In Vitro Fumonisin Biosynthesis and Genetic Structure of Fusarium verticillioides Strains from Five Mediterranean Countries
Abstract
1. Introduction
- (i)
- investigate the abilities of selected F. verticillioides strains isolated from maize kernels in five Mediterranean countries to in vitro biosynthesize FB1, FB2 and FB3;
- (ii)
- characterize the genetic structure of these selected strains to assess for possible variability within strains originating from each of the surveyed countries and between the strains originating from different countries.
2. Materials and Methods
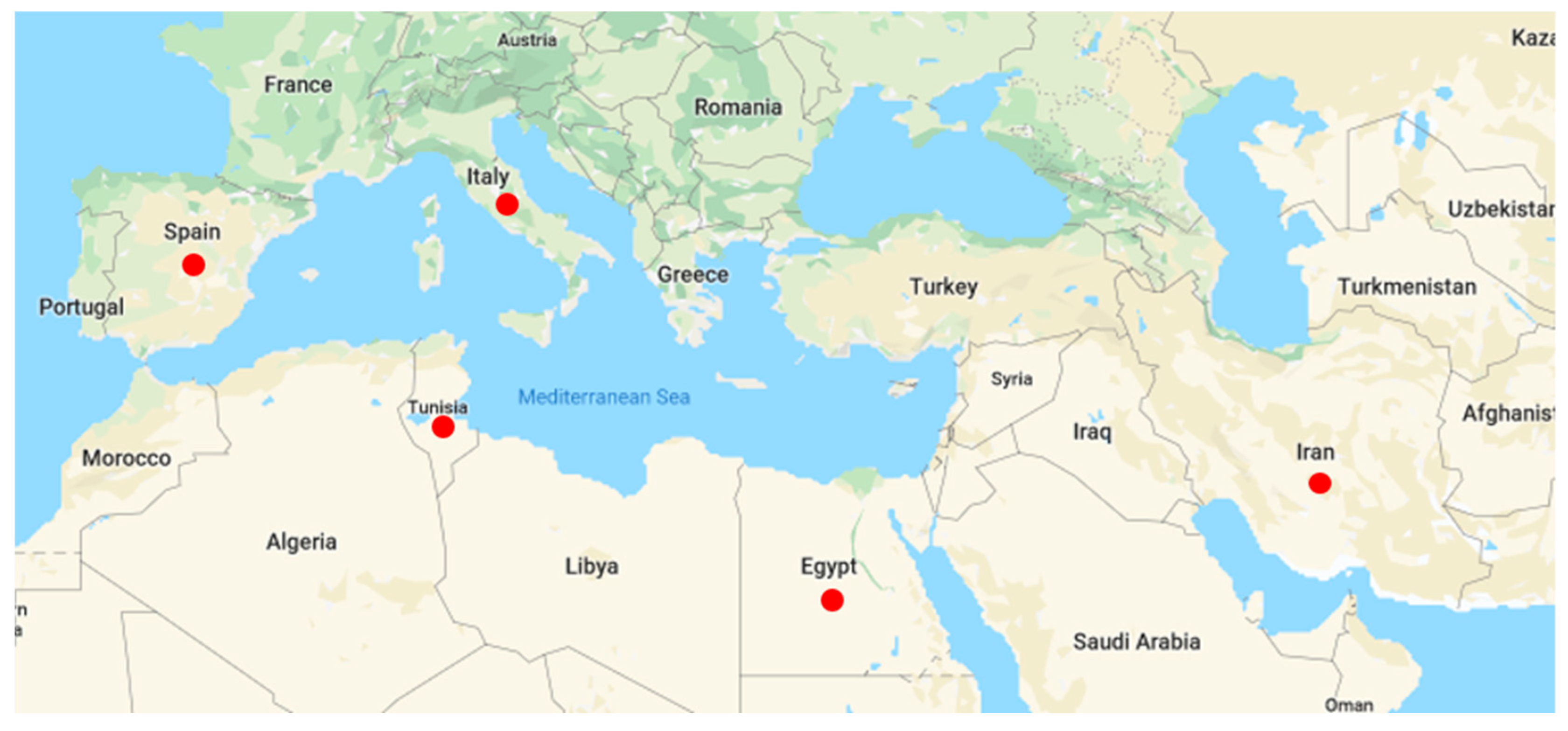
2.1. Fungal Strains
2.2. Confirmation of F. verticillioides Identity by PCR Assays
2.3. Determiantion of Fumonisin Biosynthesis by F. verticillioides In Vitro
2.3.1. F. verticillioides Cultures
2.3.2. Fumonisin Extraction and LC-MS/MS Analysis
2.4. Genetic Structure of Different F. verticillioides Populations
2.5. Statistical Analysis
3. Results
3.1. Identity Confirmation of F. verticillioides
3.2. Fumonisin Biosynthesis by F. verticillioides In Vitro
3.3. Genetic Structure and Variability of F. verticillioides Populations
4. Discussion
5. Conclusions
- (i)
- the presence of an Egyptian population which differed from the others for its low percentage of fumonisin-producing strains;
- (ii)
- the presence of significant differences in fumonisin production within the strains isolated in each of the surveyed countries and, in some cases, also among populations isolated from different countries;
- (iii)
- the high level of genetic uniformity inside the populations analyzed;
- (iv)
- the general absence of correlation between geographical origin and/or fumonisin production ability with the genetic diversity of the strain set;
- (v)
- the presence of four Egyptian strains that were distinguished from the other strains at a bootstrap value of 60.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Donnell, K.; Nirenberg, H.I.; Aoki, T.; Cigelnik, E. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetic distinct species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- Palacios, S.A.; Susca, A.; Haidukowski, M.; Stea, G.; Cendoya, E.; Ramírez, M.L.; Chulze, S.N.; Farnochi, M.C.; Moretti, A.; Torres, A.M. Genetic variability and fumonisin production by Fusarium proliferatum isolated from durum wheat grains in Argentina. Int. J. Food Microbiol. 2015, 18, 35–41. [Google Scholar] [CrossRef]
- Kvas, M.; Marasas, W.F.O.; Wingfield, B.D.; Wingfield, M.J.; Steenkamp, E.T. Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Divers. 2009, 34, 1–21. [Google Scholar]
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Folcher, L.; Jarry, M.; Weissenberger, A.; Gérault, F.; Eychenne, N.; Delos, M.; Regnault-Roger, C. Comparative activity of agrochemical treatments on mycotoxin levels with regard to corn borers and Fusarium mycoflora in maize (Zea mays L.) fields. Crop Prot. 2009, 28, 302–308. [Google Scholar] [CrossRef]
- Bottalico, A. Fusarium diseases of cereals: Species complex and related mycotoxins profiles, in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar]
- Oldenburg, E.; Höppner, F.; Ellner, F.; Weinert, J. Fusarium disease of maize associated with mycotoxin contamination of agricultural products intended to be used for food and feed. Mycotoxin Res. 2017, 33, 167–182. [Google Scholar] [CrossRef]
- Marín, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Reduction of mycotoxins and toxigenic fungi in the Mediterranean basin maize chain. Phytopathol. Mediterr. 2012, 51, 93–118. [Google Scholar]
- Fandohan, P.; Hell, K.; Marasas, W.F.O.; Wingfield, M.J. Infection of maize by Fusarium species and contamination with fumonisin in africa. Afr. J. Biotechnol. 2003, 2, 570–579. [Google Scholar]
- Mirzadi Gohari, A.M.; Javan-Nikkhah, M.; Hedjaroude, G.A.; Abbasi, M.; Rahjoo, V.; Sedaghat, N. Genetic diversity of Fusarium verticillioides isolates from maize in Iran based on vegetative compatibility grouping. J. Plant Pathol. 2008, 90, 113–116. [Google Scholar]
- Covarelli, L.; Beccari, G.; Salvi, S. Infection by mycotoxigenic fungal species and mycotoxin contamination of maize grain in Umbria, central Italy. Food Chem. Toxicol. 2011, 49, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Venturini, G.; Assante, G.; Vercesi, A. Fusarium verticillioides contamination patterns in northern Italian maize during the growing season. Phytopathologia Mediterr. 2011, 50, 110–120. [Google Scholar]
- Lazzaro, I.; Moretti, A.; Giorni, P.; Brera, C.; Battilani, P. Organic vs conventional farming: differences in infection by mycotoxin-producing fungi on maize and wheat in Northern and Central Italy. Crop Prot. 2015, 72, 22–30. [Google Scholar] [CrossRef]
- Sala, N.; Sanchis, V.; Vilaro, P.; Viladrich, R.; Torres, M.; Viñas, I.; Canela, R. Fumonisin producing capacity of Fusarium strains isolated from cereals in Spain. J. Food Prot. 1994, 57, 915–917. [Google Scholar] [CrossRef]
- Ariño, A.; Juan, T.; Estopañan, G.; González-Cabo, J.F. Natural occurrence of Fusarium species, fumonisin production by toxigenic strains, and concentrations of fumonisins B1 and B2 in conventional and organic maize grown in Spain. J. Food Protect. 2007, 70, 151–156. [Google Scholar] [CrossRef]
- Aguín, O.; Cao, A.; Pintos, C.; Santiago, R.; Mansilla, P.; Butrón, A. Occurence of Fusarium species in maize kernels grown in northwestern Spain. Plant Pathol. 2014, 63, 946–951. [Google Scholar] [CrossRef]
- Fadl Allah, E.M. Occurrence and toxigenicity of Fusarium moniliforme from freshly harvested maize ears with special references to fumonisin production in Egypt. Mycopathologia 1998, 140, 99–103. [Google Scholar] [CrossRef]
- Aboul-Nasr, M.B.; Obied-Allah, M.R.A. Biological and chemical detection of fumonisins produced on agar medium by Fusarium verticillioides isolates collected from corn in Sohag, Egypt. Microbiology 2013, 159, 1720–1724. [Google Scholar] [CrossRef]
- Abd El-Fatah, S.I.; Naguib, M.M.; El-Hossiny, E.N.; Sultan, Y.Y. Occurrence of Fusarium species and the potential accumulation of its toxins in Egyptian maize grains. Int. J. Adv. Res. 2015, 3, 1435–1444. [Google Scholar]
- Abd-El Fatah, S.I.; Naguib, M.M.; El-Hossiny, E.N.; Sultan, Y.Y.; Abodalam, T.H.; Yli-Mattila, T. Molecular versus morphological identification of Fusarium spp. isolated from Egyptian corn. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1813–1822. [Google Scholar]
- Hussien, T.; Carlobos-Lopez, A.L.; Cumagun, C.J.R.; Yli-Mattila, T. Identification and quantification of fumonisin-producing Fusarium species in grain and soil samples from Egypt and the Philippines. Phytopathol. Mediterr. 2017, 56, 146–153. [Google Scholar]
- Ghiasian, S.A.; Rezayat, S.M.; Kord-Bacheh, P.; Hossein, M.; Yazdanpanah, H.; Shephard, G.S.; van der Westhuizen, L.; Vismer, H.; Marasas, W.F.O. Fumonisin production by Fusarium species isolated from freshly harvested corn in Iran. Mycopathologia 2005, 159, 31–40. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Jaskiewicz, J.; Marasas, W.F.O.; Thiel, P.G.; Horak, R.M.; Vleggar, R.; Kriek, N.P.J. Fumonisins—Novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988, 54, 1806–1811. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Shephard, G.S.; Marasas, W.F.O.; Leggott, N.L.; Yazdanpanah, H.; Rahimian, H. Natural occurrence of fumonisins in corn from Iran. J. Agric. Food Chem. 2000, 48, 1860–1864. [Google Scholar] [CrossRef]
- African Development Bank Group. Annual Core Data. Available online: http://high5.opendataforafrica.org (accessed on 28 June 2018).
- Food and Agriculture Organization of the United Nations. Statistic Division Database 2014. Available online: http://faostat.fao.org (accessed on 3 January 2018).
- Lanubile, A.; Maschietto, V.; Borrelli, V.M.; Stagnati, L.; Logrieco, A.F.; Marocco, A. Molecular basis of resistance to Fusarium ear rot in maize. Front. Plant Sci. 2017, 8, 1774. [Google Scholar] [CrossRef]
- Marasas, W.F.O. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2011, 109, 239–243. [Google Scholar]
- Rheeder, J.P.; Marasas, W.O.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef]
- Summary and Conclusions. In Proceedings of the Eighty-Third Meeting, Joint FAO/WHO Expert Committee on Food Additives, Rome, Italy, 8–17 November 2016; p. 15.
- Bondy, G.; Mehta, R.; Caldwell, D.; Coady, L.; Armstrong, C.; Savard, M.; Miller, J.D.; Chomyshyn, E.; Bronson, R.; Zitomer, N.; et al. Effect of long term exposure to the mycotoxin fumonisin B1 in p53 heterozygous and p53 homozygous transgenic mice. Food Chem. Toxicol. 2012, 50, 3604–3613. [Google Scholar] [CrossRef]
- Escriva, L.; Font, G.; Manyes, L. In vivo studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef]
- Müller, S.; Dekant, W.; Mally, A. Fumonisin B1 and the kidney: Modes of action for renal tumor formation by fumonisin B1 in rodents. Food Chem. Toxicol. 2012, 50, 3833–3846. [Google Scholar]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merril, A.H., Jr.; Rothman, K.J.; Hendricks, K.A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Recommendation (EC) 2006/576/CE on the presence of deoxynivalenol, zearalenone, ocharatoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- European Commission. Commission Regulation (EC) No. 1126/2007 on maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L255, 14–17. [Google Scholar]
- Silva, J.J.; Viaro, H.P.; Ferranti, L.S.; Oliveira, A.L.M.; Ferreira, J.M.; Ruas, C.F.; Ono, E.Y.S.; Fungaro, M.H.P. Genetic structure of Fusarium verticillioides populations and occurrence of fumonisins in maize grown in Southern Brazil. Crop Prot. 2017, 99, 160–167. [Google Scholar] [CrossRef]
- Sewram, V.; Mshicileli, N.; Shephard, G.S.; Vismer, H.F.; Rheeder, J.P.; Lee, Y.; Leslie, J.F.; Marasas, W.F.O. Production of fumonisin B and C analogues by several Fusarium species. J. Agric. Food Chem. 2005, 53, 4861–4866. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Perrone, G.; Mulè, G. Biodiversity of complexes of mycotoxigenic fungal species associated with Fusarium ear rot of maize and Aspergillus rot of grape. Int. J. Food Microbiol. 2007, 119, 11–16. [Google Scholar] [CrossRef]
- Lanza, F.E.; Zambolim, L.; da Costa, R.V.; Queiroz, V.A.V.; Cota, L.V.; da Silva, D.D.; de Souza, A.G.C.; Figueiredo, J.E.F. Prevalence of fumonisin-producing Fusarium species in Brazilian corn grains. Crop Prot. 2014, 65, 232–237. [Google Scholar] [CrossRef]
- Falavigna, C.; Lazzaro, I.; Galaverna, G.; Dall’Asta, C.; Battilani, P. Oleoyl and linoleoyl esters of fumonisin B1 are differently produced by Fusarium verticillioides on maize and rice based media. Int. J. Food Microbiol. 2016, 217, 79–84. [Google Scholar] [CrossRef]
- Marín, S.; Magan, N.; Ramos, A.J.; Sanchis, V. Fumonisin-producing strains of Fusarium: A review of their ecophysiology. J. Food Prot. 2004, 67, 1792–1805. [Google Scholar]
- Rocha, L.O.; Barroso, V.M.; Andrade, L.J.; Pereira, G.H.A.; Ferreira-Castro, F.L.; Duarte, A.P.; Michelotto, M.D.; Correa, B. FUM gene expression profile and fumonisin production by Fusarium verticillioides inoculated in Bt and non-Bt Maize. Front. Microbiol. 2015, 6, 1503. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Brown, D.W.; Plattner, R.D.; Desjardins, A.E. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genet. Biol. 2003, 38, 237–249. [Google Scholar] [CrossRef]
- Proctor, R.H.; Plattner, R.D.; Desjardins, A.E.; Busman, M.; Butchko, R.A.E. Fumonisin production in the maize pathogen Fusarium verticillioides: Genetic basis of naturally occurring chemical variation. J. Agric. Food Chem. 2006, 54, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. FUM cluster divergence in fumonisins-producing Fusarium species. Fungal Biol. 2011, 115, 112–123. [Google Scholar] [CrossRef]
- Moretti, A.; Mulè, G.; Susca, A.; González-Jaén, M.T.; Logrieco, A. Toxin profile, fertility and AFLP analysis of Fusarium verticillioides from banana fruits. Eur. J. Plant Pathol. 2004, 110, 601–609. [Google Scholar] [CrossRef]
- da Silva, V.N.; Fernandes, F.M.C.; Cortez, A.; Ribeiro, D.H.B.; de Almeida, A.P.; Hassegawa, R.H.; Corrêa, B. Characterization and genetic variability of Fusarium verticillioides strains isolated from corn and sorghum in Brazil based on fumonisins production, microsatellites, mating type locus, and mating cross. Can. J. Microbiol. 2006, 52, 798–804. [Google Scholar] [CrossRef]
- Covarelli, L.; Stifano, S.; Beccari, G.; Raggi, L.; Lattanzio, V.M.T.; Albertini, E. Characterization of Fusarium verticillioides strains isolated from maize in Italy: Fumonisins production, pathogenicity and genetic variability. Food Microbiol. 2012, 31, 17–24. [Google Scholar] [CrossRef]
- Reynoso, M.M.; Chulze, S.N.; Zeller, K.A.; Torres, A.M.; Leslie, J.F. Genetic structure of Fusarium verticillioides populations isolated from maize in Argentina. Eur. J. Plant Pathol. 2009, 123, 207–215. [Google Scholar] [CrossRef]
- Momeni, H.; Nazari, F. Population genetic structure among Iranian isolates of Fusarium verticillioides. J. Plant Pathol. Microbiol. 2016, 7, 6. [Google Scholar] [CrossRef]
- Tsehaye, H.; Elameen, A.; Tronsmo, A.M.; Sundheim, L.; Tronsmo, A.; Assefa, D.; Brurberg, M.B. Genetic variation among Fusarium verticillioides isolates associated with Ethiopian maize kernels as revealed by AFLP analysis. Eur. J. Plant Pathol. 2016, 146, 807–816. [Google Scholar] [CrossRef]
- Olowe, O.M.; Odebode, A.C.; Olawuyi, O.J.; Sobowale, A.A. Molecular variability of Fusarium verticillioides (Sacc.) in maize from three agro-ecological zones of southwest Nigeria. Am. J. Mol. Biol. 2017, 7, 30–40. [Google Scholar] [CrossRef]
- Reynoso, M.M.; Torres, A.M.; Chulze, S.N. Fusaproliferin, beauvericin and fumonisin production by different mating populations among Gibberella fujikuroi complex isolated from maize. Mycol. Res. 2004, 108, 154–160. [Google Scholar] [CrossRef]
- Beccari, G.; Caproni, L.; Tini, F.; Uhlig, S.; Covarelli, L. Presence of Fusarium species and other toxigenic fungi in malting barley and multi-mycotoxin analysis by liquid chromatography-high-resolution mass spectrometry. J. Agric. Food Chem. 2016, 64, 4390–4399. [Google Scholar] [CrossRef]
- Beccari, G.; Colasante, V.; Tini, F.; Senatore, M.T.; Prodi, A.; Sulyok, M.; Covarelli, L. Causal agents of Fusarium head blight of durum wheat (Triticum durum Desf.) in central Italy and their in vitro biosynthesis of secondary metabolites. Food Microbiol. 2018, 70, 17–27. [Google Scholar] [CrossRef]
- Patiño, B.; Mirete, S.; González-Jaén, M.T.; Mulé, G.; Rodríguez, M.T.; Vázquez, C. PCR detection assay of fumonisin-producing Fusarium verticillioides strains. J. Food Prot. 2004, 67, 1278–1283. [Google Scholar] [CrossRef]
- SANTE/12089/2016. Guidance Document on Identification of Mycotoxins in Food and Feed; SANTE: Warszawa, Poland, 2016. [Google Scholar]
- Stępień, Ł.; Jestoi, M.; Chełkowski, J. Cyclic hexadepsipeptides in wheat field samples and esyn1 gene divergence among enniatin producing Fusarium avenaceum strains. World Mycotoxin J. 2013, 6, 399–409. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Stępień, Ł.; Wilman, K.; Kachlicki, P. Diversity of pea-associated F. proliferatum and F. verticillioides populations revealed by FUM1 sequence analysis and fumonisin biosynthesis. Toxins 2013, 5, 488–503. [Google Scholar] [CrossRef]
- Stępień, Ł.; Waśkiewicz, A.; Wilman, K. Host extract modulates metabolism and fumonisin biosynthesis by the plant-pathogenic fungus Fusarium proliferatum. Int. J. Food Microbiol. 2015, 193, 74–81. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-Plus; Springer: New York, NY, USA, 2000. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org (accessed on 10 September 2018).
- Welch, B.L. The generalization of “Student’s” problem when several different population variances are involved. Biometrika 1947, 34, 28–35. [Google Scholar] [CrossRef]
- Segvic, M.; Pepeljnjak, S. Distribution and fumonisin B1 production capacity of Fusarium moniliforme isolated from corn in Croatia. Period. Biol. 2003, 105, 275–279. [Google Scholar]
- Castella, G.; Bragulat, M.R.; Cabañes, F.J. Mycoflora and fumonisin-producing strains of Fusarium moniliforme in mixed poultry and component raw material. Mycopathologia 1996, 133, 181–184. [Google Scholar] [CrossRef]
- Lee, U.S.; Lee, M.Y.; Shin, W.S.; Min, Y.S.; Cho, C.M.; Yoshio, U. Production of fumonisin B1 and B2 by Fusarium moniliforme isolated from Korean corn kernels for feed. Mycotoxin Res. 1994, 10, 67–72. [Google Scholar]
- Nelson, P.E.; Plattner, R.D.; Shackelford, D.D.; Desjardins, A.E. Production of fumonisins by Fusarium moniliforme strains from various substrates and geographic areas. Appl. Environ. Microbiol. 1991, 57, 2410–2412. [Google Scholar] [CrossRef]
- Chulze, S.; Ramirez, M.L.; Pascale, M.; Visconti, A. Fumonisin production by, and mating type population of, Fusarium section Liseola isolates from maize in Argentina. Mycol. Res. 1998, 102, 141–144. [Google Scholar] [CrossRef]
- Cvetnić, Z.; Pepeljkjak, S.; šegvić, M. Toxigenic potential of Fusarium species isolated from non-harvested maize. Arh. Hig. Rada. Toksikol. 2005, 56, 275–280. [Google Scholar]
- Tseng, T.C.; Lee, K.L.; Deng, T.S.; Liu, T.S.; Liu, C.Y.; Huang, J.W. Production of fumonisins by Fusarium species of Taiwan. Mycopathologia 1995, 130, 117–121. [Google Scholar] [CrossRef]
- Sanchis, V.; Abadias, M.; Oncins, L.; Sala, N.; Viñas, I.; Canela, R. Fumonisins B1 and B2 and toxigenic Fusarium strains in feeds from the Spanish market. Int. J. Food Microbiol. 1995, 27, 37–44. [Google Scholar] [CrossRef]
- Moretti, A.; Bennett, G.A.; Logrieco, A.; Bottalico, A.; Beremand, M.N. Fertility of Fusarium moniliforme from maize and sorghum related to fumonisin production in Italy. Mycopathologia 1995, 131, 25–29. [Google Scholar] [CrossRef]
- Plattner, R.D.; Desjardins, A.E.; Leslie, J.F.; Nelson, P.E. Identification and characterization of strains of Gibberella fujikuroi mating population A with rare fumonisin production phenotypes. Mycologia 1996, 88, 416–424. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Sulyok, M.; Bäzinger, I.; Krska, R.; Schuhmacher, R.; Forrer, H.R. Effect of fungal strain and cereal susbtrate on in vitro mycotoxin production by Fusarium poae and Fusarium avenaceum. Food Add. Contam. 2008, 25, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.S.; Richards, C.; Terry, A.; Parra, J.; Won-Bo, S. Genetic variability and geographical distribution of mycotoxigenic Fusarium verticillioides strains isolated from maize fields in Texas. Plant Pathol. J. 2015, 31, 203–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gálvez, L.; Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł.; Palmero, D. Fusarium proliferatum – Causal agent of garlic bulb rot in Spain: Genetic variability and mycotoxin production. Food Microbiol. 2017, 67, 41–48. [Google Scholar] [CrossRef]
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species. J. Appl. Genet. 2011, 52, 487–496. [Google Scholar] [CrossRef]
- Glenn, A.E.; Zitomer, N.C.; Zimeri, A.M.; Williams, L.D.; Riley, R.T.; Proctor, R.H. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol. Plant Microbe Interact. 2008, 21, 87–97. [Google Scholar] [CrossRef]



| Strain ID | Origin | Fumonisin Production (μg/g) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fumonisin B1 | Fumonisin B2 | Fumonisin B3 | Total Fumonisins **,§ | |||||||
| PG 21C | Italy | nd † | - | nd | - | nd | - | nd | - | - |
| PG 39B | Italy | nd | - | nd | - | nd | - | nd | - | - |
| ITEM 9313 | Italy | 0.03 | (±0.01) | nd | - | nd | - | 0.03 | (±0.01) | a |
| ITEM 9319 | Italy | 0.16 | (±0.08) | 0.03 | (±0.01) | 0.05 | (±0.02) | 0.24 | (±0.11) | ab |
| PG 60A1 | Italy | 0.20 | (±0.02) | 0.04 | (±0.01) | 0.05 | (±0.01) | 0.29 | (±0.02) | b |
| ITEM 9330 | Italy | 0.30 | (±0.08) | 0.05 | (±0.01) | 0.06 | (±0.01) | 0.41 | (±0.09) | ab |
| ITEM 9320 | Italy | 0.63 | (±0.60) | 0.10 | (±0.10) | 0.08 | (±0.07) | 0.81 | (±0.77) | ab |
| ITEM 9300 | Italy | 0.65 | (±0.37) | 0.11 | (±0.06) | 0.11 | (±0.05) | 0.87 | (±0.48) | ab |
| PG 28A | Italy | 1.01 | (±0.40) | 0.22 | (±0.10) | 0.25 | (±0.08) | 1.49 | (±0.58) | ab |
| ITEM 9318 | Italy | 1.03 | (±0.68) | 0.22 | (±0.15) | 0.35 | (±0.24) | 1.59 | (±1.07) | ab |
| PG 22A | Italy | 1.67 | (±1.52) | 0.24 | (±0.23) | 0.25 | (±0.22) | 2.16 | (±1.97) | ab |
| PG 20A | Italy | 2.81 | (±1.50) | 0.66 | (±0.35) | 0.40 | (±0.16) | 3.87 | (±2) | abc |
| ITEM 9310 | Italy | 6.56 | (±3.09) | 2.46 | (±1.19) | 0.68 | (±0.29) | 9.69 | (±4.56) | abcd |
| PG 5A | Italy | 6.99 | (±0.89) | 2.35 | (±0.37) | 0.85 | (±0.06) | 10.19 | (±1.27) | cd |
| ITEM 9309 | Italy | 7.70 | (±3.45) | 2.23 | (±1) | 0.80 | (±0.30) | 10.74 | (±4.74) | abcd |
| PG 76A1 | Italy | 8.78 | (±4.50) | 2.32 | (±1.29) | 1.24 | (±0.60) | 12.34 | (±6.39) | abcde |
| PG 30B | Italy | 10.36 | (±1.25) | 2.95 | (±0.45) | 1.26 | (±0.26) | 14.57 | (±1.92) | d |
| ITEM 9329 | Italy | 10.71 | (±2.32) | 3.04 | (±0.71) | 0.84 | (±0.16) | 14.59 | (±3.16) | cd |
| PG 35A | Italy | 13.30 | (±6.96) | 4.39 | (±2.26) | 1.78 | (±0.80) | 19.47 | (±10) | abcde |
| PG 58A1 | Italy | 19.39 | (±5.28) | 7.51 | (±1.73) | 2.16 | (±0.15) | 29.07 | (±7.05) | abcde |
| ITEM 10027 | Italy | 23.64 | (±1.57) | 7.22 | (±0.44) | 2.49 | (±0.05) | 33.35 | (±1.99) | e |
| PG 36B | Italy | 23.87 | (±0.44) | 5.63 | (±1.56) | 4.23 | (±0.19) | 33.73 | (±1.49) | e |
| 03-2/A | Spain | 0.24 | (±0.17) | nd | - | nd | - | 0.24 | (±0.17) | |
| FVMM 3-2 | Spain | 0.78 | (±0.29) | 0.03 | (±0.03) | 0.01 | (±0.01) | 0.82 | (±0.33) | a |
| C1-2 SEV | Spain | 2.24 | (±1.19) | 0.53 | (±0.42) | 0.01 | - | 2.77 | (±1.61) | ab |
| FVMM 2-1 | Spain | 2.60 | (±1.60) | 0.55 | (±0.46) | 0.24 | (±0.13) | 3.38 | (±2.17) | ab |
| FVMM AD 2-4 | Spain | 6.38 | (±3.28) | 1.61 | (±0.91) | 0.20 | (±0.05) | 8.19 | (±4.19) | ab |
| 03-5/B SEV.1 | Spain | 6.63 | (±1.08) | 1.31 | (±0.31) | 0.31 | (±0.05) | 8.24 | (±1.43) | b |
| 03-5/B SEV | Spain | 7.70 | (±3.57) | 1.81 | (±0.92) | 1.06 | (±0.66) | 10.57 | (±5.01) | ab |
| FVMM 1-1 | Spain | 15.63 | (±4.19) | 4.68 | (±1.25) | 1.77 | (±0.33) | 22.08 | (±5.74) | ab |
| 0-C-1-3 2/2 | Spain | 56.12 | (±5.31) | 10.67 | (±1.35) | 3.04 | (±0.21) | 69.84 | (±6.57) | c |
| M16 | Tunisia | nd | - | nd | - | nd | - | nd | - | - |
| M11 | Tunisia | 0.29 | (±0.07) | 0.04 | (±0.02) | nd | - | 0.33 | (±0.09) | a |
| M19 | Tunisia | 0.30 | (±0.07) | 0.03 | (±0.02) | 0.11 | (±0.03) | 0.45 | (±0.11) | a |
| M12 | Tunisia | 0.56 | (±0.23) | 0.12 | (±0.05) | 0.06 | (±0.02) | 0.74 | (±0.30) | ab |
| M15 | Tunisia | 0.47 | (±0.17) | 0.06 | (±0.02) | 0.27 | (±0.08) | 0.80 | (±0.28) | ab |
| M20 | Tunisia | 0.92 | (±0.13) | nd | - | 0.01 | - | 0.93 | (±0.13) | b |
| M17 | Tunisia | 0.91 | (±0.21) | 0.12 | (±0.03) | 0.55 | (±0.12) | 1.58 | (±0.36) | ab |
| M5 | Tunisia | 2.55 | (±1.43) | 0.27 | (±0.26) | nd | - | 2.83 | (±1.69) | ab |
| M2 | Tunisia | 3.21 | (±1.32) | 0.61 | (±0.31) | 0.01 | - | 3.82 | (±1.63) | ab |
| M8 | Tunisia | 3.53 | (±1.80) | 1.01 | (±0.58) | 0.01 | - | 4.55 | (±2.39) | abc |
| M7 | Tunisia | 3.80 | (±3.05) | 0.77 | (±0.75) | 0.40 | (±0.32) | 4.97 | (±4.11) | abc |
| M22 | Tunisia | 6.85 | (±3.59) | 1.15 | (±0.40) | 2.07 | (±0.47) | 10.07 | (±4.45) | abc |
| M21 | Tunisia | 7.10 | (±4.93) | 1.47 | (±1.24) | 1.72 | (±1.09) | 10.29 | (±7.24) | abc |
| M1 | Tunisia | 8.82 | (±1.28) | 2.16 | (±0.35) | 0.68 | (±0.23) | 11.66 | (±1.81) | c |
| M14 | Tunisia | 10.50 | (±0.10) | 1.72 | (±0.12) | 1.07 | (±0.10) | 13.28 | (±0.18) | c |
| M10 | Tunisia | 11.07 | (±1.71) | 2.48 | (±0.55) | 0.04 | (±0.03) | 13.59 | (±2.23) | c |
| F2 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F6 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F7 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F10 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F12 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F19 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F22 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F23 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F25 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F26 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F27 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F30 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F36 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F38 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F41 | Egypt | nd | - | nd | - | nd | - | nd | - | - |
| F39 | Egypt | 0.22 | (±0.02) | nd | - | nd | - | 0.22 | (±0.02) | a |
| F29 | Egypt | 0.81 | (±0.05) | 0.19 | (±0.04) | 0.12 | (±0.03) | 1.12 | (±0.11) | b |
| F8 | Egypt | 0.96 | (±0.90) | 0.34 | (±0.33) | nd | - | 1.29 | (±1.23) | ab |
| F4 | Egypt | 1.18 | (±0.08) | 0.10 | (±0.02) | 0.08 | - | 1.35 | (±0.11) | b |
| F28 | Egypt | 1.08 | (±0.69) | 0.21 | (±0.13) | 0.09 | (±0.05) | 1.38 | (±0.87) | ab |
| F9 | Egypt | 1.14 | (±0.79) | 0.15 | (±0.13) | 0.32 | (±0.25) | 1.61 | (±1.17) | ab |
| F32 | Egypt | 1.11 | (±0.34) | 0.72 | (±0.27) | 0.38 | (±0.20) | 2.21 | (±0.80) | ab |
| F5 | Egypt | 4.10 | (±2.16) | 0.70 | (±0.40) | 0.05 | (±0.03) | 4.85 | (±2.60) | abc |
| F11 | Egypt | 3.56 | (±1.88) | 0.70 | (±0.44) | 0.58 | (±0.37) | 4.85 | (±2.68) | abc |
| F17 | Egypt | 4.35 | (±3.24) | 2.03 | (±1.57) | nd | - | 6.38 | (±4.81) | abc |
| F13 | Egypt | 6.02 | (±1.45) | 0.88 | (±0.11) | 0.33 | (±0.12) | 7.23 | (±1.67) | abc |
| F15 | Egypt | 6.32 | (±4.25) | 1.29 | (±0.98) | 0.38 | (±0.22) | 7.99 | (±5.45) | abc |
| F3 | Egypt | 7.52 | (±0.08) | 1.95 | (±0.15) | 1.75 | (±0.15) | 11.23 | (±0.32) | c |
| 35 | Iran | nd | - | nd | - | nd | - | nd | - | - |
| 4 | Iran | 0.03 | (±0.02) | nd | - | nd | - | 0.03 | (±0.02) | a |
| 25 | Iran | 0.10 | (±0.02) | nd | - | nd | - | 0.10 | (±0.02) | b |
| 2 | Iran | 0.27 | (±0.08) | nd | - | nd | - | 0.27 | (±0.08) | ab |
| 9 | Iran | 0.47 | (±0.37) | nd | - | nd | - | 0.47 | (±0.37) | ab |
| 18 | Iran | 1.21 | (±0.25) | 0.10 | (±0.05) | 0.09 | (±0.04) | 1.40 | (±0.35) | abc |
| 39 | Iran | 1.65 | (±0.45) | 0.19 | (±0.18) | 0.42 | (±0.12) | 2.26 | (±0.73) | abc |
| 56 | Iran | 2.21 | (±1.12) | 0.34 | (±0.18) | 0.30 | (±0.16) | 2.85 | (±1.42) | abc |
| 1 | Iran | 3.94 | (±0.76) | 0.56 | (±0.18) | 0.22 | (±0.07) | 4.72 | (±1) | c |
| 3 | Iran | 4.48 | (±1.22) | 0.76 | (±0.22) | 0.47 | (±0.16) | 5.71 | (±1.59) | abc |
| 22 | Iran | 4.61 | (±1.38) | 1.65 | (±0.53) | nd | - | 6.26 | (±1.91) | abc |
| 16 | Iran | 4.66 | (±1.63) | 1.48 | (±0.58) | 0.40 | (±0.18) | 6.55 | (±2.39) | abc |
| 5 | Iran | 9.92 | (±5.52) | 2.15 | (±1.35) | 1.17 | (±0.71) | 13.25 | (±7.59) | abcd |
| 7 | Iran | 13.65 | (±4.74) | 3.23 | (±1.15) | 1.45 | (±0.50) | 18.33 | (±6.40) | abcd |
| 89 | Iran | 30.81 | (±4.39) | 7.23 | (±1.01) | 1.75 | (±0.28) | 39.79 | (±5.25) | d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beccari, G.; Stępień, Ł.; Onofri, A.; Lattanzio, V.M.T.; Ciasca, B.; Abd-El Fatah, S.I.; Valente, F.; Urbaniak, M.; Covarelli, L. In Vitro Fumonisin Biosynthesis and Genetic Structure of Fusarium verticillioides Strains from Five Mediterranean Countries. Microorganisms 2020, 8, 241. https://doi.org/10.3390/microorganisms8020241
Beccari G, Stępień Ł, Onofri A, Lattanzio VMT, Ciasca B, Abd-El Fatah SI, Valente F, Urbaniak M, Covarelli L. In Vitro Fumonisin Biosynthesis and Genetic Structure of Fusarium verticillioides Strains from Five Mediterranean Countries. Microorganisms. 2020; 8(2):241. https://doi.org/10.3390/microorganisms8020241
Chicago/Turabian StyleBeccari, Giovanni, Łukasz Stępień, Andrea Onofri, Veronica M. T. Lattanzio, Biancamaria Ciasca, Sally I. Abd-El Fatah, Francesco Valente, Monika Urbaniak, and Lorenzo Covarelli. 2020. "In Vitro Fumonisin Biosynthesis and Genetic Structure of Fusarium verticillioides Strains from Five Mediterranean Countries" Microorganisms 8, no. 2: 241. https://doi.org/10.3390/microorganisms8020241
APA StyleBeccari, G., Stępień, Ł., Onofri, A., Lattanzio, V. M. T., Ciasca, B., Abd-El Fatah, S. I., Valente, F., Urbaniak, M., & Covarelli, L. (2020). In Vitro Fumonisin Biosynthesis and Genetic Structure of Fusarium verticillioides Strains from Five Mediterranean Countries. Microorganisms, 8(2), 241. https://doi.org/10.3390/microorganisms8020241