Anaplasma and Theileria Pathogens in Cattle of Lambwe Valley, Kenya: A Case for Pro-Active Surveillance in the Wildlife–Livestock Interface
Abstract
1. Introduction
2. Materials and Methods
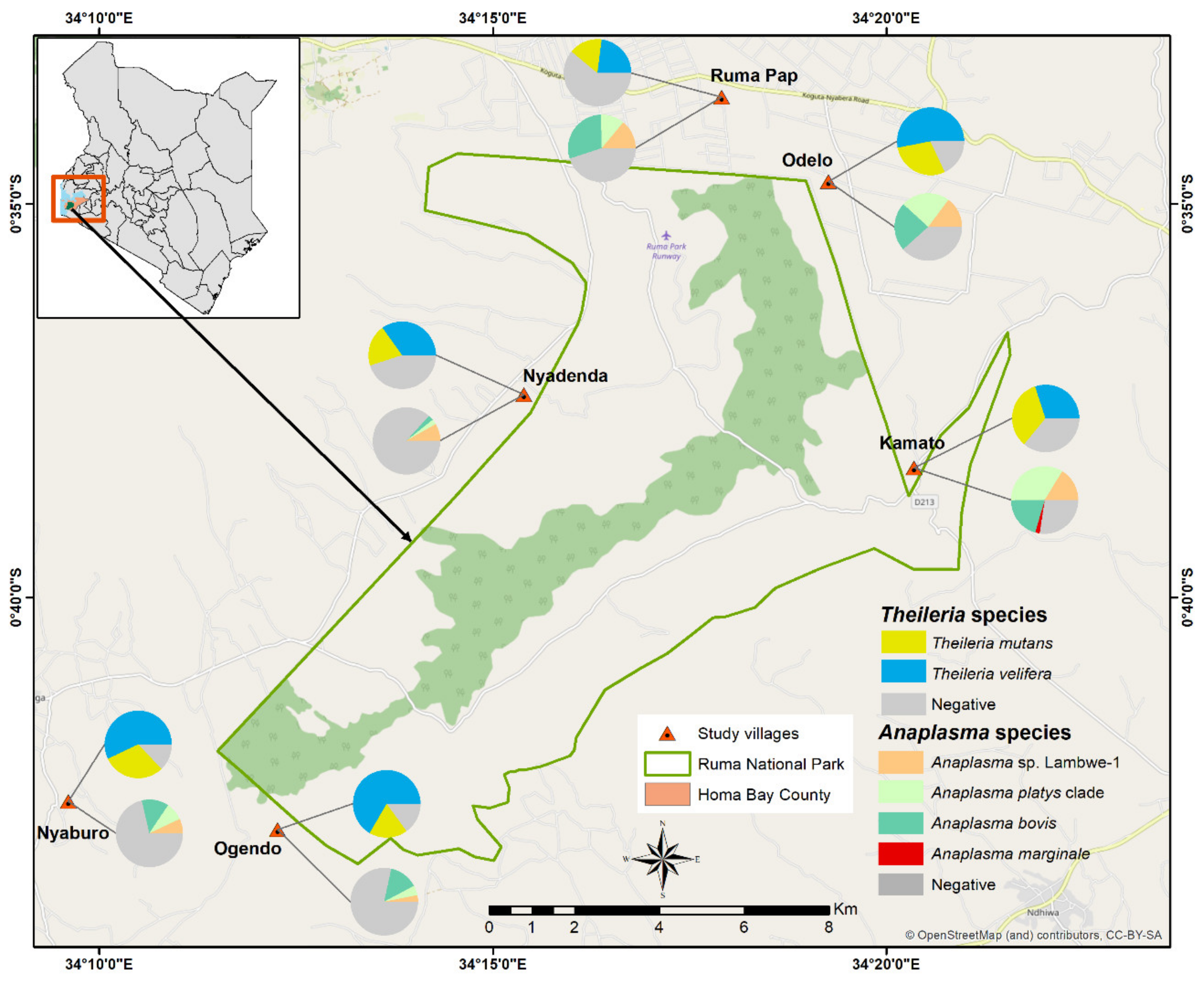
2.1. Study Area
2.2. Study Design and Sample Size Determination
2.3. Ethical Approval
2.4. Blood Sample Collection and Processing
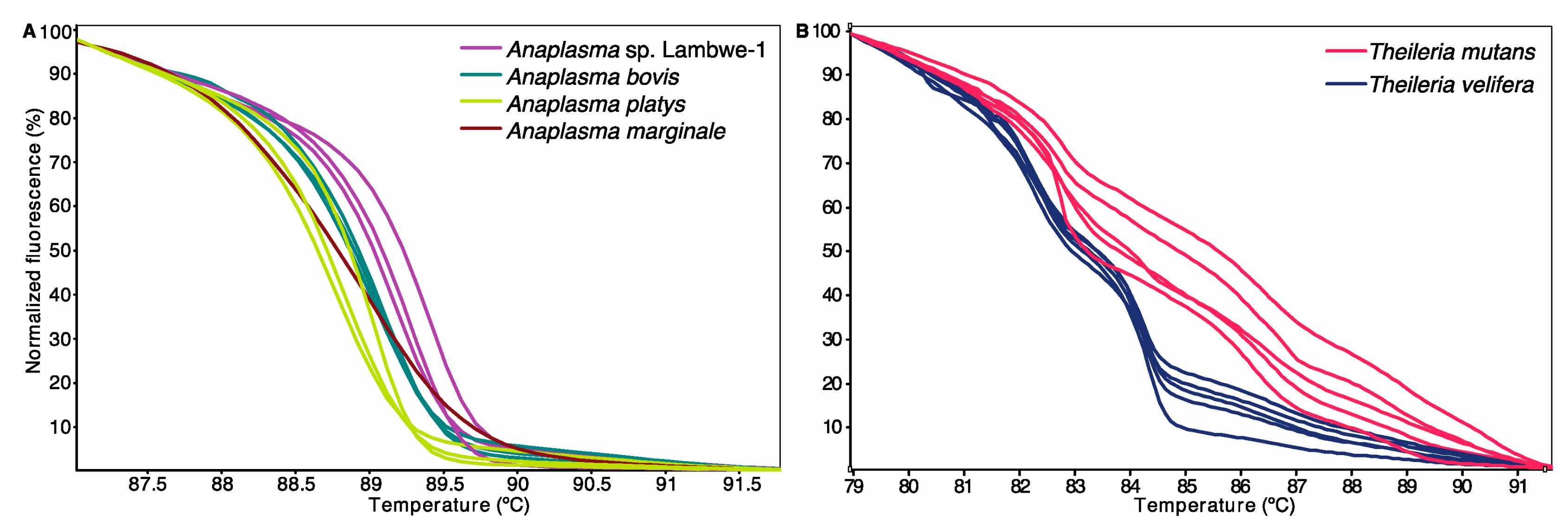
2.5. PCR and High-Resolution Melting (HRM) Analysis
2.6. Nested PCR for Anaplasma-Positive Samples
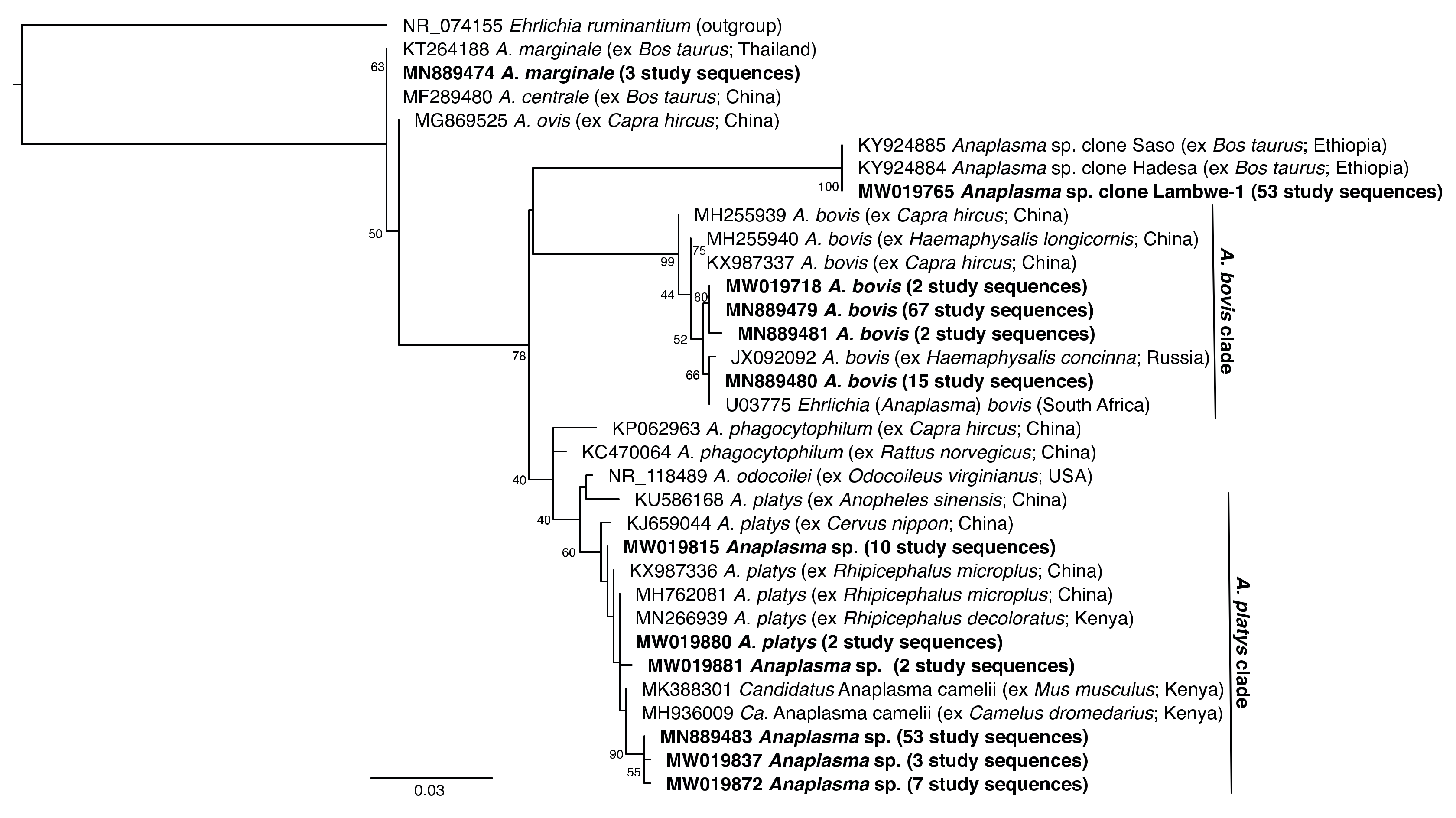
2.7. Phylogenetic Analysis
2.8. Data Management and Analysis
3. Results
3.1. Pathogen Diversity and Prevalence
3.2. Risk Factors Associated with Anaplasma and Theileria Infections
3.3. Prevalence of Co-Infections
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grootenhuis, J.G.; Olubayo, R.O. Disease research in the wildlife-livestock interface in Kenya. Vet. Q. 1993, 15, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Miguel, E.; Gomo, C.; Makaya, P.; Pfukenyi, D.M.; Foggin, C.; Hove, T.; De Garine-Wichatitsky, M. Relationship between burden of infection in ungulate populations and wildlife/livestock interfaces. Epidemiol. Infect. 2013, 141, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.S.; Farnsworth, M.L.; Malmberg, J.L. Diseases at the livestock–wildlife interface: Status, challenges, and opportunities in the United States. Prev. Vet. Med. 2013, 110, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Mwamuye, M.M.; Kariuki, E.; Omondi, D.; Kabii, J.; Odongo, D.; Masiga, D.; Villinger, J. Novel Rickettsia and emergent tick-borne pathogens: A molecular survey of ticks and tick-borne pathogens in Shimba Hills National Reserve, Kenya. Ticks Tick Borne Dis. 2017, 8, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife- threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Minjauw, B.; McLeod, A. Tick-Borne Diseases and Poverty. The Impact of Ticks and Tick-Borne Diseases on the Livelihood of Small-Scale and Marginal Livestock Owners in India and Eastern and Southern Africa; Research Report, DFID Animal Health Programme; University of Edinburgh: Edinburgh, UK, 2003. [Google Scholar]
- Ngeranwa, J.J.; Shompole, S.P.; Venter, E.H.; Wambugu, A.; Crafford, J.E.; Penzhorn, B.L. Detection of Anaplasma antibodies in wildlife and domestic species in wildlife-livestock interface areas of Kenya by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. Onderstepoort J. Vet. Res. 2008, 75, 199–205. [Google Scholar] [CrossRef]
- Ndeereh, D.; Muchemi, G.; Thaiyah, A.; Otiende, M.; Angelone-Alasaad, S.; Jowers, M.J. Molecular survey of Coxiella burnetii in wildlife and ticks at wildlife-livestock interfaces in Kenya. Exp. Appl. Acarol. 2017, 72, 277–289. [Google Scholar] [CrossRef]
- Omondi, D.; Masiga, D.K.; Ajamma, Y.U.; Fielding, B.C.; Njoroge, L.; Villinger, J. Unraveling host-vector-arbovirus interactions by two-gene high resolution melting mosquito bloodmeal analysis in a Kenyan wildlife-livestock interface. PLoS ONE 2015, 10, e0134375. [Google Scholar] [CrossRef]
- Oundo, J.W.; Villinger, J.; Jeneby, M.; Ong’amo, G.; Otiende, M.Y.; Makhulu, E.E.; Musa, A.A.; Ouso, D.O.; Wambua, L. Pathogens, endosymbionts, and blood-meal sources of host-seeking ticks in the fast-changing Maasai Mara wildlife ecosystem. PLoS ONE 2020, 15, e0228366. [Google Scholar] [CrossRef]
- Njiiri, N.E.; Bronsvoort, B.M.d.; Collins, N.E.; Steyn, H.C.; Troskie, M.; Vorster, I.; Thumbi, S.M.; Sibeko, K.P.; Jennings, A.; van Wyk, I.C.; et al. The epidemiology of tick-borne haemoparasites as determined by the reverse line blot hybridization assay in an intensively studied cohort of calves in western Kenya. Vet. Parasitol. 2015, 210, 69–76. [Google Scholar] [CrossRef]
- Kock, R.A. What is this infamous “wildlife/livestock disease interface?” A review of current knowledge for the African continent. In Conservation and Development Interventions at the Wildlife/Livestock Interface: Implications for Wildlife, Livestock and Human Health; Osofsky, S.A., Cleaveland, S., Karesh, W.B., Kock, M.D., Nyhus, P.J., Star, L., Yang, A., Eds.; International Union for Conservation of Nature: Cambridge, UK, 2005; pp. 1–13. [Google Scholar]
- Morrison, W.I.; Hemmink, J.D.; Toye, P.G. Theileria parva: A parasite of African buffalo, which has adapted to infect and undergo transmission in cattle. Int. J. Parasitol. 2020, 50, 403–412. [Google Scholar] [CrossRef]
- Lwande, O.W.; Lutomiah, J.; Obanda, V.; Gakuya, F.; Mutisya, J.; Mulwa, F.; Michuki, G.; Chepkorir, E.; Fischer, A.; Venter, M.; et al. Isolation of tick and mosquito-borne arboviruses from ticks sampled from livestock and wild animal hosts in Ijara District, Kenya. Vector-Borne Zoonotic Dis. 2013, 13, 637–642. [Google Scholar] [CrossRef]
- Kenya Wildlife Conservancies Association. State of Wildlife Conservancies in Kenya Report. 2016. Available online: https://kwcakenya.com/download/state-of-wildlife-conservancies-in-kenya-report/ (accessed on 28 October 2020).
- Muriuki, G.W.; Njoka, T.J.; Reid, R.S.; Nyariki, D.M. Tsetse control and land-use change in Lambwe Valley, south-western Kenya. Agric. Ecosyst. Environ. 2005, 106, 99–107. [Google Scholar] [CrossRef]
- Wellde, B.T.; Chumo, D.A.; Reardon, M.J.; Waema, D.; Smith, D.H.; Gibson, W.C.; Wanyama, L.; Siongok, T.A. Epidemiology of Rhodesian sleeping sickness in the Lambwe Valley, Kenya. Ann. Trop. Med. Parasitol. 1989, 83, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Ogutu, J.O.; Piepho, H.P.; Said, M.Y.; Ojwang, G.O.; Njino, L.W.; Kifugo, S.C.; Wargute, P.W. Extreme wildlife declines and concurrent increase in livestock numbers in Kenya: What are the causes? PLoS ONE 2016, 11, e0163249. [Google Scholar] [CrossRef]
- Morse, S.S. Factors in the Emergence of Infectious Diseases; Price-Smith, A.T., Ed.; Palgrave Macmillan UK: London, UK, 2001; pp. 8–26. [Google Scholar] [CrossRef]
- Otieno, D.O.; K’Otuto, G.O.; Jákli, B.; Schröttle, P.; Maina, J.N.; Jung, E.; Onyango, J.C. Spatial heterogeneity in ecosystem structure and productivity in a moist Kenyan savanna. Plant. Ecol. 2011, 212, 769–783. [Google Scholar] [CrossRef]
- Bennett, S.; Woods, T.; Liyanage, W.M.; Smith, D.L. A simplified general method for cluster-sample surveys of health in developing countries. World Health Stat. Q. 1991, 44, 98–106. [Google Scholar]
- Dohoo, I.R.; Martin, S.W.; Stryhn, H. Veterinary Epidemiologic Research; VER, Inc.: Charlottetown, PE, Canada, 2009; pp. 33–55. [Google Scholar]
- Thrusfield, M.V.; Christley, R. Veterinary Epidemiology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 864. [Google Scholar]
- Otte, M.J.; Gumm, I.D. Intra-cluster correlation coefficients of 20 infections calculated from the results of cluster-sample surveys. Prev. Vet. Med. 1997, 31, 147–150. [Google Scholar] [CrossRef]
- Nijhof, A.M.; Bodaan, C.; Postigo, M.; Nieuwenhuijs, H.; Opsteegh, M.; Franssen, L.; Jebbink, F.; Jongejan, F. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. 2007, 7, 585–595. [Google Scholar] [CrossRef]
- Bastos, A.D.S.; Mohammed, O.B.; Bennett, N.C.; Petevinos, C.; Alagaili, A.N. Molecular detection of novel Anaplasmataceae closely related to Anaplasma platys and Ehrlichia canis in the dromedary camel (Camelus dromedarius). Vet. Microbiol. 2015, 179, 310–314. [Google Scholar] [CrossRef]
- Georges, K.; Loria, G.R.; Riili, S.; Greco, A.; Caracappa, S.; Jongejan, F.; Sparagano, O. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 2001, 99, 273–286. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree; Version 1.4. 2; University of Edinburgh: Edinburgh, UK, 2014. [Google Scholar]
- Ringo, A.E.; Rizk, M.A.; Moumouni, P.F.A.; Liu, M.; Galon, E.M.; Li, Y.; Ji, S.; Tumwebaze, M.; Byamukama, B.; Thekisoe, O. Molecular detection and characterization of tick-borne haemoparasites among cattle on Zanzibar Island, Tanzania. Acta Trop. 2020, 211, 105598. [Google Scholar] [CrossRef]
- Tayebwa, D.S.; Vudriko, P.; Tuvshintulga, B.; Guswanto, A.; Nugraha, A.B.; Gantuya, S.; Batiha, G.E.-S.; Musinguzi, S.P.; Komugisha, M.; Bbira, J.S. Molecular epidemiology of Babesia species, Theileria parva, and Anaplasma marginale infecting cattle and the tick control malpractices in Central and Eastern Uganda. Ticks Tick Borne Dis. 2018, 9, 1475–1483. [Google Scholar] [CrossRef]
- Hailemariam, Z.; Krucken, J.; Baumann, M.; Ahmed, J.S.; Clausen, P.H.; Nijhof, A.M. Molecular detection of tick-borne pathogens in cattle from Southwestern Ethiopia. PLoS ONE 2017, 12, e0188248. [Google Scholar] [CrossRef]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar] [CrossRef]
- Omondi, D.; Masiga, D.K.; Fielding, B.C.; Kariuki, E.; Ajamma, Y.U.; Mwamuye, M.M.; Ouso, D.O.; Villinger, J. Molecular detection of tick-borne pathogen diversities in ticks from livestock and reptiles along the shores and adjacent islands of Lake Victoria and Lake Baringo, Kenya. Front. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Arraga-Alvarado, C.M.; Qurollo, B.A.; Parra, O.C.; Berrueta, M.A.; Hegarty, B.C.; Breitschwerdt, E.B. Case report: Molecular evidence of Anaplasma platys infection in two women from venezuela. Am. J. Trop. Med. Hyg. 2014, 91, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; D’Amico, G.; Yao, P.K.; Ionică, A.M.; Kanyari, P.W.N.; Daskalaki, A.A.; Dumitrache, M.O.; Sándor, A.D.; Gherman, C.M.; Qablan, M. Molecular detection of Anaplasma platys infection in free-roaming dogs and ticks from Kenya and Ivory Coast. Parasit Vectors. 2016, 9, 157. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, K.; Sun, Y.; Shi, J.; Li, H.; Chen, Y.; Yang, H.; Li, X.; Wu, B.; Li, X. Molecular epidemiology and risk factors of Anaplasma spp., Babesia spp. and Theileria spp. infection in cattle in Chongqing, China. PLoS ONE 2019, 14, e0215585. [Google Scholar] [CrossRef]
- Said, M.B.; Belkahia, H.; El Mabrouk, N.; Saidani, M.; Alberti, A.; Zobba, R.; Cherif, A.; Mahjoub, T.; Bouattour, A.; Messadi, L. Anaplasma platys-like strains in ruminants from Tunisia. Infect. Genet. Evol. 2017, 49, 226–233. [Google Scholar] [CrossRef]
- André, M.R.; Calchi, A.C.; Herrera, H.M.; de Souza Zanatto, D.C.; Horta, B.d.C.L.S.; Tasso, J.B.; de Souza Ramos, I.A.; de Mello, V.V.C.; Machado, R.Z. The co-infection with Ehrlichia minasensis, Anaplasma marginale and Anaplasma platys is not associated with anemia in beef cattle in the Brazilian Pantanal. Vet. Parasitol. Reg. Stud. Reports 2020, 21, 100437. [Google Scholar] [CrossRef]
- Peter, S.G.; Aboge, G.O.; Kariuki, H.W.; Kanduma, E.G.; Gakuya, D.W.; Maingi, N.; Mulei, C.M.; Mainga, A.O. Molecular prevalence of emerging Anaplasma and Ehrlichia pathogens in apparently healthy dairy cattle in peri-urban Nairobi, Kenya. BMC Vet. Res. 2020, 16, 364. [Google Scholar] [CrossRef]
- Adjou Moumouni, P.F.; Aboge, G.O.; Terkawi, M.A.; Masatani, T.; Cao, S.; Kamyingkird, K.; Jirapattharasate, C.; Zhou, M.; Wang, G.; Liu, M.; et al. Molecular detection and characterization of Babesia bovis, Babesia bigemina, Theileria species and Anaplasma marginale isolated from cattle in Kenya. Parasit. Vectors 2015, 8, 496. [Google Scholar] [CrossRef]
- Byaruhanga, C.; Collins, N.E.; Knobel, D.; Chaisi, M.E.; Vorster, I.; Steyn, H.C.; Oosthuizen, M.C. Molecular investigation of tick-borne haemoparasite infections among transhumant zebu cattle in Karamoja Region, Uganda. Vet. Parasitol. Reg. Stud. Reports 2016, 3-4, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chiuya, T.; Masiga, D.; Falzon, L.; Bastos, A.; Fevre, E.; Villinger, J. Tick-borne pathogens, including Crimean-Congo haemorrhagic fever virus, at livestock markets and slaughterhouses in western Kenya. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef]
- Simuunza, M.; Weir, W.; Courcier, E.; Tait, A.; Shiels, B. Epidemiological analysis of tick-borne diseases in Zambia. Vet. Parasitol. 2011, 175, 331–342. [Google Scholar] [CrossRef]
- Moll, G.; Lohding, A.; Young, A.S.; Leitch, B.L. Epidemiology of theileriosis in calves in an endemic area of Kenya. Vet. Parasitol. 1986, 19, 255–273. [Google Scholar] [CrossRef]
- Ringo, A.E.; Aboge, G.O.; Adjou Moumouni, P.F.; Lee, S.H.; Jirapattharasate, C.; Liu, M.; Gao, Y.; Guo, H.; Zheng, W.; Efstratiou, A.; et al. Molecular detection and genetic characterisation of pathogenic Theileria, Anaplasma and Ehrlichia species among apparently healthy sheep in central and western Kenya. Onderstepoort J. Vet. Res. 2019, 86, 1630. [Google Scholar] [CrossRef] [PubMed]
- Telfer, S.; Lambin, X.; Birtles, R.; Beldomenico, P.; Burthe, S.; Paterson, S.; Begon, M. Species interactions in a parasite community drive infection risk in a wildlife population. Science 2010, 330, 243–246. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Thumbi, S.M.; Jennings, A.; Chase-Topping, M.; Callaby, R.; Kiara, H.; Oosthuizen, M.C.; Mbole-Kariuki, M.N.; Conradie, I.; Handel, I.G.; et al. Co-infections determine patterns of mortality in a population exposed to parasite infection. Sci. Adv. 2015, 1, e1400026. [Google Scholar] [CrossRef] [PubMed]
- Darby, A.C.; Armstrong, S.D.; Bah, G.S.; Kaur, G.; Hughes, M.A.; Kay, S.M.; Koldkjær, P.; Rainbow, L.; Radford, A.D.; Blaxter, M.L. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res. 2012, 22, 2467–2477. [Google Scholar] [CrossRef]
- Ola-Fadunsin, S.D.; Gimba, F.I.; Abdullah, D.A.; Sharma, R.S.K.; Abdullah, F.J.F.; Sani, R.A. Epidemiology and risk factors associated with Anaplasma marginale infection of cattle in Peninsular Malaysia. Parasitol. Int. 2018, 67, 659–665. [Google Scholar] [CrossRef]

| Target Pathogens | Target Gene | Primer Name | Sequence (5′ to 3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|
| Rickettsia spp. | 16S rRNA | Rick-F | GAACGCTATCGGTATGCTTAACACA | 364 | [26] |
| Rick-R | CATCACTCACTCGGTATTGCTGGA | ||||
| Ehrlichia spp. | 16S rRNA | EhrlichiaJV F | GCAACCCTCATCCTTAGTTACCA | 300 | [5] |
| EhrlichiaJV R | TGTTACGACTTCACCCTAGTCAC | ||||
| Anaplasma spp. | 16S rRNA | AnaplasmaJV F | CGGTGGAGCATGTGGTTTAATTC | 300 | [5] |
| AnaplasmaJV R | CGRCGTTGCAACCTATTGTAGTC | ||||
| Anaplasmataceae | 16S rRNA | EHR16SD | GGTACCYACAGAAGAAGTCC | [27] | |
| pH1522 | AAGGAGGTGATCCAGCCGCA | 1060 | |||
| pH1492 | GGCTACCTTGTTACGACTT | 1030 | |||
| Theileria and Babesia spp. | 18S rRNA | RLB F | GAGGTAGTGACAAGAAATAACAATA | 450 | [28] |
| RLB R | TCTTCGATCCCCTAACTTTC |
| Pathogen | Individual Prevalence | Herd Prevalence | ||||
|---|---|---|---|---|---|---|
| na | Prevalence (%) | 95% CI | nb | Prevalence (%) | 95% CI | |
| Anaplasma spp. | 311 | 45.7 | 42.0, 49.5 | 75 | 78.9 | 70.8, 87.2 |
| A. bovis | 118 | 17.4 | 14.5, 20.2 | 55 | 57.9 | 48.0, 67.8 |
| A. platys clade | 115 | 16.9 | 14.1, 19.7 | 49 | 51.6 | 41.5, 61.6 |
| A. marginale | 4 | 0.6 | 0.0, 1.2 | 4 | 4.2 | 0.2, 8.3 |
| Anaplasma sp. Lambwe-1 | 79 | 11.6 | 9.2, 14.0 | 39 | 41.1 | 31.2, 50.9 |
| Theileria spp. | 432 | 63.5 | 59.9, 67.2 | 84 | 88.4 | 82.0, 94.9 |
| T. velifera | 272 | 40.0 | 36.3, 43.7 | 72 | 75.8 | 67.2, 84.4 |
| T. mutans | 175 | 25.7 | 22.5, 29.0 | 62 | 65.3 | 55.7, 74.8 |
| Overall TBPs | 680 | 78.5 | 75.3, 81.5 | 91 | 95.8 | 91.8, 99.8 |
| Pathogen Detected | Number Positive (% Prevalence) | 95% CI |
|---|---|---|
| Single infections | 319 (46.9) | 43.7, 50.7 |
| A. marginale | 2 (0.3) | 0.0, 0.7 |
| A. bovis | 36 (5.3) | 3.6, 6.9 |
| A. platys clade | 35 (5.2) | 3.5, 6.8 |
| Anaplasma sp. Lambwe-1 | 29 (4.3) | 2.8, 5.8 |
| T. velifera | 145 (21.0) | 18.2, 24.4 |
| T. mutans | 72 (11.0) | 8.3, 12.9 |
| Double infections | 200 (29.4) | 26.0, 32.8 |
| A. bovis + T. mutans | 31 (4.6) | 3.0, 6.1 |
| A. bovis + T. velifera | 45 (6.6) | 4.8, 8.5 |
| A. marginale + T. velifera | 2 (0.3) | 0.0, 0.7 |
| A. platys clade + T. mutans | 36 (5.3) | 3.5, 6.8 |
| A. platys clade + T. velifera | 35 (5.2) | 3.5, 6.8 |
| Anaplasma sp. Lambwe-1 + T. velifera | 29 (4.3) | 2.8, 5.8 |
| Anaplasma sp. Lambwe-1 + T. mutans | 17 (2.5) | 1.3, 3.7 |
| T. velifera + T. mutans | 6 (1.0) | 0.2, 1.6 |
| Triple infections | 15 (2.2) | 1.1, 3.3 |
| A. bovis + A. platys clade + T. mutans | 1 (0.2) | 0.0, 0.4 |
| Anaplasma sp. Lambwe-1 + A. platys clade + T. mutans | 3 (0.4) | 0.0, 0.9 |
| Anaplasma sp. Lambwe-1 + A. platys clade + T. velifera | 1 (0.2) | 0.0, 0.4 |
| A. bovis + T. velifera + T. mutans | 5(0.7) | 0.0, 1.4 |
| A. platys clade + T. mutans + T. velifera | 4 (0.6) | 0.0, 1.2 |
| Total | 534 (78.5) | 75.4, 81.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okal, M.N.; Odhiambo, B.K.; Otieno, P.; Bargul, J.L.; Masiga, D.; Villinger, J.; Kalayou, S. Anaplasma and Theileria Pathogens in Cattle of Lambwe Valley, Kenya: A Case for Pro-Active Surveillance in the Wildlife–Livestock Interface. Microorganisms 2020, 8, 1830. https://doi.org/10.3390/microorganisms8111830
Okal MN, Odhiambo BK, Otieno P, Bargul JL, Masiga D, Villinger J, Kalayou S. Anaplasma and Theileria Pathogens in Cattle of Lambwe Valley, Kenya: A Case for Pro-Active Surveillance in the Wildlife–Livestock Interface. Microorganisms. 2020; 8(11):1830. https://doi.org/10.3390/microorganisms8111830
Chicago/Turabian StyleOkal, Michael N., Brenda Kisia Odhiambo, Peter Otieno, Joel L. Bargul, Daniel Masiga, Jandouwe Villinger, and Shewit Kalayou. 2020. "Anaplasma and Theileria Pathogens in Cattle of Lambwe Valley, Kenya: A Case for Pro-Active Surveillance in the Wildlife–Livestock Interface" Microorganisms 8, no. 11: 1830. https://doi.org/10.3390/microorganisms8111830
APA StyleOkal, M. N., Odhiambo, B. K., Otieno, P., Bargul, J. L., Masiga, D., Villinger, J., & Kalayou, S. (2020). Anaplasma and Theileria Pathogens in Cattle of Lambwe Valley, Kenya: A Case for Pro-Active Surveillance in the Wildlife–Livestock Interface. Microorganisms, 8(11), 1830. https://doi.org/10.3390/microorganisms8111830







