Enhanced Cypermethrin Degradation Kinetics and Metabolic Pathway in Bacillus thuringiensis Strain SG4
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Enrichment, Screening, and Identification of Cypermethrin-Degrading Bacteria
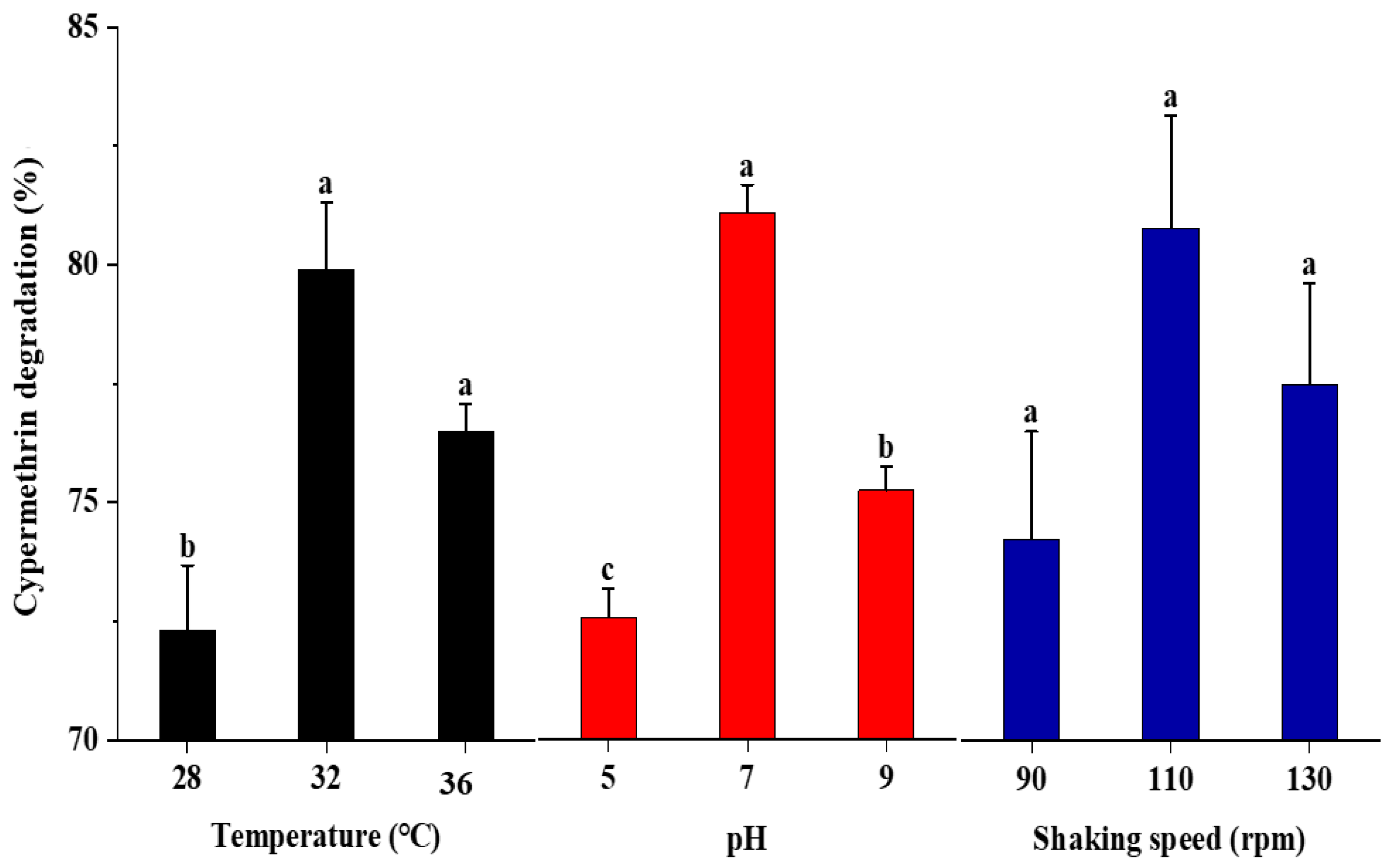
2.3. Effect of Temperature, pH, and Shaking Speed on the Degradation of Cypermethrin
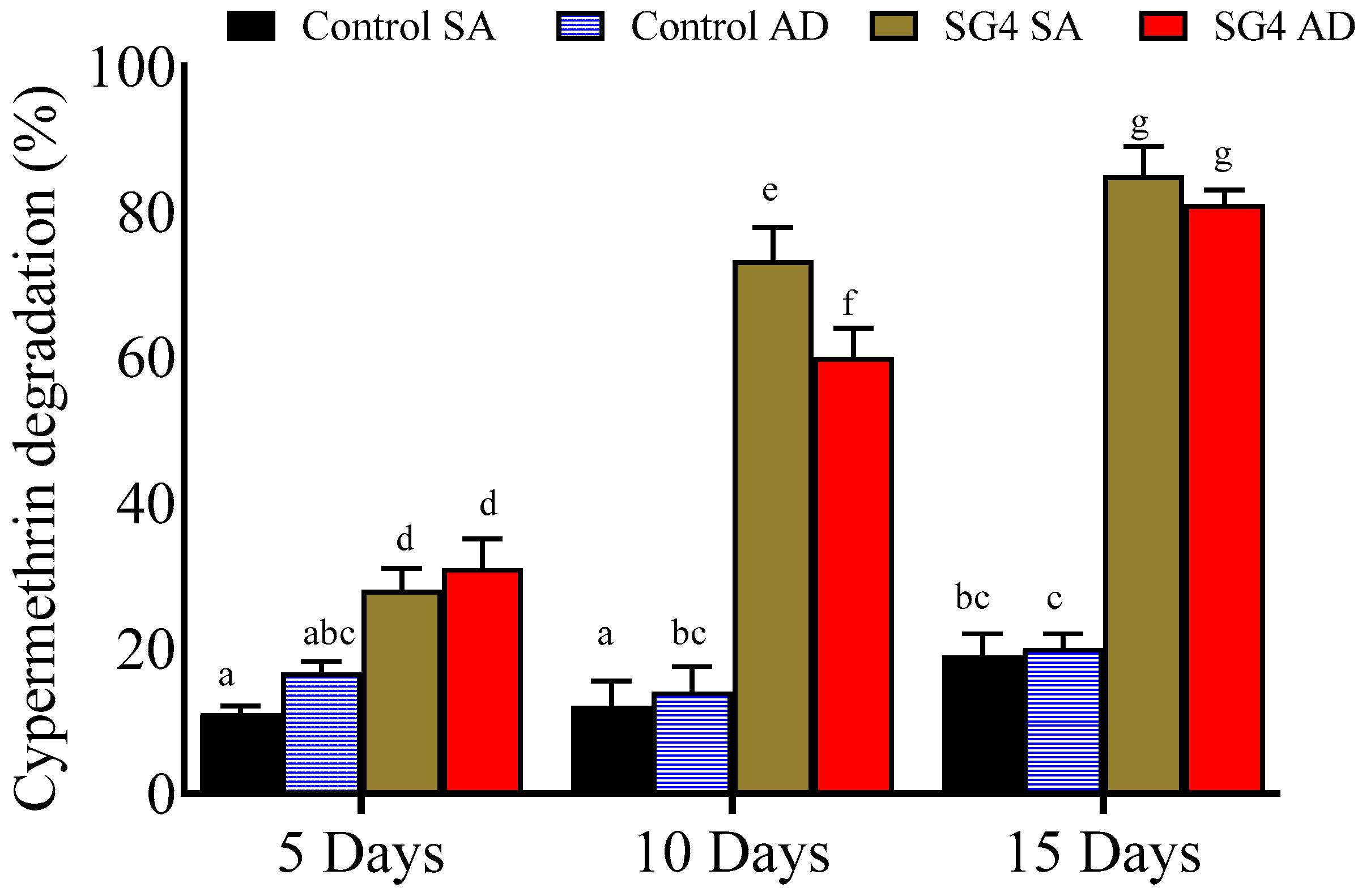
2.4. Effect of Immobilization on Cypermethrin Degradation
2.4.1. Sodium Alginate Beads
2.4.2. Agar Discs
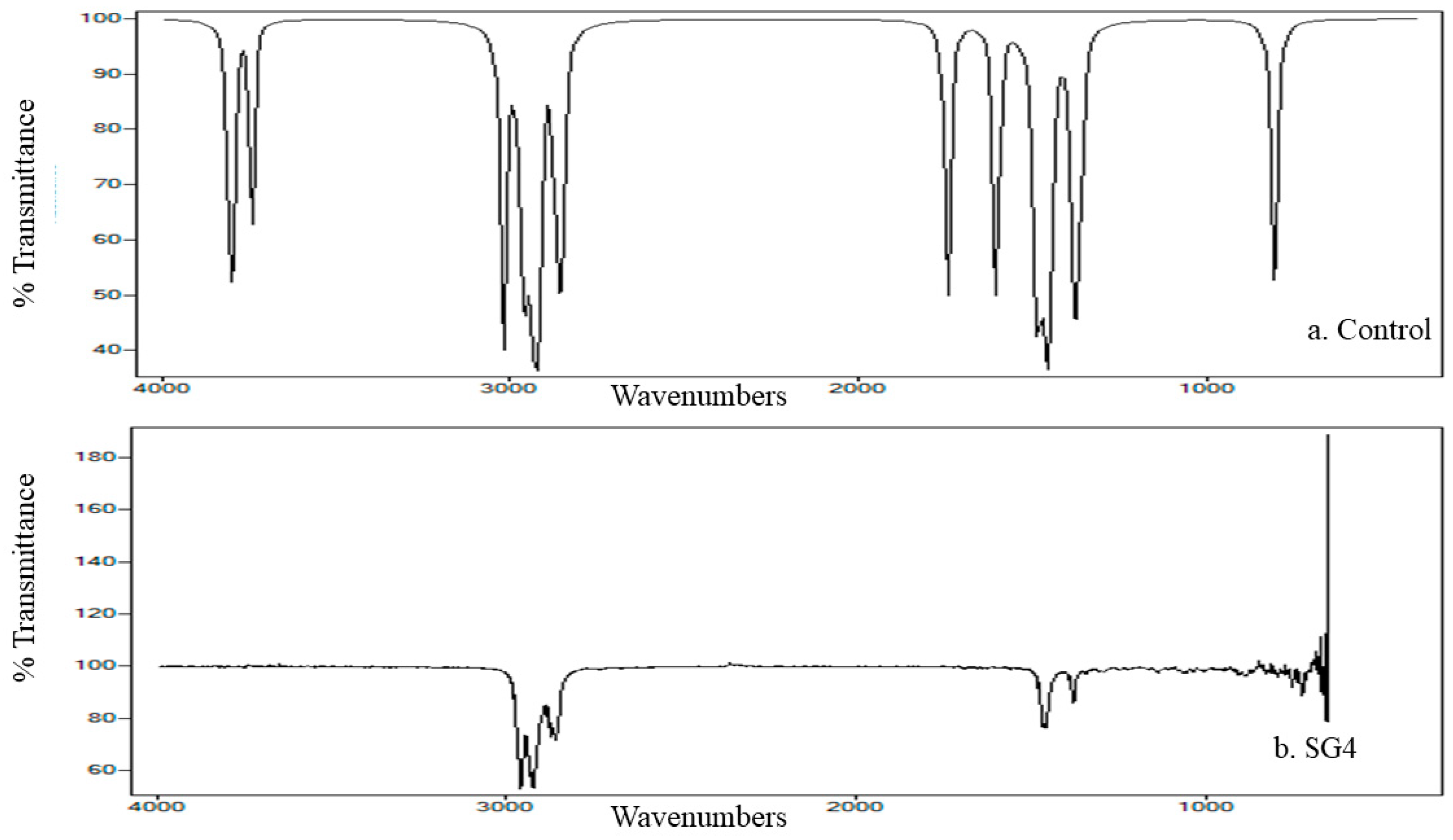
2.5. FTIR Analysis of Cypermethrin Biodegradation
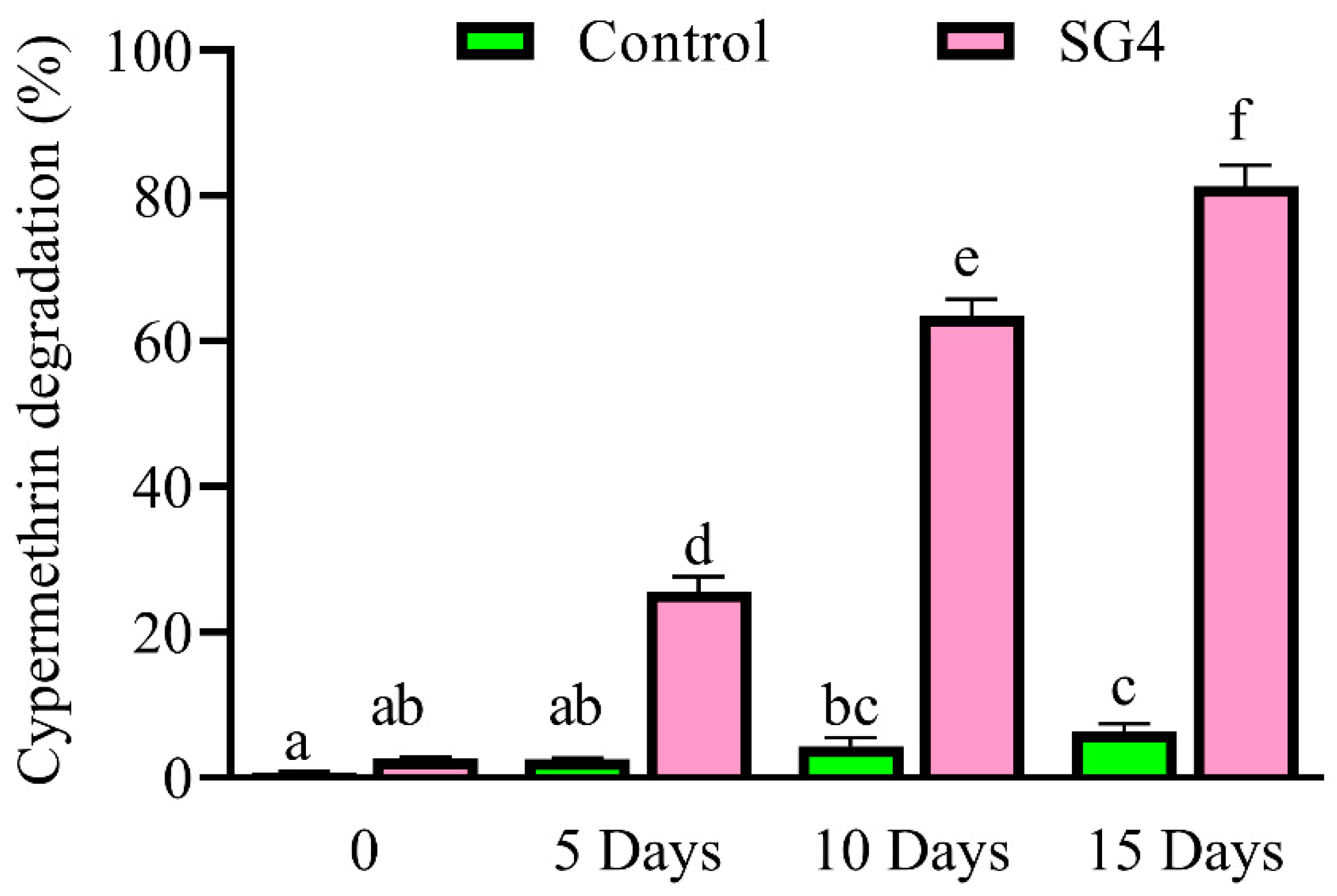
2.6. Cypermethrin Degradation in Soil Slurry and Metabolite Identification
2.7. Chemical Analysis
2.8. Kinetic Analysis of Cypermethrin
2.9. Statistical Analysis
3. Results
3.1. Enrichment of Bacterial Strain
3.2. Effect of Temperature, pH, and Shaking Speed on the Degradation of Cypermethrin by Strain SG4
3.3. Cypermethrin Degradation with Immobilized Culture
3.4. Degradation Kinetics of Cypermethrin in Soil Slurry
3.5. FTIR Analysis of Cypermethrin Metabolites
3.6. Identification of Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatt, P.; Huang, Y.; Zhan, H.; Chen, S. Insight into microbial applications for the biodegradation of pyrethroid insecticides. Front. Microbiol. 2019, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Piotrowska-Seget, Z. Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: A review. Front. Microbiol. 2016, 7, 1463. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Sultan, S.; Kertesz, M. Bacterial community analysis of cypermethrin enrichment cultures and bioremediation of cypermethrin contaminated soils. J. Basic Microbiol. 2015, 55, 819–829. [Google Scholar] [CrossRef]
- Yao, G.; Gao, J.; Zhang, C.; Jiang, W.; Wang, P.; Liu, X.; Liu, D.; Zhou, Z. Enantioselective degradation of the chiral alpha-cypermethrin and detection of its metabolites in five plants. Environ. Sci. Pollut. Res. 2018, 26, 1558–1564. [Google Scholar] [CrossRef]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. Paraquat degradation from contaminated environments: Current achievements and perspectives. Front. Microbiol. 2019, 10, 1754. [Google Scholar] [CrossRef]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 10, 5033–5043. [Google Scholar] [CrossRef]
- Bragança, I.; Mucha, A.P.; Tomasino, M.P.; Santos, F.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Deltamethrin impact in a cabbage planted soil: Degradation and effect on microbial community structure. Chemosphere 2019, 12, 1179–1186. [Google Scholar] [CrossRef]
- Wang, D.; Zou, L.; Jin, Q.; Hou, J.; Ge, G.; Yang, L. Human carboxylesterases: A comprehensive review. Acta Pharm. Sin. B 2018, 8, 699–712. [Google Scholar] [CrossRef]
- Chen, S.; Lin, Q.; Xiao, Y.; Deng, Y.; Chang, C.; Zhong, G.; Hu, M.; Zhang, L.H. Monooxygenase, a novel beta-cypermethrin degrading enzyme from Streptomyces sp. PLoS ONE 2013, 8, e75450. [Google Scholar] [CrossRef]
- Chen, S.; Geng, P.; Xiao, Y.; Hu, M. Bioremediation of β-cypermethrin and 3-phenoxybenzaldehyde contaminated soils using Streptomyces aureus HP-S-01. Appl. Microbiol. Biotechnol. 2012, 94, 505–515. [Google Scholar] [CrossRef]
- Pankaj; Sharma, A.; Gangola, S.; Khati, P.; Kumar, G.; Srivastava, A. Novel pathway of cypermethrin biodegradation in a Bacillus sp. strain SG2 isolated from cypermethrin-contaminated agriculture field. 3 Biotech 2016, 6, 45. [Google Scholar]
- Chen, S.; Hu, M.; Liu, J.; Zhong, G.; Yang, L.; Rizwan-ul-Haq, M.; Han, H. Biodegradation of beta-cypermethrin and 3-phenoxybenzoic acid by a novel Ochrobactrum lupini DG-S-01. J. Hazard. Mater. 2011, 187, 433–440. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Q.; Hu, M.; Luo, J.; Weng, Q.; Lai, K. Isolation and characterization of a fungus able to degrade pyrethroids and 3-phenoxybenzaldehyde. Bioresour. Technol. 2011, 102, 8110–8116. [Google Scholar] [CrossRef]
- Tallur, P.N.; Megadi, V.B.; Ninnekar, H.Z. Biodegradation of cypermethrin by Micrococcus sp. strain CPN 1. Biodegradation 2008, 19, 77–82. [Google Scholar] [CrossRef]
- Chen, S.; Chang, C.; Deng, Y.; An, S.; Dong, Y.H.; Zhou, J.; Hu, M.; Zhong, G.; Zhang, L.H. Fenpropathrin biodegradation pathway in Bacillus sp. DG-02 and its potential for bioremediation of pyrethroid-contaminated soils. J. Agric. Food Chem. 2014, 62, 2147–2157. [Google Scholar] [CrossRef]
- Pankaj; Negi, G.; Gangola, S.; Khati, P.; Kumar, G.; Srivastava, A.; Sharma, A. Differential expression and characterization of cypermethrin-degrading potential proteins in Bacillus thuringiensis strain, SG4. 3 Biotech 2016, 6, 255. [Google Scholar]
- Xiao, Y.; Chen, S.; Gao, Y.; Hu, W.; Hu, M.; Zhong, G. Isolation of a novel beta-cypermethrin degrading strain Bacillus subtilis BSF01 and its biodegradation pathway. Appl. Microbiol. Biotechnol. 2015, 99, 2849–2859. [Google Scholar] [CrossRef]
- Birolli, W.G.; Vacondio, B.; Alvarenga, N.; Seleghim, M.H.R.; Porto, A.L.M. Enantioselective biodegradation of the pyrethroid (±)-lambda-cyhalothrin by marine-derived fungi. Chemosphere 2018, 197, 651–660. [Google Scholar] [CrossRef]
- Cycoń, M.; Żmijowska, A.; Piotrowska-Seget, Z. Enhancement of deltamethrin degradation by soil bioaugmentation with two different strains of Serratia marcescens. Int. J. Environ. Sci. Technol. 2014, 11, 1305–1316. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, S.; Hu, M.; Rizwan-ul-Haq, M.; Yang, L.; Li, H. Biodegradation of cypermethrin by a newly isolated actinomycetes HU-S-01 from wastewater sludge. Int. J. Environ. Sci. Technol. 2011, 8, 45–56. [Google Scholar] [CrossRef]
- Chen, S.; Yang, L.; Hu, M.; Liu, J. Biodegradation of fenvalerate and 3-phenoxybenzoic acid by a novel Stenotrophomonas sp. strain ZS-S-01 and its use in bioremediation of contaminated soils. Appl. Microbiol. Biotechnol. 2011, 90, 755–767. [Google Scholar] [CrossRef]
- Guo, P.; Wang, B.; Hang, B.J.; Li, L.; Ali, S.W.; He, J.; Li, S. Pyrethroid-degrading Sphingobium sp. JZ-2 and the purification and characterization of a novel pyrethroid hydrolase. Int. Biodeterior. Biodegrad. 2009, 63, 1107–1112. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Yan, Y. Isomerization and biodegradation of beta-cypermethrin by Pseudomonas aeruginosa CH7 with biosurfactant production. Bioresour. Technol. 2011, 102, 7139–7146. [Google Scholar] [CrossRef]
- Chen, S.; Luo, J.; Hu, M.; Lai, K.; Geng, P.; Huang, H. Enhancement of cypermethrin degradation by a coculture of Bacillus cereus ZH-3 and Streptomyces aureus HP-S-01. Bioresour. Technol. 2012, 110, 97–104. [Google Scholar] [CrossRef]
- Akbar, S.; Sultan, S.; Kertesz, M. Determination of cypermethrin degradation potential of soil bacteria along with plant growth-promoting characteristics. Curr. Microbiol. 2015, 70, 75–84. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Y.H.; Chang, C.; Deng, Y.; Zhang, X.F.; Zhong, G.; Song, H.; Hu, M.; Zhang, L.H. Characterization of a novel cyfluthrin-degrading bacterial strain Brevibacterium aureum and its biochemical degradation pathway. Bioresour. Technol. 2013, 132, 16–23. [Google Scholar] [CrossRef]
- Yang, J.; Feng, Y.; Zhan, H.; Liu, J.; Zhang, K.; Zhang, L.H.; Chen, S. Characterization of a pyrethroid-degrading Pseudomonas fulva strain P31 and biochemical degradation pathway of D-phenothrin. Front. Microbiol. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Li, J.; de Toledo, R.A.; Shim, H. Multivariate optimization for the simultaneous bioremoval of BTEX and chlorinated aliphatic hydrocarbons by Pseudomonas plecoglossicida. J. Hazard. Mater. 2017, 321, 238–246. [Google Scholar] [CrossRef]
- Zhan, H.; Wang, H.; Liao, L.; Feng, Y.; Fan, X.; Zhang, L.H.; Chen, S. Kinetics and novel degradation pathway of permethrin in Acinetobacter baumannii ZH-14. Front. Microbiol. 2018, 9, 98. [Google Scholar] [CrossRef]
- Chen, S.; Liu, C.; Peng, C.; Liu, H.; Hu, M.; Zhong, G. Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PLoS ONE 2012, 7, e47205. [Google Scholar] [CrossRef]
- Doraiswamy, N.; Sarathi, M.; Pennathur, G. Cross-linked esterase aggregates (CLEAs) using nanoparticles as immobilization matrix. Prep. Biochem. Biotechnol. 2019, 49, 270–278. [Google Scholar] [CrossRef]
- Saez, J.M.; Aparicio, J.D.; Amoroso, M.J.; Benimeli, C.S. Effect of the acclimation of a Streptomyces consortium on lindane biodegradation by free and immobilized cells. Process Biochem. 2015, 50, 1923–1933. [Google Scholar] [CrossRef]
- Naidu, Y.; Idris, A.S.; Nusaibah, S.A.; Norman, K.; Siddiqui, Y. In vitro screening of biocontrol and biodegradation potential of selected hymenomycetes against Ganoderma boninense and infected oil palm waste. Forest Pathol. 2015, 45, 474–483. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, A.; Parihar, L. In vitro study of mycoremediation of cypermethrin-contaminated soils in different regions of Punjab. Ann. Microbiol. 2015, 65, 1949–1959. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, M.; Zhou, L.; Gu, Z.; Zhao, J. Bioremediation of marine oil pollution by Brevundimonas diminuta: Effect of salinity and nutrients. Desalin. Water Treat. 2016, 57, 19768–19775. [Google Scholar] [CrossRef]
- Hechmi, N.; Bosso, L.; El-Bassi, L.; Scelza, R.; Testa, A.; Jedidi, N.; Rao, M.A. Depletion of pentachlorophenol in soil microcosms with Byssochlamys nivea and Scopulariopsis brumptii as detoxification agents. Chemosphere 2016, 165, 547–554. [Google Scholar] [CrossRef]
- Maddela, N.R.; Burgos, R.; Kadiyala, V.; Carrion, A.R.; Bangeppagari, M. Removal of petroleum hydrocarbons from crude oil in solid and slurry phase by mixed soil microorganisms isolated from Ecuadorian oil fields. Int. Biodeter. Biodegr. 2016, 108, 85–90. [Google Scholar] [CrossRef]
- Negi, G.; Pankaj; Sharma, A.; Srivastava, A. In situ biodegradation of endosulfan, imidacloprid, and carbendazim using indigenous bacterial cultures of agriculture fields of Uttarakhand, India. Int. J. Bioeng. Life Sci. 2014, 8, 973–981. [Google Scholar]
- Ye, T.; Zhou, T.; Li, Q.; Xu, X.; Fan, X.; Zhang, L.; Chen, S. Cupriavidus sp. HN-2, a novel quorum quenching bacterial isolate, is a potent biocontrol agent against Xanthomonas campestris pv. campestris. Microorganisms 2020, 8, 45. [Google Scholar] [CrossRef]
- Bhatt, P.; Gangola, S.; Chaudhary, P.; Khat, P.; Kumar, G.; Sharma, A.; Srivastava, A. Pesticide induced up-regulation of esterase and aldehyde dehydrogenase in indigenous Bacillus spp. Bioremediat J. 2019, 23, 42–52. [Google Scholar] [CrossRef]
- Chen, S.; Deng, Y.; Chang, C.; Lee, J.; Cheng, Y.; Cui, Z.; Zhou, J.; He, F.; Hu, M.; Zhang, L.H. Pathway and kinetics of cyhalothrin biodegradation by Bacillus thuringiensis strain ZS-19. Sci. Rep. 2015, 5, 8784. [Google Scholar] [CrossRef]
- Gangola, S.; Sharma, A.; Bhatt, P.; Khati, P.; Chaudhary, P. Presence of esterase and laccase in Bacillus subtilis facilitates biodegradation and detoxification of cypermethrin. Sci. Rep. 2018, 8, 12755. [Google Scholar] [CrossRef]
- Tallur, P.N.; Mulla, S.I.; Megadi, V.B.; Talwar, M.P.; Ninnekar, H.Z. Biodegradation of cypermethrin by immobilized cells of Micrococcus sp. strain CPN 1. Brazilian J. Microbiol. 2015, 46, 667–672. [Google Scholar] [CrossRef]
- Tang, A.X.; Liu, H.; Liu, Y.Y.; Li, Q.Y.; Qing, Y.M. Purification and characterization of a novel β-cypermethrin-degrading aminopeptidase from Pseudomonas aeruginosa GF31. J. Agric. Food Chem. 2017, 65, 9412–9418. [Google Scholar] [CrossRef]
- Wang, B.; Guo, P.; Hang, B.; Li, L.; He, J.; Li, S.P. Cloning of a novel pyrethroid-hydrolyzing carboxylesterase gene from Sphingobium sp. strain JZ-1. Appl. Environ. Microbiol. 2009, 75, 5496–5500. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhatt, K.; Huang, Y.; Lin, Z.; Chen, S. Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemosphere 2020, 244, 125507. [Google Scholar] [CrossRef]
- Bhatt, P. Smart Bioremediation Technologies: Microbial Enzymes; Amesterdam Elsevier Science: London, UK, 2019. [Google Scholar] [CrossRef]
- Armenta, S.; Quintás, G.; Garrigues, S.; De La Guardia, M. A validated and fast procedure for FTIR determination of cypermethrin and chlorpyrifos. Talanta 2005, 67, 634–639. [Google Scholar] [CrossRef]
- Segal-Rosenheimer, M.; Dubowski, Y. Heterogeneous ozonolysis of cypermethrin using real-time monitoring FTIR techniques. J. Phys. Chem. C 2007, 111, 11682–11691. [Google Scholar] [CrossRef]
- Fiorenza, R.; Mauro, A.D.; Cantarella, M.; Laria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Datillo, S. Preferential removal of pesticides from water by molecular imprinting on TiO2 photocatalysts. Chem. Engi. J. 2020, 389, 122309. [Google Scholar] [CrossRef]
- Chen, S.; Hu, W.; Xiao, Y.; Deng, Y.; Jia, J.; Hu, M. Degradation of 3-phenoxybenzoic acid by a Bacillus sp. PLoS ONE 2012, 7, e50456. [Google Scholar] [CrossRef]
- Yang, L.; Chen, S.; Hu, M.; Liu, J. Biodegradation of carbofuran by Pichia anomala strain HQ-C-01 and its application for bioremediation of contaminated soils. Biol. Fert. Soils 2011, 47, 917–923. [Google Scholar] [CrossRef]
- Zhao, H.M.; Hu, R.W.; Huang, H.B.; Wen, H.F.; Du, H.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Liu, J.S.; et al. Enhanced dissipation of DEHP in soil and simultaneously reduced bioaccumulation of DEHP in vegetable using bioaugmentation with exogenous bacteria. Biol. Fertil. Soils 2017, 53, 663–675. [Google Scholar] [CrossRef]
- Purkarthofer, T.; Skranc, W.; Schuster, C.; Griengl, H. Potential and capabilities of hydroxynitrile lyases as biocatalysts in the chemical industry. Appl. Microbiol. Biotechnol. 2007, 76, 309–320. [Google Scholar] [CrossRef]
- Birolli, W.G.; Borges, E.M.; Nitschke, M.; Romão, L.P.C.; Porto, A.L.M. Biodegradation pathway of the pyrethroid pesticide esfenvalerate by bacteria from different biomes. Water Air Soil Poll. 2016, 227, 271. [Google Scholar] [CrossRef]
- Birolli, W.G.; Arai, M.S.; Nitschke, M.; Porto, A.L.M. The pyrethroid (±)-lambda-cyhalothrin enantioselective biodegradation by a bacterial consortium. Pest. Biochem. Physiol. 2019, 156, 129–137. [Google Scholar] [CrossRef]
- Nakamura, T.; Ichinose, H.; Wariishi, H. Cloning and heterologous expression of two aryl-aldehyde dehydrogenases from the white-rot basidiomycete Phanerochaete chrysosporium. Biochem. Biophys. Res. Commun. 2010, 394, 470–475. [Google Scholar] [CrossRef]
- Zhao, J.; Chi, Y.; Xu, Y.; Jia, D.; Yao, K. Co-metabolic degradation of β-cypermethrin and 3-phenoxybenzoic acid by co-culture of Bacillus licheniformis B-1 and Aspergillus oryzae M-4. PLoS ONE 2016, 11, e0166796. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, K.; Song, J.; Shi, Y.; Yan, Y. Molecular cloning, purification and biochemical characterization of a novel pyrethroid-hydrolyzing carboxylesterase gene from Ochrobactrum anthropi YZ-1. J. Hazard. Mater. 2012, 221–222, 206–212. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, L.; Liu, Y.; Zhang, D.; Luo, X.; Cheng, J.; Cheng, F.; Dai, J. Cometabolic biotransformation of fenpropathrin by Clostridium species strain ZP3. Biodegradation 2011, 22, 869–875. [Google Scholar] [CrossRef]
- Fan, X.; Liu, X.; Huang, R.; Liu, Y. Identification and characterization of a novel thermostable pyrethroid-hydrolyzing enzyme isolated through metagenomic approach. Microb. Cell Fact. 2012, 11, 33. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, X.; Huo, S.; Lin, W.; Xia, X.; Liu, K.; Duan, B. Isolation and identification of the Raoultella ornithinolytica-ZK4 degrading pyrethroid pesticides within soil sediment from an abandoned pesticide plant. Arch. Microbiol. 2019. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, Y.; Lin, Z.; Bhatt, P.; Chen, S. New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environ. Res. 2020. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Ding, J.; Xiao, Y.; Han, H.; Zhong, G. Sugarcane bagasse as support for immobilization of Bacillus pumilus HZ-2 and its use in bioremediation of mesotrione-contaminated soils. Appl. Microbiol. Biotechnol. 2015, 99, 10839–10851. [Google Scholar] [CrossRef]

| Treatment | Regression Equation | k (day−1) | R2 | t1/2 (days) |
|---|---|---|---|---|
| MSM | ln(Ct/C0) = –0.0042x + 4.6074 | 0.0042 | 0.990 | 165.0 |
| MSM + SG4 | ln(Ct/C0) = –0.1035x + 4.725 | 0.1035 | 0.954 | 6.7 |
| Soil slurry | ln(Ct/C0) = –0.0039x + 4.599 | 0.0039 | 0.999 | 177.7 |
| Soil slurry + SG4 | ln(Ct/C0) = –0.1134x + 4.702 | 0.113 | 0.968 | 0.70 |
| Treatment | Regression Equation | k (day−1) | R2 | t1/2 (days) |
|---|---|---|---|---|
| MSM + SA | ln(Ct/Co) = –0.0131x + 4.5868 | 0.0131 | 0.933 | 52.9 |
| MSM + SA + SG4 | ln(Ct/Co) = –0.1336x + 4.7126 | 0.133 | 0.966 | 5.3 |
| MSM + AD | ln(Ct/Co) = –0.0136x + 4.5732 | 0.0136 | 0.859 | 50.9 |
| MSM + AD + SG4 | ln(Ct/Co) = –0.1089x + 4.6867 | 0.1089 | 0.977 | 6.4 |
| Cypermethrin Degradation Metabolites Sequence | Retention Time (min) | Identified Metabolites | Molecular Weight (MW) | Chemical Structure |
|---|---|---|---|---|
| CP1 | 4.123 | Phenol | 94 | 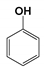 |
| CP2 | 11.050 | Benzoic acid, 2,5-dimethyl | 150.17 |  |
| CP3 | 13.724 | 2-Hydroxy-3- phenoxy- benzeneacetonitrile | 225 | 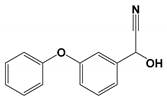 |
| CP4 | 13.728 | 3-Phenoxybenzaldehyde | 198 | 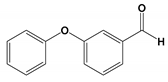 |
| CP5 | 14.289 | Phthalic acid | 166.14 | 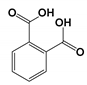 |
| CP6 | 15.942 | 2-Pentadecanone | 226.4 | 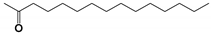 |
| CP7 | 20.00 | 3-Phenoxybenzoate | 228 | 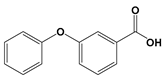 |
| CP8 | 23.960 | Cypermethrin | 415 | 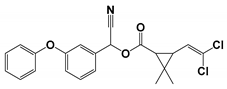 |
| CP9 | 24.098 | 3-(2,2-Dichloroethenyl)-2,2-dimethyl cyclopropanecarboxylate | 236 | 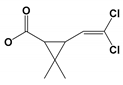 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, P.; Huang, Y.; Zhang, W.; Sharma, A.; Chen, S. Enhanced Cypermethrin Degradation Kinetics and Metabolic Pathway in Bacillus thuringiensis Strain SG4. Microorganisms 2020, 8, 223. https://doi.org/10.3390/microorganisms8020223
Bhatt P, Huang Y, Zhang W, Sharma A, Chen S. Enhanced Cypermethrin Degradation Kinetics and Metabolic Pathway in Bacillus thuringiensis Strain SG4. Microorganisms. 2020; 8(2):223. https://doi.org/10.3390/microorganisms8020223
Chicago/Turabian StyleBhatt, Pankaj, Yaohua Huang, Wenping Zhang, Anita Sharma, and Shaohua Chen. 2020. "Enhanced Cypermethrin Degradation Kinetics and Metabolic Pathway in Bacillus thuringiensis Strain SG4" Microorganisms 8, no. 2: 223. https://doi.org/10.3390/microorganisms8020223
APA StyleBhatt, P., Huang, Y., Zhang, W., Sharma, A., & Chen, S. (2020). Enhanced Cypermethrin Degradation Kinetics and Metabolic Pathway in Bacillus thuringiensis Strain SG4. Microorganisms, 8(2), 223. https://doi.org/10.3390/microorganisms8020223






