Microfluidic Microbial Bioelectrochemical Systems: An Integrated Investigation Platform for a More Fundamental Understanding of Electroactive Bacterial Biofilms
Abstract
1. Introduction
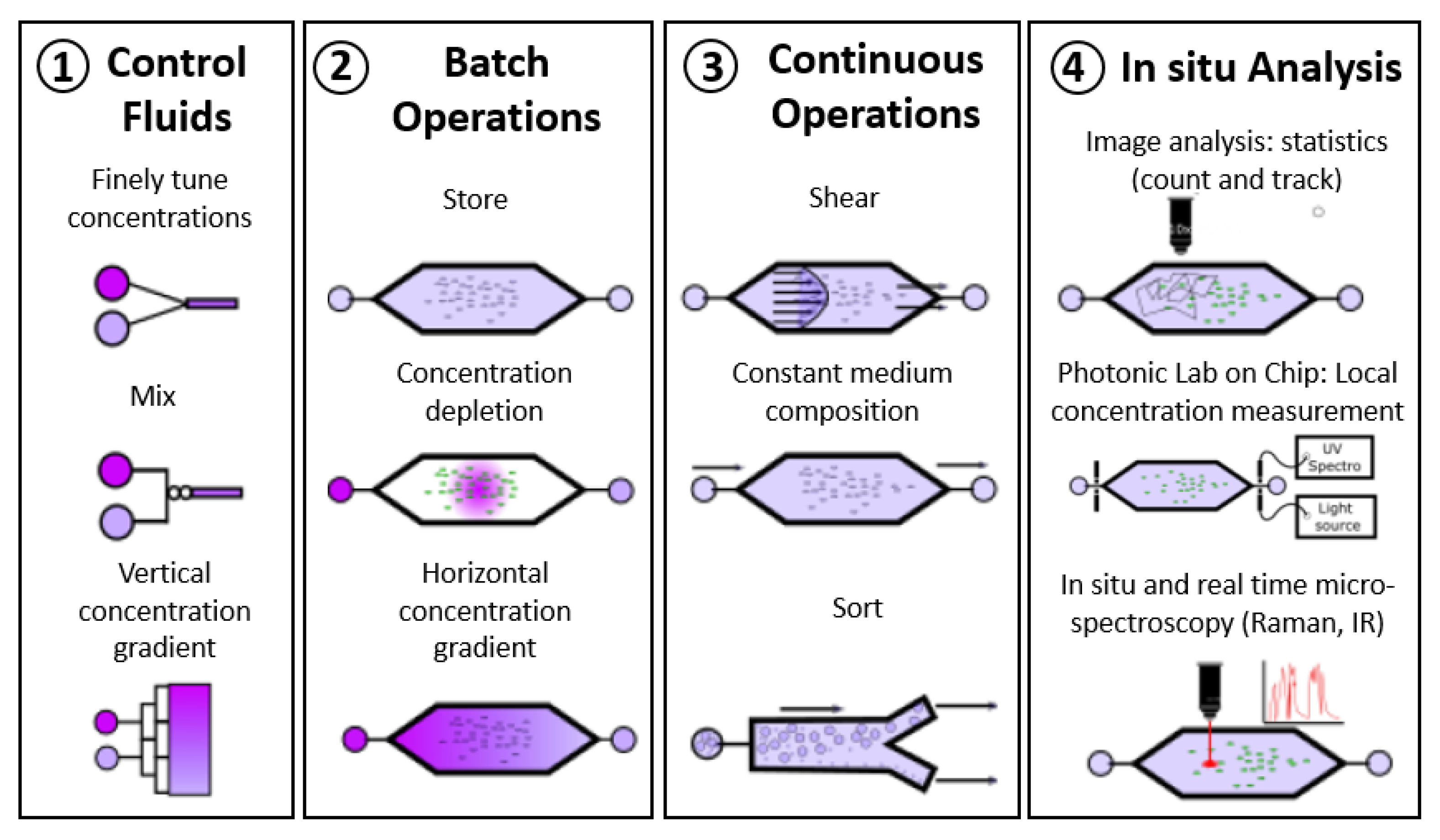
2. Microfluidic Microbial BES
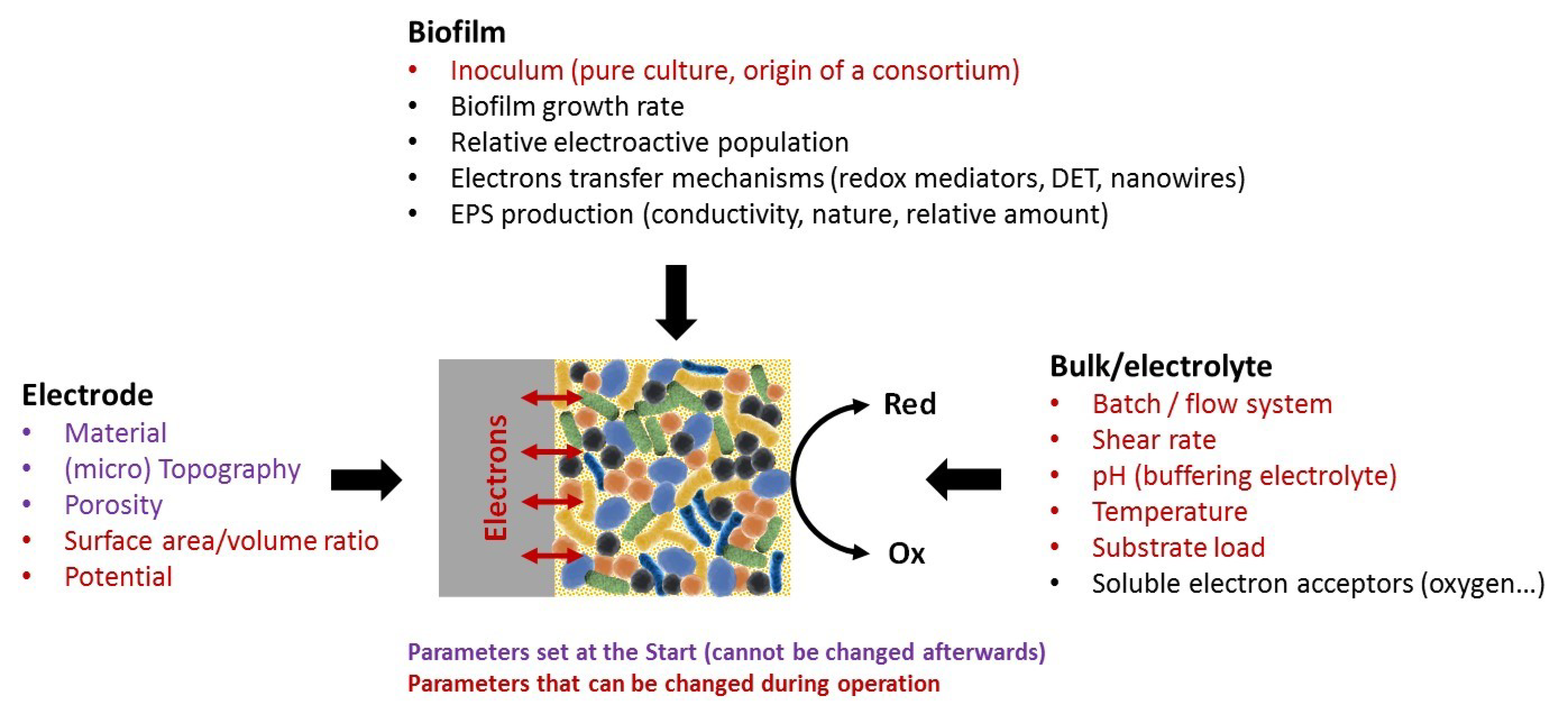
2.1. Scaling Down the BES
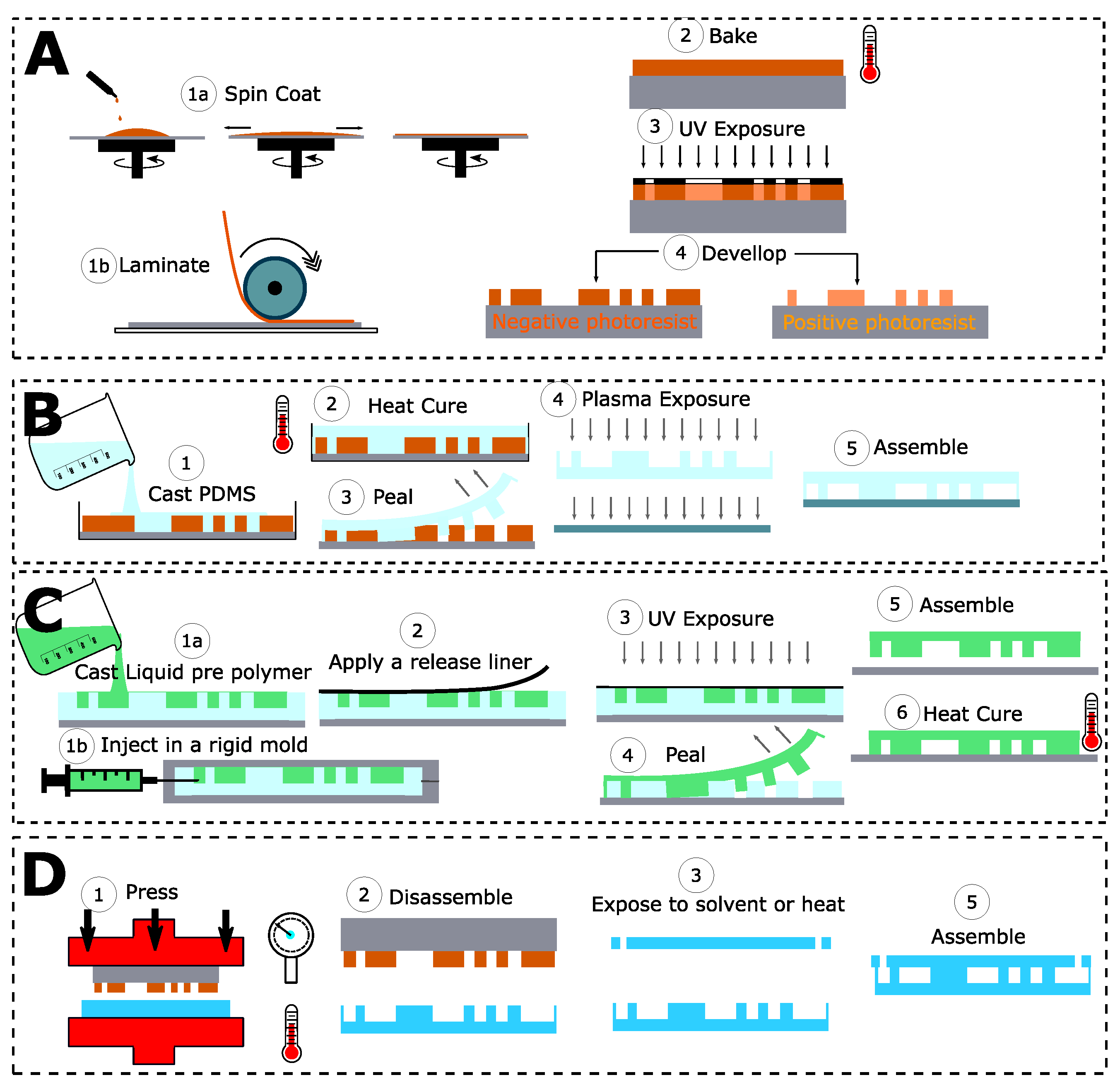
2.2. A Wide Range of Designs and Materials
2.3. The Different Types of BES
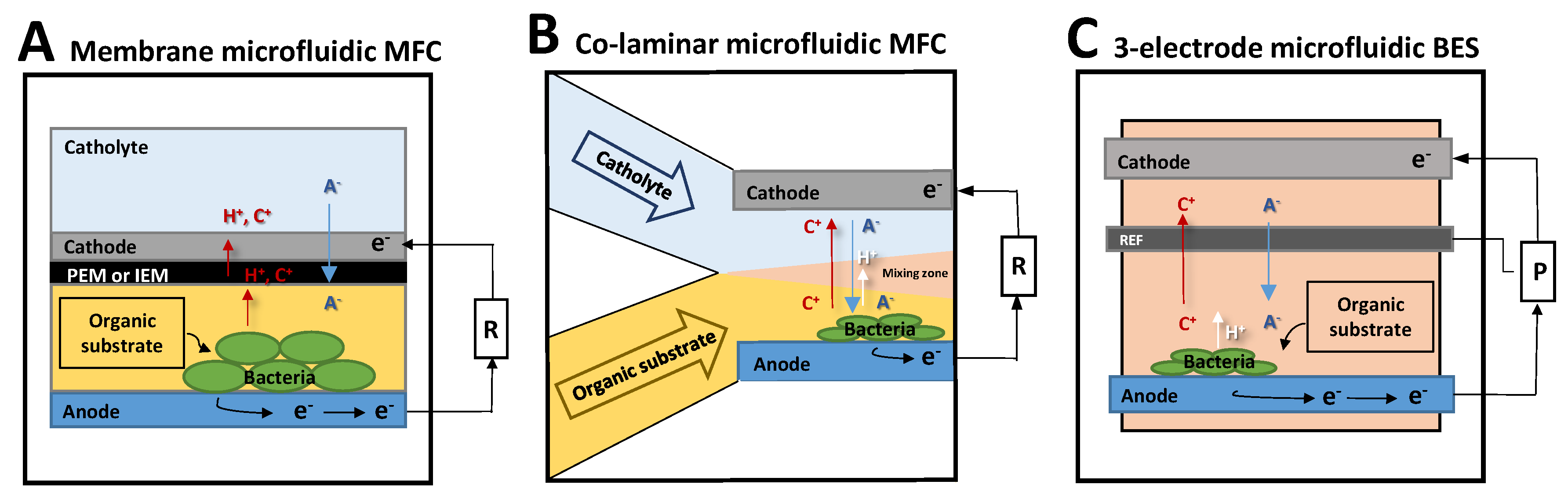
2.3.1. Scaled Down MFC: Membrane Microfluidic MFC
2.3.2. Membraneless Microfluidic MFC: The Co-Laminar Microfluidic MFC
2.3.3. Three-Electrode Microfluidic BES
3. Contribution of Microfluidic Investigations to the Fundamental Knowledge of Electroactive Biofilms
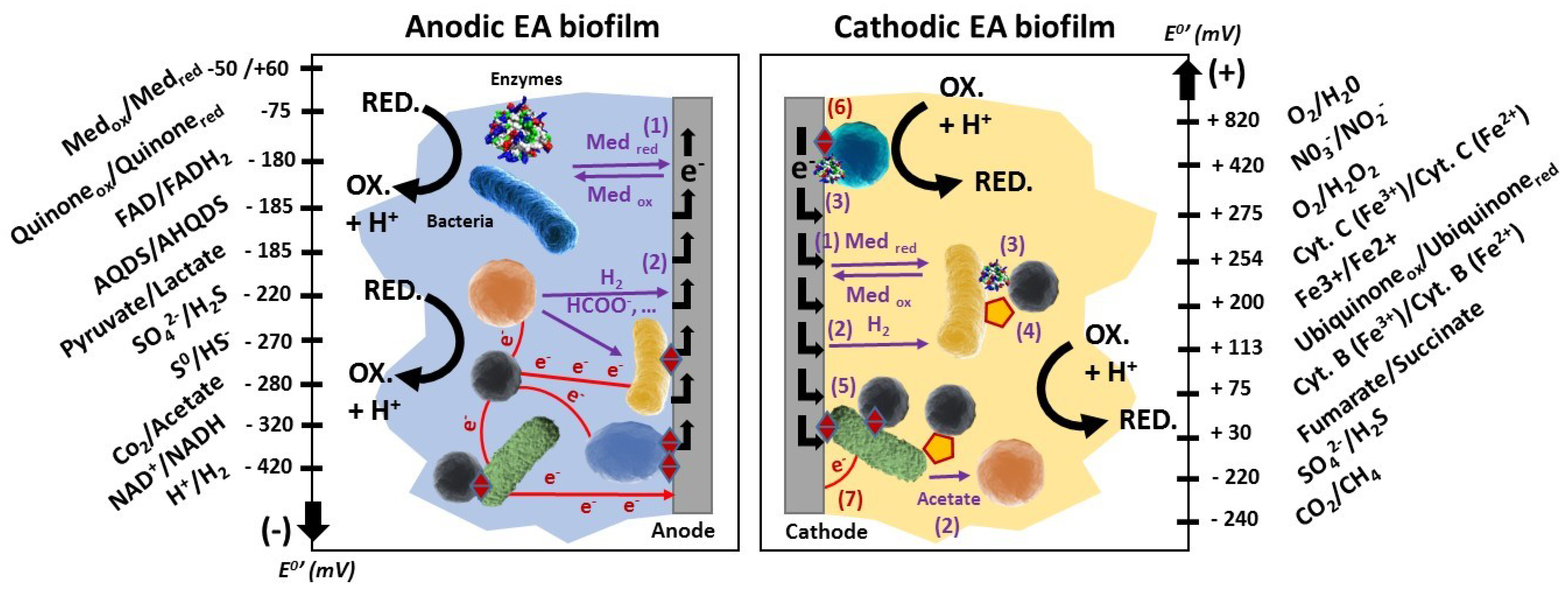
3.1. Contributions to Disentangle the EET Mechanisms
3.2. Contributions to Our Understanding of Electroactive Biofilm Formation
3.3. Contributions to Spatial Probing of the Electrochemical Activity of Biofilms
- -
- -
- -
- - A stratification of the coexisting direct mechanisms of EET: pili become the main electron discharge mechanism in the upper region far from the anode (>10 μm), where the c-type cytochromes are mainly in the reduced state [14].
3.4. Contributions Examining the Impact of Electrolyte Conditions
4. Outlook
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kato, S. Microbial extracellular electron transfer and its relevance to iron corrosion. Microb. Biotechnol. 2016, 9, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, J.K.; Zachara, J.M. Electron transfer at the microbe–mineral interface: A grand challenge in biogeochemistry. Geobiology 2008, 6, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Park, J.-D.; Ren, Z.J. Practical Energy Harvesting for Microbial Fuel Cells: A Review. Environ. Sci. Technol. 2015, 49, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Nevin, K.P. Electrobiocommodities: Powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr. Opin. Biotechnol. 2013, 24, 385–390. [Google Scholar] [CrossRef]
- Abrevaya, X.C.; Sacco, N.J.; Bonetto, M.C.; Hilding-Ohlsson, A.; Cortón, E. Analytical applications of microbial fuel cells. Part II: Toxicity, microbial activity and quantification, single analyte detection and other uses. Biosens. Bioelectron. 2015, 63, 591–601. [Google Scholar] [CrossRef]
- Pinck, S.; Jorand, F.; Etienne, M. Electrochemistry of Biofilms; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Aghababaie, M.; Farhadian, M.; Jeihanipour, A.; Biria, D. Effective factors on the performance of microbial fuel cells in wastewater treatment—A review. Environ. Technol. Rev. 2015, 4, 71–89. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.D.; Colprim, J.; Puig, S. On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874. [Google Scholar] [CrossRef]
- Lies, D.P.; Hernandez, M.E.; Kappler, A.; Mielke, R.E.; Gralnick, J.A.; Newman, D.K. Shewanella oneidensis MR-1 Uses Overlapping Pathways for Iron Reduction at a Distance and by Direct Contact under Conditions Relevant for Biofilms. Appl. Environ. Microbiol. 2005, 71, 4414–4426. [Google Scholar] [CrossRef]
- Basséguy, R.; Délia, M.-L.; Erable, B.; Bergel, A. Electroactive biofilms. Underst. Biocorrosion 2014, 2014, 107–143. [Google Scholar] [CrossRef]
- Barchinger, S.E.; Pirbadian, S.; Sambles, C.M.; Baker, C.S.; Leung, K.M.; Burroughs, N.J.; El-Naggar, M.Y.; Golbeck, J.H. Regulation of Gene Expression in Shewanella oneidensis MR-1 during Electron Acceptor Limitation and Bacterial Nanowire Formation. Appl. Environ. Microbiol. 2016, 82, 5428–5443. [Google Scholar] [CrossRef] [PubMed]
- Steidl, R.J.; Lampa-Pastirk, S.; Reguera, G. Mechanistic stratification in electroactive biofilms of Geobacter sulfurreducens mediated by pilus nanowires. Nat. Commun. 2016, 7, 12217. [Google Scholar] [CrossRef] [PubMed]
- Kotloski, N.J.; Gralnick, J.A. Flavin Electron Shuttles Dominate Extracellular Electron Transfer by Shewanella oneidensis. mBio 2013, 4, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Nakamura, R.; Nealson, K.H.; Hashimoto, K. Bound Flavin Model Suggests Similar Electron-Transfer Mechanisms inShewanellaandGeobacter. ChemElectroChem 2014, 1, 1808–1812. [Google Scholar] [CrossRef]
- Yang, G.; Huang, L.; You, L.; Zhuang, L.; Zhou, S. Electrochemical and spectroscopic insights into the mechanisms of bidirectional microbe-electrode electron transfer in Geobacter soli biofilms. Electrochem. Commun. 2017, 77, 93–97. [Google Scholar] [CrossRef]
- Shi, L.; Rosso, K.M.; Clarke, T.A.; Richardson, D.J.; Zachara, J.M.; Fredrickson, J.K. Molecular Underpinnings of Fe(III) Oxide Reduction by Shewanella Oneidensis MR-1. Front. Microbiol. 2012, 3, 50. [Google Scholar] [CrossRef]
- Richter, H.; Nevin, K.P.; Jia, H.; Lowy, D.A.; Lovley, D.R.; Tender, L.M. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ. Sci. 2009, 2, 506–516. [Google Scholar] [CrossRef]
- Rimboud, M.; Pocaznoi, D.; Erable, B.; Bergel, A. Electroanalysis of microbial anodes for bioelectrochemical systems: Basics, progress and perspectives. Phys. Chem. Chem. Phys. 2014, 16, 16349–16366. [Google Scholar] [CrossRef]
- Erable, B.; Féron, D.; Bergel, A. Microbial Catalysis of the Oxygen Reduction Reaction for Microbial Fuel Cells: A Review. ChemSusChem 2012, 5, 975–987. [Google Scholar] [CrossRef]
- Debuy, S.; Pécastaings, S.; Bergel, A.; Erable, B. Oxygen-reducing biocathodes designed with pure cultures of microbial strains isolated from seawater biofilms. Int. Biodeterior. Biodegrad. 2015, 103, 16–22. [Google Scholar] [CrossRef]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Schaap, J.D.; Kampman, C.; Buisman, C.J.N. Bioelectrochemical Ethanol Production through Mediated Acetate Reduction by Mixed Cultures. Environ. Sci. Technol. 2010, 44, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Hydrogen Production with a Microbial Biocathode. Environ. Sci. Technol. 2008, 42, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Soussan, L.; Riess, J.; Erable, B.; Delia, M.-L.; Bergel, A. Electrochemical reduction of CO2 catalysed by Geobacter sulfurreducens grown on polarized stainless steel cathodes. Electrochem. Commun. 2013, 28, 27–30. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Genet. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry 2010, 78, 39–43. [Google Scholar] [CrossRef]
- Luo, H.; Teng, W.; Liu, G.; Zhang, R.; Lu, Y. Sulfate reduction and microbial community of autotrophic biocathode in response to acidity. Process. Biochem. 2017, 54, 120–127. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Baawain, M.S.; Egger, F.; Al-Muhtaseb, A.H.; Ng, H.Y. Optimization of a baffled-reactor microbial fuel cell using autotrophic denitrifying bio-cathode for removing nitrogen and recovering electrical energy. Biochem. Eng. J. 2017, 120, 93–102. [Google Scholar] [CrossRef]
- Pocaznoi, D.; Erable, B.; Delia, M.-L.; Bergel, A. Ultra microelectrodes increase the current density provided by electroactive biofilms by improving their electron transport ability. Energy Environ. Sci. 2012, 5, 5287–5296. [Google Scholar] [CrossRef]
- Champigneux, P.; Renault-Sentenac, C.; Bourrier, D.; Rossi, C.; Delia, M.-L.; Bergel, A. Effect of surface roughness, porosity and roughened micro-pillar structures on the early formation of microbial anodes. Bioelectrochemistry 2019, 128, 17–29. [Google Scholar] [CrossRef]
- Li, Z.J.; Venkataraman, A.; Rosenbaum, M.A.; Angenent, L.T. A Laminar-Flow Microfluidic Device for Quantitative Analysis of Microbial Electrochemical Activity. ChemSusChem 2012, 5, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, D.; Liao, Q.; Zhang, P.; Zhu, X.; Li, J.; Fu, Q. Enhanced biofilm distribution and cell performance of microfluidic microbial fuel cells with multiple anolyte inlets. Biosens. Bioelectron. 2016, 79, 406–410. [Google Scholar] [CrossRef]
- Pousti, M.; Zarabadi, M.P.; Amirdehi, M.A.; Paquet-Mercier, F.; Greener, J. Microfluidic bioanalytical flow cells for biofilm studies: A review. Analyst 2019, 144, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Rochex, A.; Godon, J.-J.; Bernet, N.; Escudié, R. Role of shear stress on composition, diversity and dynamics of biofilm bacterial communities. Water Res. 2008, 42, 4915–4922. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, L.K.; Hermanowicz, S.W. Detachment of biofilm bacteria due to variations in nutrient supply. Water Sci. Technol. 1998, 37, 211–214. [Google Scholar] [CrossRef]
- Dickschat, J.S. Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 2010, 27, 343–369. [Google Scholar] [CrossRef]
- Richter, H.; McCarthy, K.; Nevin, K.P.; Johnson, J.P.; Rotello, V.M.; Lovley, D.R. Electricity Generation byGeobacter sulfurreducensAttached to Gold Electrodes. Langmuir 2008, 24, 4376–4379. [Google Scholar] [CrossRef] [PubMed]
- Ringeisen, B.R.; Henderson, E.; Wu, P.K.; Pietron, J.; Ray, R.; Little, B.; Biffinger, J.C.; Jones-Meehan, J.M. Erratum: High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ. Sci. Technol. 2006, 40, 2629–2634. [Google Scholar] [CrossRef]
- Biffinger, J.C.; Pietron, J.; Ray, R.; Little, B.; Ringeisen, B.R. A biofilm enhanced miniature microbial fuel cell using Shewanella oneidensis DSP10 and oxygen reduction cathodes. Biosens. Bioelectron. 2007, 22, 1672–1679. [Google Scholar] [CrossRef]
- Qian, F.; Baum, M.; Gu, Q.; Morse, D.E. A 1.5 µL microbial fuel cell for on-chip bioelectricity generation. Lab Chip 2009, 9, 3076–3081. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Zhao, Y.; Qiu, K.-Q.; Chu, J.; Lu, R.; Sun, M.; Liu, X.-W.; Sheng, G.-P.; Yu, H.-Q.; Chen, J.; et al. An innovative miniature microbial fuel cell fabricated using photolithography. Biosens. Bioelectron. 2011, 26, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; Mascia, M.; Fernandez-Morales, F.J.; Rodrigo, M.A.; Di Lorenzo, M. Assessing the impact of design factors on the performance of two miniature microbial fuel cells. Electrochim. Acta 2019, 297, 297–306. [Google Scholar] [CrossRef]
- Chouler, J.; Di Lorenzo, M. Pesticide detection by a miniature microbial fuel cell under controlled operational disturbances. Water Sci. Technol. 2019, 79, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xie, W.; Wang, R.; Wu, X.; Yu, L.; Qiao, Y. Fast Start-Up Microfluidic Microbial Fuel Cells with Serpentine Microchannel. Front. Microbiol. 2018, 9, 2816. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Kiran, P.S.; Rewatkar, P.; Krishnamurthy, B.; Ganesh, P.S.; Goel, S. Escherichia Coli Fed Paper-Based Microfluidic Microbial Fuel Cell With MWCNT Composed Bucky Paper Bioelectrodes. IEEE Trans. NanoBiosci. 2019, 18, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Mardanpour, M.M.; Yaghmaei, S. Characterization of a microfluidic microbial fuel cell as a power generator based on a nickel electrode. Biosens. Bioelectron. 2016, 79, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhang, P.; Zhu, X.; Yang, Y.; Li, J.; Fu, Q.; Chen, R.; Liao, Q.; Zhang, B. Electricity generation of a laminar-flow microbial fuel cell without any additional power supply. RSC Adv. 2018, 8, 33637–33641. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; LeDuc, P.R.; Gregory, K.B. Microbial electricity generation via microfluidic flow control. Biotechnol. Bioeng. 2011, 108, 2061–2069. [Google Scholar] [CrossRef]
- Li, F.; Zheng, Z.; Yang, B.; Zhang, X.; Li, Z.; Lei, L. A laminar-flow based microfluidic microbial three-electrode cell for biosensing. Electrochim. Acta 2016, 199, 45–50. [Google Scholar] [CrossRef]
- Zarabadi, M.P.; Charette, S.J.; Greener, J. Flow-Based Deacidification of Geobacter sulfurreducens Biofilms Depends on Nutrient Conditions: A Microfluidic Bioelectrochemical Study. ChemElectroChem 2018, 5, 3645–3653. [Google Scholar] [CrossRef]
- Bruchmann, J.; Sachsenheimer, K.; Rapp, B.E.; Schwartz, T. Multi-Channel Microfluidic Biosensor Platform Applied for Online Monitoring and Screening of Biofilm Formation and Activity. PLoS ONE 2015, 10, e0117300. [Google Scholar] [CrossRef] [PubMed]
- Zarabadi, M.P.; Paquet-Mercier, F.; Charette, S.J.; Greener, J. Hydrodynamic Effects on Biofilms at the Biointerface Using a Microfluidic Electrochemical Cell: Case Study of Pseudomonas sp. Langmuir 2017, 33, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Su, J.Y.; Huang, C.Y.; Wang, H.Y. Microfluidic microbial fuel cells for rapid screening of electroactive microorganisms. In Proceedings of the 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2012, Okinawa, Japan, 28 October–1 November 2012; pp. 1435–1437. [Google Scholar]
- Dang, T.C.; Yin, Y.; Yu, Y.; Phan, D.-T.; Yang, C.; Cao, B.; Song, H.; Kang, Y. A membrane-free micro-fluidic microbial fuel cell for rapid characterization of exoelectrogenic bacteria. Microfluid. Nanofluidics 2016, 20, 144. [Google Scholar] [CrossRef]
- Hou, H.; Li, L.; Cho, Y.; De Figueiredo, P.; Han, A. Microfabricated Microbial Fuel Cell Arrays Reveal Electrochemically Active Microbes. PLoS ONE 2009, 4, e6570. [Google Scholar] [CrossRef] [PubMed]
- Dávila, D.; Esquivel, J.P.; Sabaté, N.; Mas, J. Silicon-based microfabricated microbial fuel cell toxicity sensor. Biosens. Bioelectron. 2011, 26, 2426–2430. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Thomson, A.R.; Schneider, K.; Cameron, P.J.; Ieropoulosc, I. A small-scale air-cathode microbial fuel cell for on-line monitoring of water quality. Biosens. Bioelectron. 2014, 62, 182–188. [Google Scholar] [CrossRef]
- Yang, W.; Wei, X.; Fraiwan, A.; Coogan, C.G.; Lee, H.; Choi, S. Fast and sensitive water quality assessment: A μL-scale microbial fuel cell-based biosensor integrated with an air-bubble trap and electrochemical sensing functionality. Sens. Actuators B Chem. 2016, 226, 191–195. [Google Scholar] [CrossRef]
- Michelson, K.; Sanford, R.A.; Valocchi, A.J.; Werth, C.J. Nanowires ofGeobacter sulfurreducensRequire Redox Cofactors to Reduce Metals in Pore Spaces Too Small for Cell Passage. Environ. Sci. Technol. 2017, 51, 11660–11668. [Google Scholar] [CrossRef]
- Kim, B.J.; Chu, I.; Jusuf, S.; Kuo, T.; TerAvest, M.A.; Angenent, L.T.; Wu, M. Oxygen Tension and Riboflavin Gradients Cooperatively Regulate the Migration of Shewanella oneidensis MR-1 Revealed by a Hydrogel-Based Microfluidic Device. Front. Microbiol. 2016, 7, 1438. [Google Scholar] [CrossRef]
- He, L.; Du, P.; Chen, Y.; Lu, H.W.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Bernarda, A.; Huang, C.-Y.; Lee, D.-J.; Chang, J.-S. Micro-sized microbial fuel cell: A mini-review. Bioresour. Technol. 2011, 102, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, D.; Li, J.; Zhu, X.-M.; Liao, Q.; Zhang, B. Microfluidic microbial fuel cells: From membrane to membrane free. J. Power Sources 2016, 324, 113–125. [Google Scholar] [CrossRef]
- Elmekawy, A.; Hegab, H.M.; Dominguez-Benetton, X.; Pant, D. Internal resistance of microfluidic microbial fuel cell: Challenges and potential opportunities. Bioresour. Technol. 2013, 142, 672–682. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Hindatu, Y.; Annuar, M.; Gumel, A.M. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Uday, U.S.P.; Bandyopadhyay, T.K.; Ray, R.N.; Bhunia, B. Performance improvement of microbial fuel cell (MFC) using suitable electrode and Bioengineered organisms: A review. Bioengineered 2017, 8, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Rusli, S.F.N.; Abu Bakar, M.H.; Loh, K.S.; Masdar, M.S. Review of high-performance biocathode using stainless steel and carbon-based materials in Microbial Fuel Cell for electricity and water treatment. Int. J. Hydrogen Energy 2019, 44, 30772–30787. [Google Scholar] [CrossRef]
- Ahmed, I.; Iqbal, H.M.N.; Akram, Z. Microfluidics Engineering: Recent Trends, Valorization, and Applications. Arab. J. Sci. Eng. 2018, 43, 23–32. [Google Scholar] [CrossRef]
- Qian, F.; He, Z.; Thelen, M.P.; Li, Y. A microfluidic microbial fuel cell fabricated by soft lithography. Bioresour. Technol. 2011, 102, 5836–5840. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Ahn, Y.; Schröder, U. Parylene C-coated PDMS-based microfluidic microbial fuel cells with low oxygen permeability. J. Power Sources 2018, 398, 209–214. [Google Scholar] [CrossRef]
- Mousavi, M.R.; Ghasemi, S.; Sanaee, Z.; Nejad, Z.G.; Mardanpour, M.M.; Yaghmaei, S.; Ghorbanzadeh, M. Improvement of the microfluidic microbial fuel cell using a nickel nanostructured electrode and microchannel modifications. J. Power Sources 2019, 437. [Google Scholar] [CrossRef]
- Ye, D.; Yang, Y.; Li, J.; Zhu, X.; Liao, Q.; Deng, B.; Chen, R. Performance of a microfluidic microbial fuel cell based on graphite electrodes. Int. J. Hydrogen Energy 2013, 38, 15710–15715. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, D.; Li, J.; Zhu, X.; Liao, Q.; Zhang, B. Biofilm distribution and performance of microfluidic microbial fuel cells with different microchannel geometries. Int. J. Hydrogen Energy 2015, 40, 11983–11988. [Google Scholar] [CrossRef]
- Abgrall, P.; Conedera, V.; Camon, H.; Gue, A.-M.; Nguyen, N.-T. SU-8 as a structural material for labs-on-chips and microelectromechanical systems. Electrophoresis 2007, 28, 4539–4551. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, I.; Llobera, A.; Vila-Planas, J.; Johnson, D.W.; Gómez-Morales, J.; Garcia-Ruiz, J.M. Analysis of the Structural Integrity of SU-8-Based Optofluidic Systems for Small-Molecule Crystallization Studies. Anal. Chem. 2013, 85, 9678–9685. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, I.; Teychené, S.; Van Pham, N.; Radajewski, D.; Lamadie, F.; Llobera, A.; Charton, S. Broadcasting photonic lab on a chip concept through a low cost manufacturing approach. Talanta 2017, 170, 180–184. [Google Scholar] [CrossRef]
- Mcdonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T. Review General Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Carlborg, C.F.; Moraga, F.; Saharil, F.; Van Der Wijngaart, W.; Haraldsson, T. Rapid permanent hydrophilic and hydrophobic patterning of polymer surfaces via off-stoichiometry thiol-ene (OSTE) photografting. In Proceedings of the 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2012, Okinawa, Japan, 28 October–1 November 2012; pp. 677–679. [Google Scholar]
- Ogończyk, D.; Węgrzyn, J.; Jankowski, P.; Dąbrowski, B.; Garstecki, P. Bonding of microfluidic devices fabricated in polycarbonate. Lab Chip 2010, 10, 1324–1327. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; De Groot, T.E.; Wan, A.M.D.; Beebe, D.J.; Young, E.W.K. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Bressan, L.P.; Adamo, C.B.; Quero, R.F.; de Jesus, D.P.; da Silva, J.A. A simple procedure to produce FDM-based 3D-printed microfluidic devices with an integrated PMMA optical window. Anal. Methods 2019, 11, 1014–1020. [Google Scholar] [CrossRef]
- Fraiwan, A.; Choi, S. A stackable, two-chambered, paper-based microbial fuel cell. Biosens. Bioelectron. 2016, 83, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Veerubhotla, R.; Bandopadhyay, A.; Das, D.; Chakraborty, S. Instant power generation from an air-breathing paper and pencil based bacterial bio-fuel cell. Lab Chip 2015, 15, 2580–2583. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, A.; Mukherjee, S.; Sundermier, S.; Lee, H.-S.; Choi, S. A paper-based microbial fuel cell: Instant battery for disposable diagnostic devices. Biosens. Bioelectron. 2013, 49, 410–414. [Google Scholar] [CrossRef]
- Lee, H.; Choi, S. An origami paper-based bacteria-powered battery. Nano Energy 2015, 15, 549–557. [Google Scholar] [CrossRef]
- Jeuken, L.J.C. Structure and Modification of Electrode Materials for Protein Electrochemistry. In Biophotoelectrochemistry: From Bioelectrochemistry to Biophotovoltaics; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Ren, H.; Pyo, S.; Lee, J.-I.; Park, T.-J.; Gittleson, F.S.; Leung, F.C.C.; Kim, J.; Taylor, A.D.; Lee, H.-S.; Chae, J. A high power density miniaturized microbial fuel cell having carbon nanotube anodes. J. Power Sources 2015, 273, 823–830. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Ling, Y.; Qian, F.; Song, Y.; Lu, X.; Chen, S.; Tong, Y.; Li, Y. High power density microbial fuel cell with flexible 3D graphene–nickel foam as anode. Nanoscale 2013, 5, 10283–10290. [Google Scholar] [CrossRef]
- Dominguez-Benetton, X.; Sevda, S.; Vanbroekhoven, K.; Pant, D. The accurate use of impedance analysis for the study of microbial electrochemical systems. Chem. Soc. Rev. 2012, 41, 7228–7246. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, T.; Tao, K.; Chang, H. Generating Electricity on Chips: Microfluidic Biofuel Cells in Perspective. Ind. Eng. Chem. Res. 2018, 57, 2746–2758. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.-S.; Yang, Y.; Parameswaran, P.; Torres, C.I.; Rittmann, B.E.; Chae, J. A μL-scale micromachined microbial fuel cell having high power density. Lab Chip 2011, 11, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Ismagilov, R.F.; Stroock, A.D.; Kenis, P.J.A.; Whitesides, G.M.; Stone, H.A. Experimental and theoretical scaling laws for transverse diffusive broadening in two-phase laminar flows in microchannels. Appl. Phys. Lett. 2000, 76, 2376–2378. [Google Scholar] [CrossRef]
- Tian, W.C.; Finehout, E. Microfluidics for Biological Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Jayashree, R.S.; Gancs, L.; Choban, E.R.; Primak, A.; Natarajan, D.; Markoski, L.J.; Kenis, P.J.A. Air-Breathing Laminar Flow-Based Microfluidic Fuel Cell. J. Am. Chem. Soc. 2005, 127, 16758–16759. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Brumley, D.R.; Stocker, R. Live from under the lens: Exploring microbial motility with dynamic imaging and microfluidics. Nat. Rev. Microbiol. 2015, 13, 761–775. [Google Scholar] [CrossRef]
- Vigolo, D.; Al-Housseiny, T.T.; Shen, Y.; Akinlawon, F.O.; Al-Housseiny, S.T.; Hobson, R.K.; Sahu, A.; Bedkowski, K.I.; DiChristina, T.J.; Stone, H.A. Flow dependent performance of microfluidic microbial fuel cells. Phys. Chem. Chem. Phys. 2014, 16, 12535–12543. [Google Scholar] [CrossRef]
- Södergren, S. Electrochemical Microsensor with In-Situ Fabricated Ag/AgCl Reference Electrode for High-Pressure Microfluidics. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, September 2017. [Google Scholar]
- Kim, J.; Elsnab, J.R.; Gehrke, C.; Li, J.; Gale, B.K. Microfluidic integrated multi-walled carbon nanotube (MWCNT) sensor for electrochemical nucleic acid concentration measurement. Sens. Actuators B Chem. 2013, 185, 370–376. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical Detection for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Haluk, B.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, J.-S.; Fitzgerald, L.A.; Biffinger, J.C.; Xie, P.; Ringeisen, B.R.; Lieber, C.M. Probing electron transfer mechanisms in Shewanella oneidensis MR-1 using a nanoelectrode platform and single-cell imaging. Proc. Natl. Acad. Sci. USA 2010, 107, 16806–16810. [Google Scholar] [CrossRef]
- Humphries, J.; Xiong, L.; Liu, J.; Prindle, A.; Yuan, F.; Arjes, H.A.; Tsimring, L.; Süel, G.M. Species-Independent Attraction to Biofilms through Electrical Signaling. Cell 2017, 168, 200–209.e12. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C. Staying in Touch while on the Go. Cell 2017, 168, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iii, A.-A.D.J.; Gralnick, J.A.; Lin, L.; Buie, C.R. Microfluidic dielectrophoresis illuminates the relationship between microbial cell envelope polarizability and electrochemical activity. Sci. Adv. 2019, 5, eaat5664. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Mu, Q.; Cheng, T.; Li, N.; Wang, X. Real-Time Imaging Revealed That Exoelectrogens from Wastewater Are Selected at the Center of a Gradient Electric Field. Environ. Sci. Technol. 2018, 52, 8939–8946. [Google Scholar] [CrossRef]
- Zhang, X.; Prévoteau, A.; Louro, R.O.; Paquete, C.M.; Rabaey, K. Periodic polarization of electroactive biofilms increases current density and charge carriers concentration while modifying biofilm structure. Biosens. Bioelectron. 2018, 121, 183–191. [Google Scholar] [CrossRef]
- Ter Heijne, A.; Pereira, M.A.; Pereira, J.; Sleutels, T. Electron Storage in Electroactive Biofilms. Trends Biotechnol. 2020. [Google Scholar] [CrossRef]
- Erable, B.; Bergel, A. First air-tolerant effective stainless steel microbial anode obtained from a natural marine biofilm. Bioresour. Technol. 2009, 100, 3302–3307. [Google Scholar] [CrossRef][Green Version]
- Erable, B.; Vandecandelaere, I.; Faimali, M.; Delia, M.-L.; Etcheverry, L.; Vandamme, P.; Bergel, A. Marine aerobic biofilm as biocathode catalyst. Bioelectrochemistry 2010, 78, 51–56. [Google Scholar] [CrossRef]
- Virdis, B.; Millo, D.; Donose, B.C.; Batstone, D.J. Real-Time Measurements of the Redox States of c-Type Cytochromes in Electroactive Biofilms: A Confocal Resonance Raman Microscopy Study. PLoS ONE 2014, 9, e89918. [Google Scholar] [CrossRef]
- Commault, A.S.; Lear, G.; Packer, M.; Weld, R.J. Influence of anode potentials on selection of Geobacter strains in microbial electrolysis cells. Bioresour. Technol. 2013, 139, 226–234. [Google Scholar] [CrossRef]
- Zhu, X.; Yates, M.D.; Hatzell, M.C.; Rao, H.A.; Saikaly, P.E.; Logan, B.E. Microbial Community Composition Is Unaffected by Anode Potential. Environ. Sci. Technol. 2014, 48, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, E.; Desmond, E.; Erable, B.; Bridier, A.; Bouchez, T.; Bergel, A. Comparison of synthetic medium and wastewater used as dilution medium to design scalable microbial anodes: Application to food waste treatment. Bioresour. Technol. 2015, 185, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.E.; Glaven, S.M.; Lovley, D.R. Real-Time Spatial Gene Expression Analysis within Current-Producing Biofilms. ChemSusChem 2012, 5, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.S.; Bradley, D.F.; Schrott, G.D.; Busalmen, J.P. Limitations for Current Production in Geobacter sulfurreducens Biofilms. ChemSusChem 2013, 6, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, J.; Huang, H.; Liu, W.; Ye, Y.; Cheng, S. The effect of biofilm thickness on electrochemical activity of Geobacter sulfurreducens. Int. J. Hydrogen Energy 2016, 41, 16523–16528. [Google Scholar] [CrossRef]
- Franks, A.E.; Nevin, K.P.; Jia, H.; Izallalen, M.; Woodard, T.L.; Lovley, D.R. Novel strategy for three-dimensional real-time imaging of microbial fuel cell communities: Monitoring the inhibitory effects of proton accumulation within the anode biofilm. Energy Environ. Sci. 2009, 2, 113–119. [Google Scholar] [CrossRef]
- Torres, C.I.; Marcus, A.K.; Rittmann, B.E. Proton transport inside the biofilm limits electrical current generation by anode-respiring bacteria. Biotechnol. Bioeng. 2008, 100, 872–881. [Google Scholar] [CrossRef]
- Renslow, R.S.; Babauta, J.T.; Dohnalkova, A.C.; Boyanov, M.I.; Kemner, K.M.; Majors, P.D.; Fredrickson, J.K.; Beyenal, H. Metabolic spatial variability in electrode-respiring Geobacter sulfurreducens biofilms. Energy Environ. Sci. 2013, 6, 1827–1836. [Google Scholar] [CrossRef]
- Jain, A.; Gazzola, G.; Panzera, A.; Zanoni, M.; Marsili, E. Visible spectroelectrochemical characterization of Geobacter sulfurreducens biofilms on optically transparent indium tin oxide electrode. Electrochimica Acta 2011, 56, 10776–10785. [Google Scholar] [CrossRef]
- Robuschi, L.; Tomba, J.P.; Schrott, G.D.; Bonanni, P.S.; DeSimone, P.M.; Busalmen, J.P. Spectroscopic Slicing to Reveal Internal Redox Gradients in Electricity-Producing Biofilms. Angew. Chem. Int. Ed. 2013, 52, 925–928. [Google Scholar] [CrossRef]
- Robuschi, L.; Tomba, J.P.; Busalmen, J.P. Proving Geobacter biofilm connectivity with confocal Raman microscopy. J. Electroanal. Chem. 2017, 793, 99–103. [Google Scholar] [CrossRef]
- Babauta, J.T.; Nguyen, H.D.; Harrington, T.D.; Renslow, R.; Beyenal, H. pH, redox potential and local biofilm potential microenvironments within Geobacter sulfurreducens biofilms and their roles in electron transfer. Biotechnol. Bioeng. 2012, 109, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, G.L.; Otero, F.J.; Gralnick, J.A.; Bond, D.R.; Orphan, V.J. NanoSIMS imaging reveals metabolic stratification within current-producing biofilms. Proc. Natl. Acad. Sci. USA 2019, 116, 20716–20724. [Google Scholar] [CrossRef] [PubMed]
- Biffinger, J.C.; Byrd, J.N.; Dudley, B.L.; Ringeisen, B.R. Oxygen exposure promotes fuel diversity for Shewanella oneidensis microbial fuel cells. Biosens. Bioelectron. 2008, 23, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, R.; Guasto, J.S.; Stocker, R. Bacterial transport suppressed by fluid shear. Nat. Phys. 2014, 10, 212–217. [Google Scholar] [CrossRef]
- Ren, H.; Torres, C.I.; Parameswaran, P.; Rittmann, B.E.; Chae, J. Improved current and power density with a micro-scale microbial fuel cell due to a small characteristic length. Biosens. Bioelectron. 2014, 61, 587–592. [Google Scholar] [CrossRef]
- Mikkonen, S.; Rokhas, M.K.; Jacksén, J.; Emmer, Å. Sample preconcentration in open microchannels combined with MALDI-MS. Electrophoresis 2012, 33, 3343–3350. [Google Scholar] [CrossRef]
- Choi, S.; Chae, J. Optimal biofilm formation and power generation in a micro-sized microbial fuel cell (MFC). Sens. Actuators A Phys. 2013, 195, 206–212. [Google Scholar] [CrossRef]
- Molenaar, S.D.; Sleutels, T.; Pereira, J.; Iorio, M.; Borsje, C.; Zamudio, J.A.; Fabregat-Santiago, F.; Buisman, C.J.; Ter Heijne, A. In situ Biofilm Quantification in Bioelectrochemical Systems by using Optical Coherence Tomography. ChemSusChem 2018, 11, 2171–2178. [Google Scholar] [CrossRef]
- Diao, J.; Young, L.; Kim, S.; Fogarty, E.A.; Heilman, S.M.; Zhou, P.; Shuler, M.L.; Wu, M.; Delisa, M.P. A three-channel microfluidic device for generating static linear gradients and its application to the quantitative analysis of bacterial chemotaxis. Lab Chip 2006, 6, 381–388. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.-D.; Chung, S. Microfluidic Approaches to Bacterial Biofilm Formation. Molecules 2012, 17, 9818–9834. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Akram, Z.; Bule, M.H.; Iqbal, H.M.N. Advancements and Potential Applications of Microfluidic Approaches—A Review. Chemosensors 2018, 6, 46. [Google Scholar] [CrossRef]
- Feng, J.; De La Fuente-Núñez, C.; Trimble, M.J.; Xu, J.; Hancock, R.E.W.; Lu, X. An in situ Raman spectroscopy-based microfluidic “lab-on-a-chip” platform for non-destructive and continuous characterization of Pseudomonas aeruginosa biofilms. Chem. Commun. 2015, 51, 8966–8969. [Google Scholar] [CrossRef]
- Kou, S.; Cheng, D.; Sun, F.; Hsing, I.-M. Microfluidics and microbial engineering. Lab Chip 2016, 16, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Chilkoor, G.; Vemuri, B.; Rathinam, N.; Sani, R.K.; Gadhamshetty, V. Extremophiles for microbial-electrochemistry applications: A critical review. Bioresour. Technol. 2018, 255, 318–330. [Google Scholar] [CrossRef]
- Chen, T.; Gomez-Escoda, B.; Munoz-Garcia, J.; Babic, J.; Griscom, L.; Wu, P.-Y.J.; Coudreuse, D. A drug-compatible and temperature-controlled microfluidic device for live-cell imaging. Open Biol. 2016, 6, 160156. [Google Scholar] [CrossRef]
- Straub, H.; Eberl, L.; Zinn, M.; Rossi, R.M.; Maniura-Weber, K.; Ren, Q. A Microfuidic Platform for in Situ Investigation of Bio lm Formation and Its Treatment under Controlled Conditions. J. Nanobiotechnology 2020, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhang, P.; Li, J.; Zhu, X.; Chen, R.; Liao, Q. In situ visualization of biofilm formation in a microchannel for a microfluidic microbial fuel cell anode. Int. J. Hydrog. Energy 2020, in press. [Google Scholar] [CrossRef]
- Yawata, Y.; Toda, K.; Setoyama, E.; Fukuda, J.; Suzuki, H.; Uchiyama, H.; Nomura, N. Bacterial growth monitoring in a microfluidic device by confocal reflection microscopy. J. Biosci. Bioeng. 2010, 110, 130–133. [Google Scholar] [CrossRef]
- Connell, J.L.; Kim, J.; Shear, J.B.; Bard, A.J.; Whiteley, M. Real-time monitoring of quorum sensing in 3D-printed bacterial aggregates using scanning electrochemical microscopy. Proc Natl Acad Sci USA 2014, 111, 18255–18260. [Google Scholar] [CrossRef]
- Holman, H.-Y.N.; Miles, R.; Hao, Z.; Wozei, E.; Anderson, L.M.; Yang, H. Real-time chemical imaging of bacterial activity in biofilms using open-channel microfluidics and synchrotron FTIR spectromicroscopy. Anal. Chem. 2009, 81, 8564–8570. [Google Scholar] [CrossRef]
- Renslow, R.S.; Marshall, M.J.; Tucker, A.E.; Chrisler, W.B.; Yu, X.Y. In situ nuclear magnetic resonance microimaging of live biofilms in a microchannel. Analyst 2017, 142, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.T.; Roy, V.; Bentley, W.E.; Ghodssi, R. Development and validation of a microfluidic reactor for biofilm monitoring via optical methods. J. Micromech. Microeng. 2011, 21, 054023. [Google Scholar] [CrossRef]
- Kim, Y.W.; Mosteller, M.P.; Subramanian, S.; Meyer, M.T.; Bentley, W.E.; Ghodssi, R. An optical microfluidic platform for spatiotemporal biofilm treatment monitoring. J. Micromech. Microeng. 2015, 26. [Google Scholar] [CrossRef]
- Kara, A.; Reitz, A.; Mathault, J.; Mehou-Loko, S.; Abbaszadeh Amirdehi, M.; Miled, A.; Greener, J. Electrochemical imaging for microfluidics: A full-system approach. Lab Chip 2016, 16, 1081–1087. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinck, S.; Ostormujof, L.M.; Teychené, S.; Erable, B. Microfluidic Microbial Bioelectrochemical Systems: An Integrated Investigation Platform for a More Fundamental Understanding of Electroactive Bacterial Biofilms. Microorganisms 2020, 8, 1841. https://doi.org/10.3390/microorganisms8111841
Pinck S, Ostormujof LM, Teychené S, Erable B. Microfluidic Microbial Bioelectrochemical Systems: An Integrated Investigation Platform for a More Fundamental Understanding of Electroactive Bacterial Biofilms. Microorganisms. 2020; 8(11):1841. https://doi.org/10.3390/microorganisms8111841
Chicago/Turabian StylePinck, Stéphane, Lucila Martínez Ostormujof, Sébastien Teychené, and Benjamin Erable. 2020. "Microfluidic Microbial Bioelectrochemical Systems: An Integrated Investigation Platform for a More Fundamental Understanding of Electroactive Bacterial Biofilms" Microorganisms 8, no. 11: 1841. https://doi.org/10.3390/microorganisms8111841
APA StylePinck, S., Ostormujof, L. M., Teychené, S., & Erable, B. (2020). Microfluidic Microbial Bioelectrochemical Systems: An Integrated Investigation Platform for a More Fundamental Understanding of Electroactive Bacterial Biofilms. Microorganisms, 8(11), 1841. https://doi.org/10.3390/microorganisms8111841





