. [Google Scholar]
- Lakshmi, B.; Viswanath, B.; Sai Gopal, D.V.R. Probiotics as antiviral agents in shrimp aquaculture. J. Pathog. 2013, 13. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496. [Google Scholar] [CrossRef]
- Tseng, D.Y.; Ho, P.L.; Huang, S.Y.; Cheng, S.C.; Shiu, Y.L.; Chiu, C.S.; Liu, C.H. Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish Shellfish Immunol. 2009, 26, 339–344. [Google Scholar] [CrossRef]
- Zokaeifar, H.; Babaei, N.; Saad, C.R.; Kamarudin, M.S.; Sijam, K.; Balcazar, J.L. Administration of Bacillus subtilis strains in the rearing water enhances the water quality, growth performance, immune response, and resistance against Vibrio harveyi infection in juvenile white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2014, 36, 68–74. [Google Scholar] [CrossRef]
- Leyva-Madrigal, K.Y.; Luna-González, A.; Escobedo-Bonilla, C.M.; Fierro-Coronado, J.A.; Maldonado-Mendoza, I.E. Screening for potential probiotic bacteria to reduce prevalence of WSSV and IHHNV in whiteleg shrimp (Litopenaeus vannamei) under experimental conditions. Aquaculture 2011, 322, 16–22. [Google Scholar] [CrossRef]
- Adel, M.; Yeganeh, S.; Dawood, M.A.O.; Safari, R.; Radhakrishnan, S. Effects of Pediococcus pentosaceus supplementation on growth performance, intestinal microflora and disease resistance of white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2017, 23, 1401–1409. [Google Scholar] [CrossRef]
- Maeda, M.; Shibata, A.; Biswas, G.; Korenaga, H.; Kono, T.; Itami, T.; Sakai, M. Isolation of lactic acid bacteria from kuruma shrimp (Marsupenaeus japonicus) intestine and assessment of immunomodulatory role of a selected strain as probiotic. Mar. Biotechnol. 2014, 16, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; El-Sayed, A.F.M.; Yeganeh, S.; Dadar, M.; Giri, S.S. Effect of potential probiotic Lactococcus lactis subsp. lactis on growth performance, intestinal microbiota, digestive enzyme activities, and disease resistance of Litopenaeus vannamei. Probiotics Antimicro. 2017, 9, 150–156. [Google Scholar] [CrossRef]
- Lee, S.; Katya, K.; Park, Y.; Won, S.; Seong, M.; Bai, S.C. Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 2017, 61, 201–210. [Google Scholar] [CrossRef]
- Ben-David, A.; Davidson, C.E. Estimation method for serial dilution experiments. J. Microbiol. Methods 2014, 107, 214–221. [Google Scholar] [CrossRef]
- Bai, S.C.; Kim, K.W. Effects of dietary animal protein sources on growth and body composition in Korean rockfish, Sebastes schlegeli. J. Aquac. 1997, 10, 77–85. [Google Scholar]
- AOAC Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2000.
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Hossain, M.I.; Kamal, M.M.; Mannan, M.A.; Bhuyain, M.A.B. Effects of probiotics on growth and survival of shrimp (Penaeus monodon) in Coastal Pond at Khulna, Bangladesh. J. Sci. Res. 2013, 5, 363–370. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, B.; Mai, K.; Zhang, W.; Ma, H.; Ai, Q.; Liufu, Z. Dietary administration of Bacillus (B. licheniformis and B. subtilis) and isomaltooligosaccharide influences the intestinal microflora, immunological parameters and resistance against Vibrio alginolyticus in shrimp, Penaeus japonicus (Decapoda: Penaeidae). Aqua Res. 2011, 42, 943–952. [Google Scholar] [CrossRef]
- Chiu, C.H.; Guu, Y.K.; Liu, C.H.; Pan, T.M.; Cheng, W. Immune responses and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol. 2007, 23, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, M.S.; Jones, D.A.; le Vay, L.; Abidin, A.Z. Ontogenetic change in digestive enzyme activity during larval development of Macrobrachium rosenbergii. Aquaculture 1994, 123, 323–333. [Google Scholar] [CrossRef]
- Zhou, X.X.; Wang, Y.B.; Li, W.F. Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture 2009, 287, 349–353. [Google Scholar] [CrossRef]
- Berges, J.A.; Mulholland, M.R. Enzymes and nitrogen cycling. Nitrogen Mar. Environ. 2018, 1385–1444. [Google Scholar] [CrossRef]
- Carrillo-Farnés, O.; Vega-Villasante, F.; Forrellat-Barrios, A.; Guerrero-Galván, S. A review of digestive enzyme activity in penaeid shrimps. Crustaceana 2007, 80, 257–275. [Google Scholar] [CrossRef]
- Ziaei-Nejad, S.; Rezaei, M.H.; Takami, G.A.; Lovett, D.L.; Mirvaghefi, A.R.; Shakouri, M. The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 2006, 252, 516–524. [Google Scholar] [CrossRef]
- Wang, Y.B. Effect of probiotics on growth performance and digestive enzyme activity of the shrimp Penaeus vannamei. Aquaculture 2007, 269, 259–264. [Google Scholar] [CrossRef]
- Kongnum, K.; Hongpattarakere, T. Effect of Lactobacillus plantarum isolated from digestive tract of wild shrimp on growth and survival of white shrimp (Litopenaeus vannamei) challenged with Vibrio harveyi. Fish Shellfish Immunol. 2012, 32, 170–177. [Google Scholar] [CrossRef]
- Castex, M.; Lemaire, P.; Wabete, N.; Chim, L. Effect of probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress of Litopenaeus stylirostris under Vibrio nigripulchritudo challenge. Fish Shellfish Immunol. 2010, 28, 622–631. [Google Scholar] [CrossRef]
- Javahery, S.; Noori, A.; Hoseinifar, S.H. Growth performance, immune response, and digestive enzyme activity in Pacific white shrimp, Penaeus vannamei Boone, 1931, fed dietary microbial lysozyme. Fish Shellfish Immunol. 2019, 92, 528–535. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of dietary Lactobacillus plantarum on growth performance, digestive enzymes and gut morphology of Litopenaeus vannamei. Probiotics Antimicrob. 2018, 10, 504–510. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Tian, L.; Yang, H.; Liang, G.; Xu, D. Effects of dietary mannan oligosaccharide on growth performance, gut morphology and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2012, 33, 1027–1032. [Google Scholar] [CrossRef]
- Khojasteh, S.M.B. The morphology of the post-gastric alimentary canal in teleost fishes: A brief review. Int. J. Aquat. Sci. 2012, 3, 71–88. [Google Scholar]
- Rengpipat, S.; Rukpratanporn, S.; Piyatiratitivorakul, S.; Menasaveta, P. Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 2000, 191, 271–288. [Google Scholar] [CrossRef]
- Ai, Q.; Xu, H.; Mai, K.; Xu, W.; Wang, J.; Zhang, W. Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture 2011, 317, 155–161. [Google Scholar] [CrossRef]
- Zhang, L.; Mai, K.; Tan, B.; Ai, Q.; Qi, C.; Xu, W.; Ma, H. Effects of dietary administration of probiotic Halomonas sp. B12 on the intestinal microflora, immunological parameters, and midgut histological structure of shrimp, Fenneropenaeus chinensis. J. World Aquac. Soc. 2009, 40, 58–66. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Tan, B.; Lao, Y.; Duan, Z.; Sun, W.; Dong, X. Isolation of a putative probiotic strain S12 and its effect on growth performance, non-specific immunity and disease-resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2014, 41, 300–307. [Google Scholar] [CrossRef]
- Salinas, I.; Cuesta, A.; Esteban, M.Á.; Meseguer, J. Dietary administration of Lactobacillus delbrüeckii and Bacillus subtilis, single or combined, on gilthead seabream cellular innate immune responses. Fish Shellfish Immunol. 2005, 19, 67–77. [Google Scholar] [CrossRef]
- Zoppi, G.; Cinquetti, M.; Benini, A.; Bonamini, E.; Minelli, E.B. Modulation of the intestinal ecosystem by probiotics and lactulose in children during treatment with ceftriaxone. Curr. Ther. Res. Clin. Exp. 2001, 62, 418–435. [Google Scholar] [CrossRef]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef]
- Sritunyalucksana, K.; Wongsuebsantati, K.; Johansson, M.W.; Söderhäll, K. Peroxinectin, a cell adhesive protein associated with the proPO system from the black tiger shrimp, Penaeus monodon. Dev. Comp. Immunol. 2001, 25, 353–363. [Google Scholar] [CrossRef]
- Thörnqvist, P.O.; Johansson, M.W.; Söderhäll, K. Opsonic activity of cell adhesion proteins and β-1, 3-glucan binding proteins from two crustaceans. Dev. Comp. Immunol. 1994, 18, 3–12. [Google Scholar] [CrossRef]
- Johansson, M.W.; Söderhäll, K. A cell adhesion factor from crayfish haemocytes has degranulating activity towards crayfish granular cells. Insect. Biochem. 1989, 19, 183–190. [Google Scholar] [CrossRef]
- Johansson, M.W.; Lind, M.I.; Holmblad, T.; Thornqvist, P.O.; Soderhall, K. Peroxinectin, a novel cell adhesion protein from crayfish blood. Biochem. Biophys. Res. Commun. 1995, 216, 1079–1087. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, J.; Dong, H.; Wang, Y.; Liu, Q.; Li, H. Effect of desiccation and resubmersion on the oxidative stress response of the kuruma shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2016, 49, 91–99. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Rojas-Luna, T.; Cunningham, D.P. Effect of the addition of four potential probiotic strains on the survival of pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. J. Invertebr. Pathol. 2007, 96, 147–150. [Google Scholar] [CrossRef]
- Sapcharoen, P.; Rengpipat, S. Effects of the probiotic Bacillus subtilis (BP 11 and BS 11) on the growth and survival of Pacific white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2013, 19, 946–954. [Google Scholar] [CrossRef]
- Zuo, Z.H.; Shang, B.J.; Shao, Y.C.; Li, W.Y.; Sun, J.S. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 86, 160–168. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Tang, Y.; Sun, B.; Huang, J.; Song, X. Pseudoalteromonas probiotics as potential biocontrol agents improve the survival of Penaeus vannamei challenged with acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Aquaculture 2018, 494, 30–36. [Google Scholar] [CrossRef]
- Lim, S.Y.; Loo, K.W.; Wong, W.L. Synergistic Antimicrobial Effect of a Seaweed-Probiotic Blend Against Acute Hepatopancreatic Necrosis Disease (AHPND)-Causing Vibrio parahaemolyticus. Probiotics Antimicrob. 2019, 1, 1–12. [Google Scholar] [CrossRef]
- Nayak, S.K. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Xu, W.; Mai, K.; Zhang, Y.; Liufu, Z. Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2012, 32, 750–755. [Google Scholar] [CrossRef]
- Liu, C.H.; Chiu, C.S.; Ho, P.L.; Wang, S.W. Improvement in the growth performance of white shrimp, Litopenaeus vannamei, by a protease-producing probiotic, Bacillus subtilis E20, from natto. J. Appl. Microbiol. 2009, 107, 1031–1041. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).