Distinct Functional Traits of Lactobacilli from Women with Asymptomatic Bacterial Vaginosis and Normal Microbiota
Abstract
1. Introduction
2. Material and Methods
2.1. Isolation of Lactobacilli
2.2. Preparation of Cell-Free Culture Supernatants
2.3. Determination of Lactic Acid
2.4. Determination of Hydrogen Peroxide
2.5. Semi-Quantitative Estimation Lactobacilli Agglutination with C. albicans
2.6. Quantitative Evaluation of Coaggregation and Autoaggregation
2.7. Antagonistic Activity of Lactobacilli Metabolites against Pathogens
2.8. Statistical Analysis
3. Results
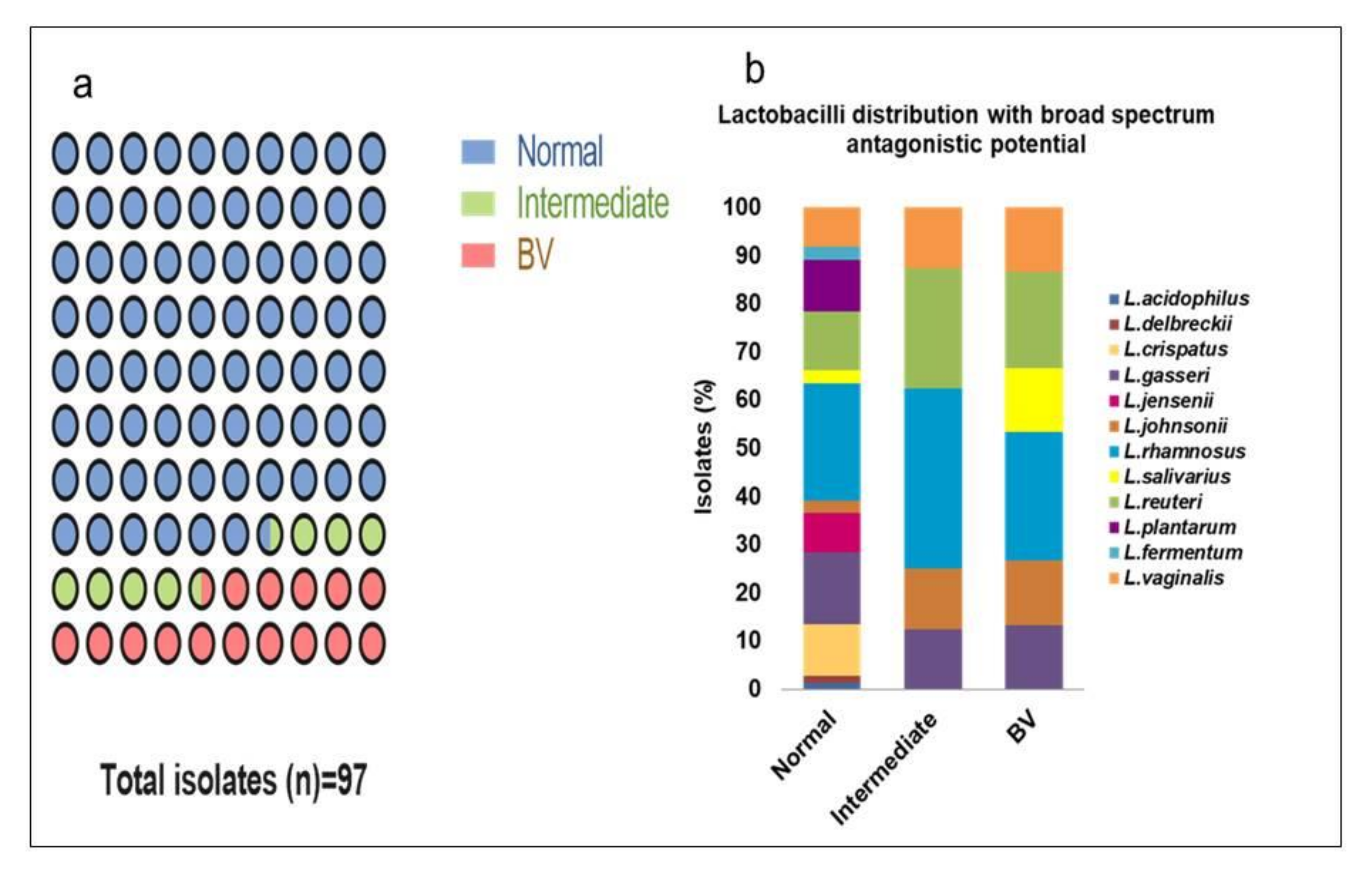
3.1. Lactobacillus Isolates Diversity
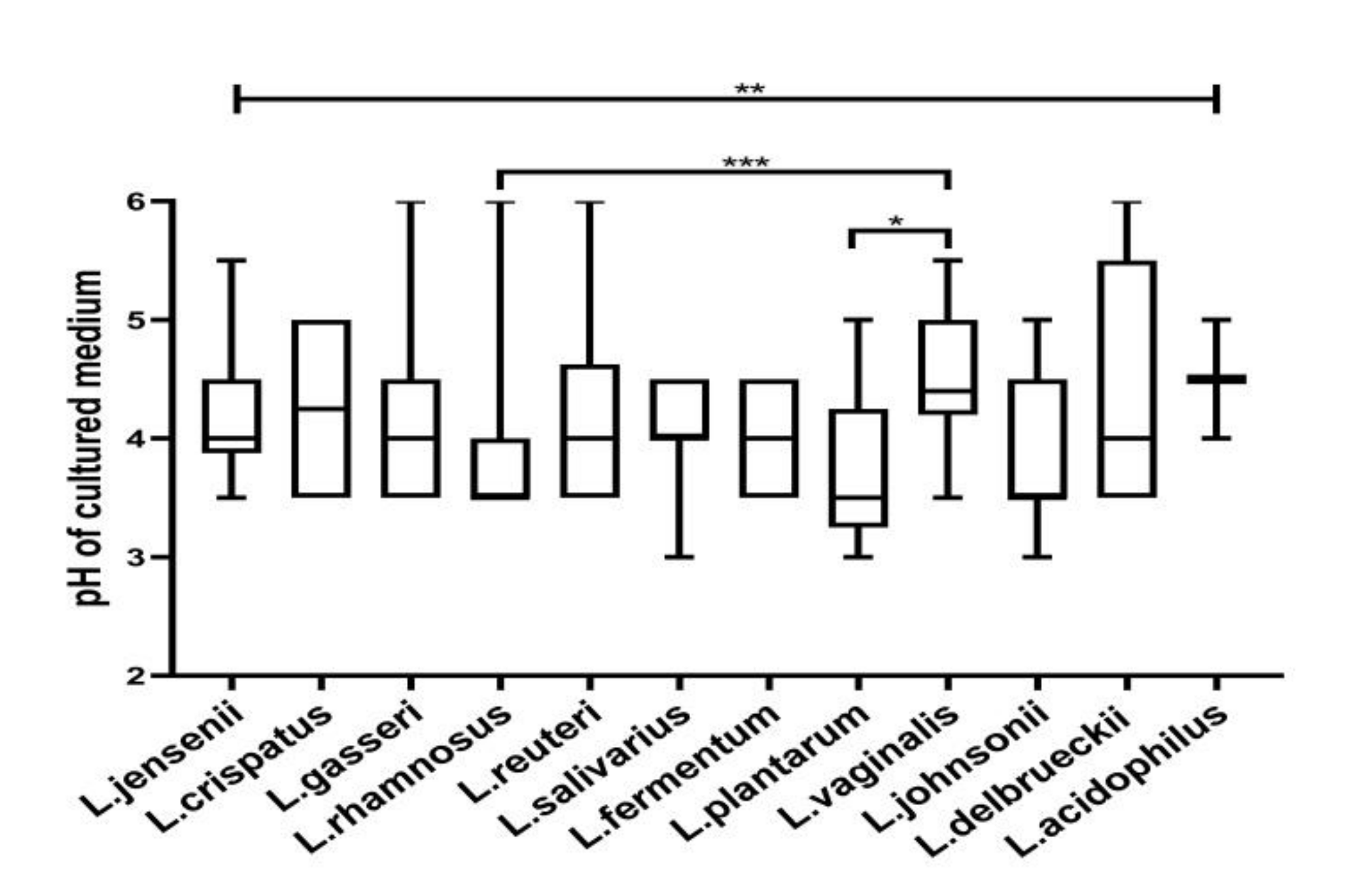
3.2. Acidification of Medium
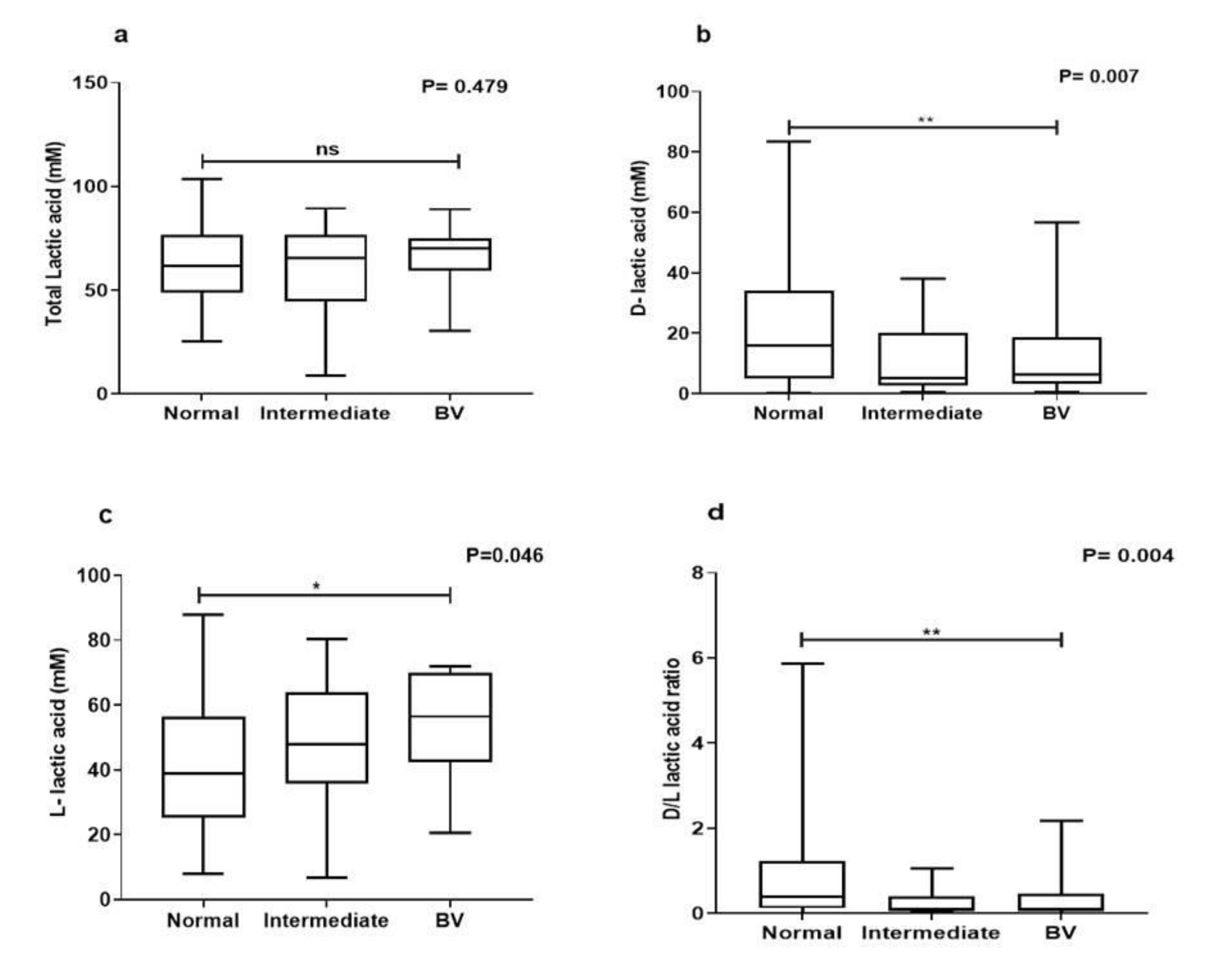
3.3. Lactic Acid Quantification
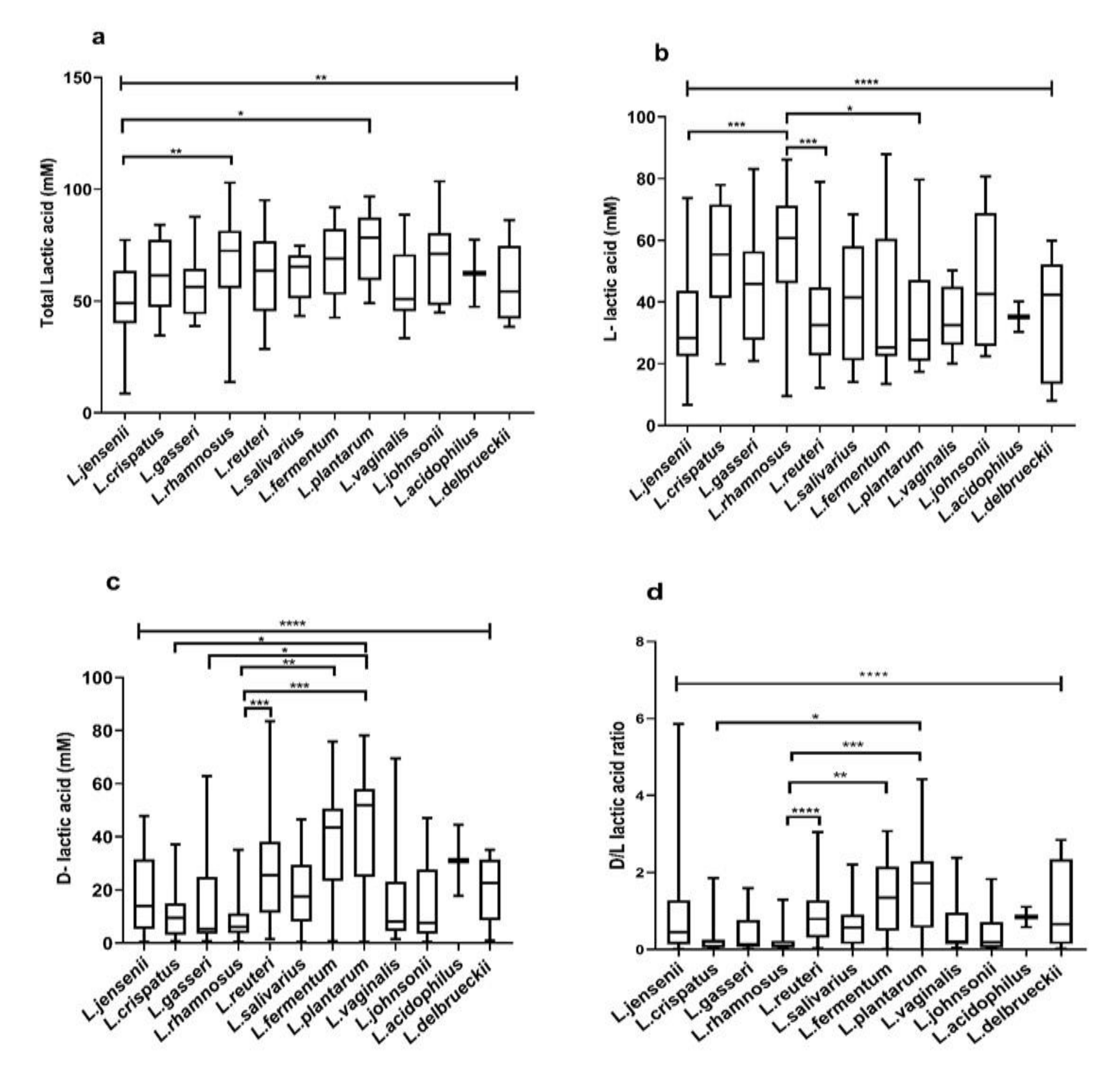
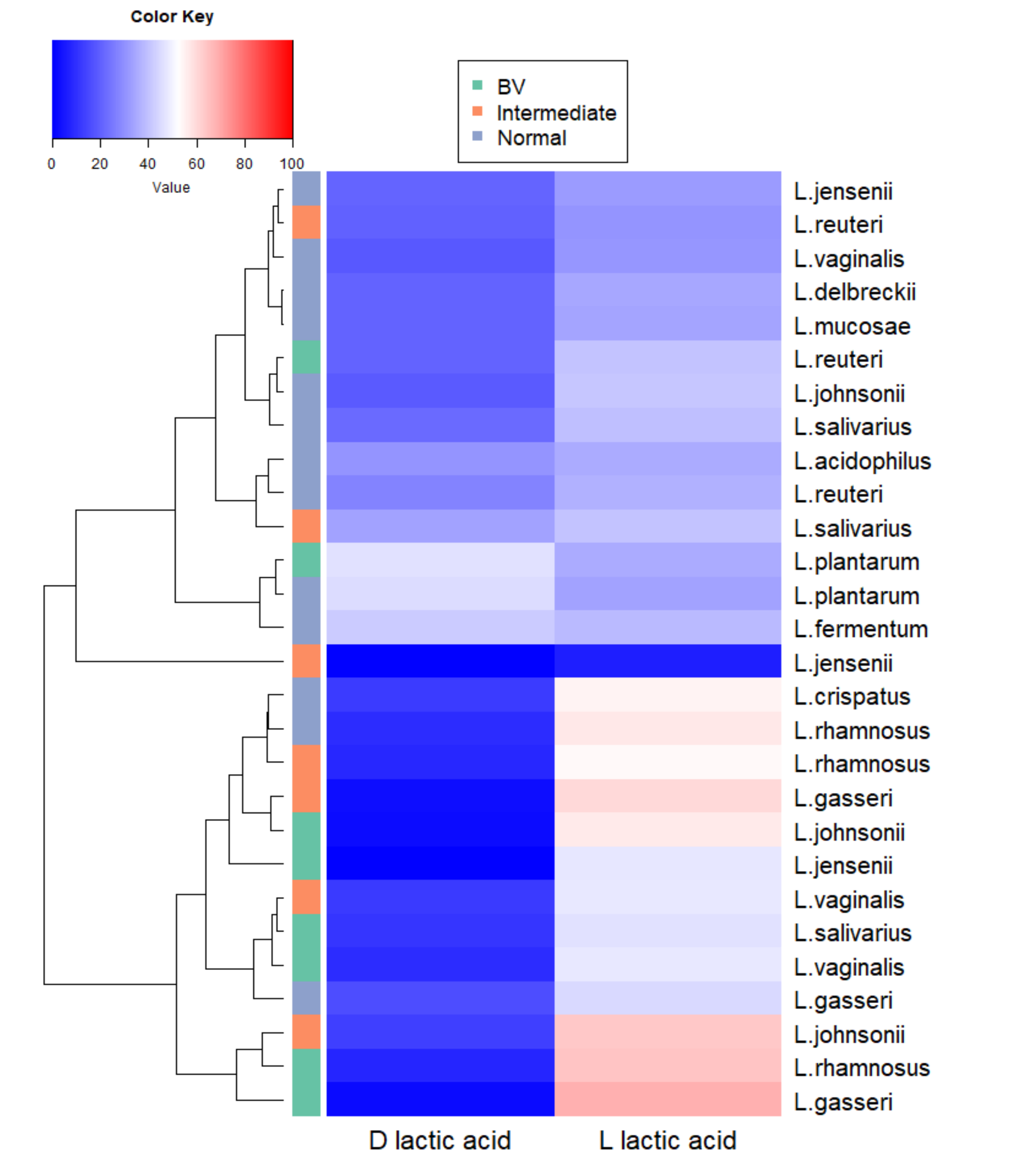
3.4. Species-Specific Lactic Acid Production
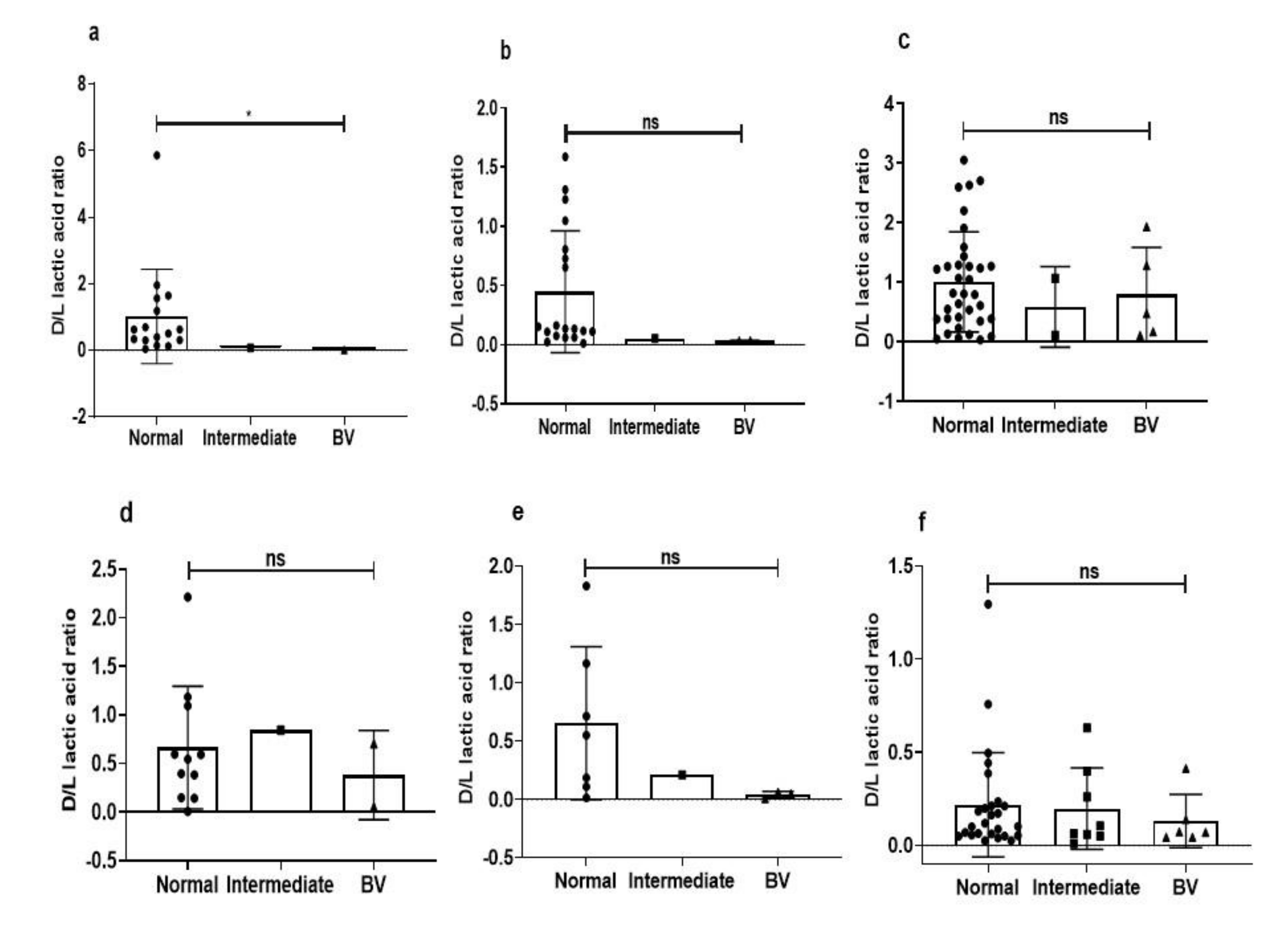
3.5. Intraspecies Comparison of Lactic Acid Production from Different Microbiota
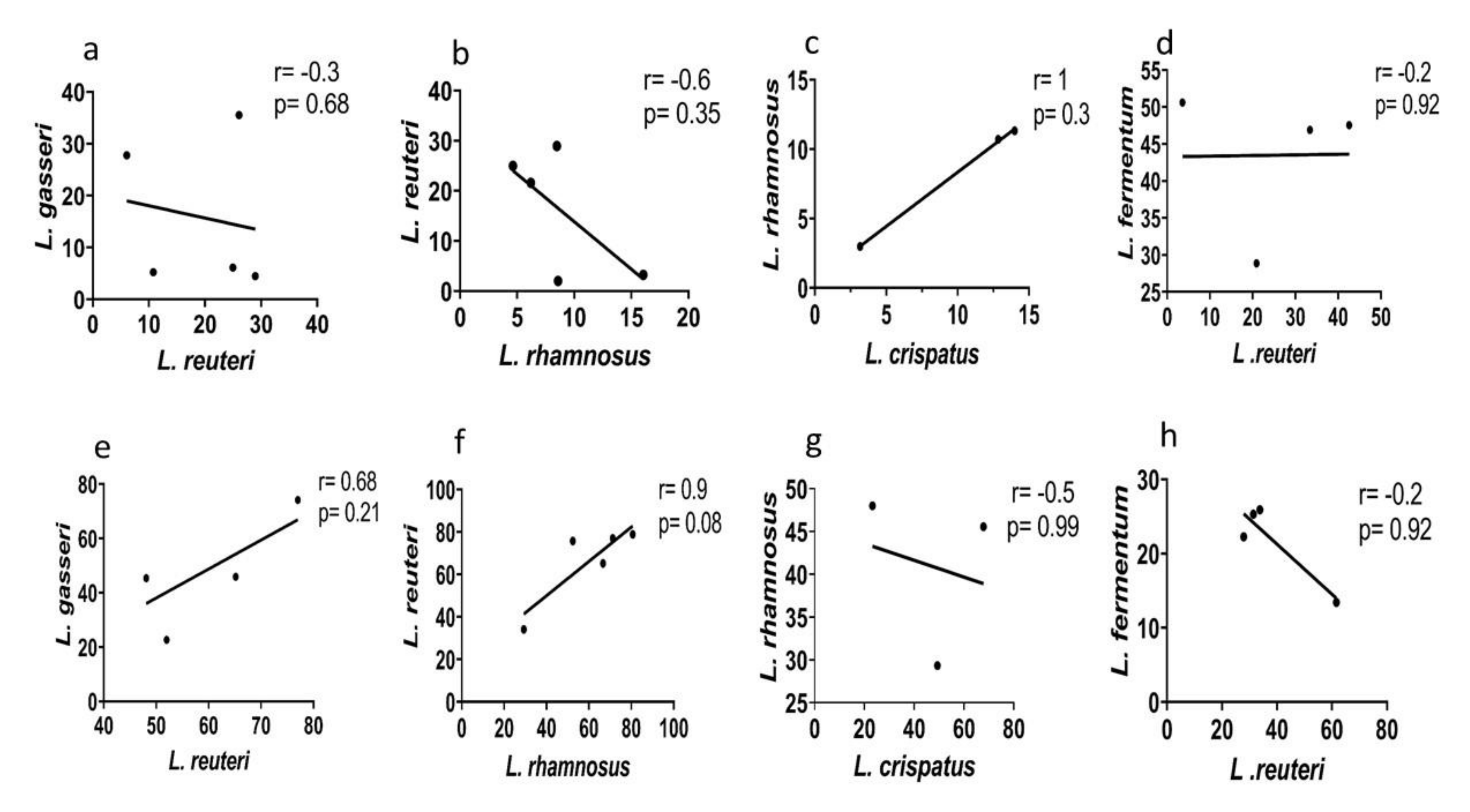
3.6. Species-Specific Correlation of Lactic Acid
3.7. Hydrogen Peroxide Evaluation
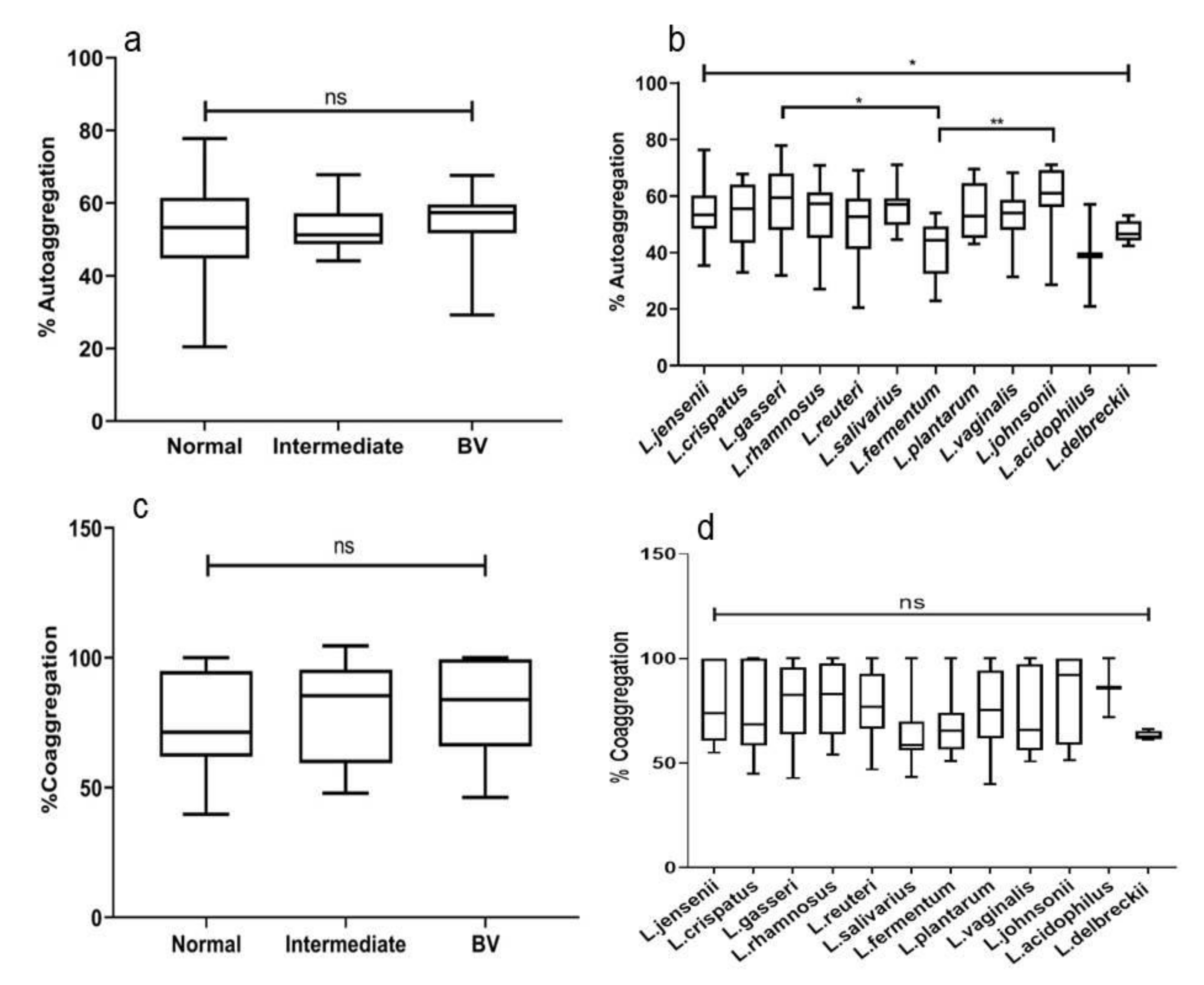
3.8. Autoaggregation of Lactobacilli
3.9. Coaggregation of Lactobacillus with C. albicans
3.10. Antagonistic Effect on Pathogens
3.11. Association of Probiotic Properties from Different Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiesenfeld, H.C.; Hillier, S.L.; Krohn, M.A.; Landers, D.V.; Sweet, R.L. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeaee and Chlamydia trachomatis infection. Clin. Infect. Dis. 2003, 36, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R. Abnormal vaginal flora as a biological risk factor for acquisitionof HIV infection and sexually transmitted diseases. J. Infect. Dis. 2005, 192, 1315–1317. [Google Scholar] [CrossRef]
- Brotman, R.M. Vaginal microbiome and sexually transmitted infections: An epidemiologic perspective. J. Clin. Investig. 2011, 121, 4610–4617. [Google Scholar] [CrossRef]
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The Prevalence of Bacterial Vaginosis in the United States, 2001–2004; Associations with Symptoms, Sexual Behaviors, and Reproductive Health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Joyisa, N.; Moodley, D.; Nkosi, T.; Talakgale, R.; Sebitloane, M.; Naidoo, M.; Karim, Q.A. Asymptomatic Bacterial Vaginosis in Pregnancy and Missed Opportunities for Treatment: A Cross-Sectional Observational Study. Infect. Dis. Obstet. Gynecol. 2019, 2019, 7808179. [Google Scholar] [CrossRef] [PubMed]
- Pramanick, R.; Mayadeo, N.; Warke, H.; Begum, S.; Aich, P.; Aranha, C. Vaginal microbiota of asymptomatic bacterial vaginosis and vulvovaginal candidiasis: Are they different from normal microbiota? Microb. Pathog. 2019, 134, 103599. [Google Scholar] [CrossRef]
- Gibbs, R.S. Asymptomatic bacterial vaginosis: Is it time to treat? Am. J. Obstet. Gynecol. 2007, 196, 495–496. [Google Scholar] [CrossRef]
- Leitich, H.; Kiss, H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 375–390. [Google Scholar] [CrossRef]
- Holzer, I.; Farr, A.; Kiss, H.; Hagmann, M.; Petricevic, L. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch. Gynecol. Obstet. 2017, 295, 891–895. [Google Scholar] [CrossRef]
- Klein, L.L.; Gibbs, R.S. Use of microbial cultures and antibiotics in the prevention of infection-associated preterm birth. Am. J. Obstet. Gynecol. 2004, 190, 1493–1502. [Google Scholar] [CrossRef]
- Kero, K.; Rautava, J.; Syrjänen, S.; Grenman, S. Association of asymptomatic bacterial vaginosis with persistence of female genital human papillomavirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2215–2219. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef] [PubMed]
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.M.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of Vaginal Bacteria and d- and l-Lactic Acid Isomers on Vaginal Extracellular Matrix Metalloproteinase Inducer: Implications for Protection against Upper Genital Tract Infections. mBio 2013, 4, 00460-13. [Google Scholar] [CrossRef] [PubMed]
- Eschenbach, D.A.; Davick, P.R.; Williams, B.L.; Klebanoff, S.J.; Young-Smith, K.; Critchlow, C.M.; Holmes, K.K. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 1989, 27, 251–256. [Google Scholar] [CrossRef]
- Kovachev, S. Defence factors of vaginal lactobacilli. Crit. Rev. Microbiol. 2018, 44, 31–39. [Google Scholar] [CrossRef]
- Wilks, M.; Wiggins, R.; Whiley, A.; Hennessy, E.; Warwick, S.; Porter, H.; Corfield, A.; Millar, M. Identification and H2O2 Production of Vaginal Lactobacilli from Pregnant Women at High Risk of Preterm Birth and Relation with Outcome. J. Clin. Microbiol. 2004, 42, 713–717. [Google Scholar] [CrossRef]
- Boris, S.; Suárez, J.E.; Vázquez, F.; Barbés, C. Adherence of Human Vaginal Lactobacilli to Vaginal Epithelial Cells and Interaction with Uropathogens. Infect. Immun. 1998, 66, 1985–1989. [Google Scholar] [CrossRef]
- Younes, J.A.; Van Der Mei, H.C.; Heuvel, E.V.D.; Busscher, H.J.; Reid, G. Adhesion Forces and Coaggregation between Vaginal Staphylococci and Lactobacilli. PLoS ONE 2012, 7, e36917. [Google Scholar] [CrossRef]
- Zeng, Z.; Zuo, F.; Marcotte, H. Putative Adhesion Factors in Vaginal Lactobacillus gasseri DSM 14869: Functional Characterization. Appl. Environ. Microbiol. 2019, 85, 00800-19. [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef]
- Pendharkar, S.; Magopane, T.; Larsson, P.G.; De Bruyn, G.; Gray, G.E.; Hammarström, L.; Marcotte, H. Identification and characterisation of vaginal lactobacilli from South African women. BMC Infect. Dis. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; McGroarty, J.A.; Gil Domingue, P.A.; Chow, A.W.; Bruce, A.W.; Eisen, A.; Costerton, J.W. Coaggregation of urogenital bacteria in vitro and in vivo. Curr. Microbiol. 1990, 20, 47–52. [Google Scholar] [CrossRef]
- Todorov, S.; Botes, M.; Guigas, C.; Schillinger, U.; Wiid, I.; Wachsman, M.; Holzapfel, W.; Dicks, L. Boza, a natural source of probiotic lactic acid bacteria. J. Appl. Microbiol. 2007, 104, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R. Asymptomatic bacterial vaginosis: Response to therapy. Am. J. Obstet. Gynecol. 2000, 183, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.S.; Gaydos, C.A. Molecular Diagnosis of Bacterial Vaginosis: An Update. J. Clin. Microbiol. 2018, 56, 00342-18. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, D.E.; Gajer, P.; Brotman, R.M.; Ravel, J. Asymptomatic Bacterial Vaginosis Is Associated With Depletion of Mature Superficial Cells Shed From the Vaginal Epithelium. Front. Cell. Infect. Microbiol. 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; O’Hanlon, D.E.; Ravel, J. The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome 2018, 6, 29. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Boskey, E.; Cone, R.; Whaley, K.; Moench, T. Origins of vaginal acidity: High d/l lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef]
- Hoang, T.; Toler, E.; Delong, K.; Mafunda, N.A.; Bloom, S.M.; Zierden, H.C.; Moench, T.R.; Coleman, J.S.; Hanes, J.; Kwon, D.; et al. The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis. PLoS Pathog. 2020, 16, e1008236. [Google Scholar] [CrossRef]
- Manhanzva, M.T.; Abrahams, A.G.; Gamieldien, H.; Froissart, R.; Jaspan, H.; Jaumdally, S.Z.; Barnabas, S.L.; Dabee, S.; Bekker, L.G.; Gray, G.; et al. Inflammatory and antimicrobial properties differ between vaginal Lactobacillus isolates from South African women with non-optimal versus optimal microbiota. Sci. Rep. 2020, 10, 6196. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Fredricks, D.; Agnew, K.; Hitti, J. Hydrogen Peroxide–Producing Lactobacilli Are Associated With Lower Levels of Vaginal Interleukin-1β, Independent of Bacterial Vaginosis. Sex. Transm. Dis. 2015, 42, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Cherpes, T.L.; Hillier, S.L.; Meyn, L.A.; Busch, J.L.; Krohn, M.A. A Delicate Balance: Risk Factors for Acquisition of Bacterial Vaginosis Include Sexual Activity, Absence of Hydrogen Peroxide-Producing Lactobacilli, Black Race, and Positive Herpes Simplex Virus Type 2 Serology. Sex. Transm. Dis. 2008, 35, 78–83. [Google Scholar] [CrossRef]
- Pramanick, R.; Parab, S.; Mayadeo, N.; Warke, H.S.; Aranha, C. Cross sectional analysis of vaginal Lactobacillus in asymptomatic women of reproductive age in Mumbai, India. J. Infect. Dev. Ctries. 2018, 12, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzona, D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000, 31, 438–442. [Google Scholar] [CrossRef]
- De Souza, E.L.; De Albuquerque, T.M.R.; Dos Santos, A.S.; Massa, N.M.L.; Alves, J.L.D.B. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Gil, N.F.; Martinez, R.C.; Gomes, B.C.; Nomizo, A.; De Martinis, E.C.P. Vaginal lactobacilli as potential probiotics against Candida spp. Braz. J. Microbiol. 2010, 41, 6–14. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef]
- Martin, L.J.E. Candidiasis (vulvovaginal). BMJ Clin. Evid. 2015, 0815, 1–23. [Google Scholar]
- Di Cerbo, A.; Palmieri, B.; Aponte, M.; Morales-Medina, J.C.; Iannitti, T. Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 2016, 69, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Ealdunate, M.; Esrbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Egugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of vaginal lactobacilli as probiotic candidates. Sci. Rep. 2019, 9, 3355. [Google Scholar] [CrossRef] [PubMed]
- Graver, M.A.; Wade, J.J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Valore, E.V.; Park, C.H.; Igreti, S.L.; Ganz, T. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 2002, 187, 561–568. [Google Scholar] [CrossRef]
- Allonsius, C.N.; Broek, M.F.L.V.D.; De Boeck, I.; Kiekens, S.; Oerlemans, E.F.M.; Kiekens, F.; Foubert, K.; Vandenheuvel, D.; Cos, P.; Delputte, P.; et al. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb. Biotechnol. 2017, 10, 1753–1763. [Google Scholar] [CrossRef]

| Lactobacillus Species | H2O2 Production | Normal | Dysbiosis | p Value |
|---|---|---|---|---|
| L. crispatus (n = 14) | H2O2 producer | 10 | 0 | _ |
| H2O2 non producer | 4 | 0 | ||
| L. gasseri (n = 22) | H2O2 producer | 15 | 3 | 0.99 |
| H2O2 non producer | 4 | 0 | ||
| L. jensenii (n = 18) | H2O2 producer | 15 | 1 | 0.22 |
| H2O2 non producer | 1 | 1 | ||
| L. johnsonii (n = 11) | H2O2 producer | 4 | 3 | 0.99 |
| H2O2 non producer | 3 | 1 | ||
| L. rhamnosus (n = 40) | H2O2 producer | 23 | 11 | 0.64 |
| H2O2 non producer | 3 | 3 | ||
| L. salivarius (n = 14) | H2O2 producer | 9 | 3 | 0.99 |
| H2O2 non producer | 2 | 0 | ||
| L. reuteri (n = 42) | H2O2 producer | 26 | 5 | 0.99 |
| H2O2 non producer | 9 | 2 | ||
| L. plantarum (n = 13) | H2O2 producer | 6 | 1 | 0.99 |
| H2O2 non producer | 5 | 1 | ||
| L. fermentum (n = 11) | H2O2 producer | 7 | 0 | _ |
| H2O2 non producer | 4 | 0 | ||
| L. vaginalis (n = 13) | H2O2 producer | 9 | 2 | 0.42 |
| H2O2 non producer | 1 | 1 |
| Lactobacillus Species | Number of H2O2 Non Producers | Number of H2O2 Producers | |||
|---|---|---|---|---|---|
| Weak | Medium | Strong | Total | ||
| L. acidophilus (n = 2) | 1(50) | 0 (0) | 1 (50) | 0 (0) | 1 (100) |
| L. delbrueckii (n = 5) | 2 (40.0) | 0 (0) | 1 (20.0) | 2 (40.0) | 3 (60.0) |
| L. crispatus (n = 14) | 4 (28.57) | 0 (0) | 5 (35.71) | 5 (35.71) | 10 (71.43) |
| L. gasseri (n = 22) | 4 (18.18) | 4 (18.18) | 5 (22.72) | 9 (40.91) | 18(81.82) |
| L. jensenii (n = 18) | 2 (11.11) | 1 (5.56) | 3 (16.67) | 12 (66.67) | 16 (88.89 |
| L. johnsonii (n = 11) | 4 (36.36) | 2 (18.18) | 2 (18.18) | 3 (27.27) | 7 (63.63) |
| L. rhamnosus (n = 40) | 6 (15.0) | 5 (12.5) | 6 (15) | 23 (57.5) | 34 (85.0) |
| L. salivarius (n = 14) | 2 (14.29) | 2 (14.29) | 4 (28.57) | 6 (48.86) | 12 (85.71) |
| L. reuteri (n = 42) | 11 (26.19) | 7 (16.67) | 6 (14.29) | 18 (42.86) | 31 (73.81) |
| L. plantarum (n = 13) | 6 (46.15) | 2 (15.38) | 0 (0) | 5 (38.46) | 7 (53.85) |
| L. fermentum (n = 11) | 4 (36.36) | 1 (9.09) | 1 (9.09) | 5 (45.45) | 7 (63.63) |
| L. vaginalis (n = 13) | 2 (15.38) | 2 (15.38) | 3 (23.08) | 6 (46.15) | 11 (84.62) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pramanick, R.; Aranha, C. Distinct Functional Traits of Lactobacilli from Women with Asymptomatic Bacterial Vaginosis and Normal Microbiota. Microorganisms 2020, 8, 1949. https://doi.org/10.3390/microorganisms8121949
Pramanick R, Aranha C. Distinct Functional Traits of Lactobacilli from Women with Asymptomatic Bacterial Vaginosis and Normal Microbiota. Microorganisms. 2020; 8(12):1949. https://doi.org/10.3390/microorganisms8121949
Chicago/Turabian StylePramanick, Rinku, and Clara Aranha. 2020. "Distinct Functional Traits of Lactobacilli from Women with Asymptomatic Bacterial Vaginosis and Normal Microbiota" Microorganisms 8, no. 12: 1949. https://doi.org/10.3390/microorganisms8121949
APA StylePramanick, R., & Aranha, C. (2020). Distinct Functional Traits of Lactobacilli from Women with Asymptomatic Bacterial Vaginosis and Normal Microbiota. Microorganisms, 8(12), 1949. https://doi.org/10.3390/microorganisms8121949





