The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. COVID-19 Crisis Management
2.3. Patient Selection
2.3.1. Cases
2.3.2. Controls
2.4. Data Collection and Endpoints
2.5. Microbiology Data
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Patients Admitted to COVID-19 ICUs
3.2. Characteristics of MDRB Acquisition
3.3. Risk of MDRB Acquisition in COVID-19 Patients Compared to the Control Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-2019) Situation Reports; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Li, R.; Rivers, C.; Tan, Q.; Murray, M.B.; Toner, E.; Lipsitch, M. Estimated Demand for US Hospital Inpatient and Intensive Care Unit Beds for Patients With COVID-19 Based on Comparisons With Wuhan and Guangzhou, China. JAMA Netw. Open 2020, 3, e208297. [Google Scholar] [CrossRef]
- Garcia Godoy, L.R.; Jones, A.E.; Anderson, T.N.; Fisher, C.L.; Seeley, K.M.L.; Beeson, E.A.; Zane, H.K.; Peterson, J.W.; Sullivan, P.D. Facial protection for healthcare workers during pandemics: A scoping review. BMJ Glob. Health 2020, 5, e002553. [Google Scholar] [CrossRef]
- The COVID19-APHP Group. Assistance Publique-Hopitaux de Paris’ response to the COVID-19 pandemic. Lancet 2020, 395, 1760–1761. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Huttner, A.; Harbarth, S.; Carlet, J.; Cosgrove, S.; Goossens, H.; Holmes, A.; Jarlier, V.; Voss, A.; Pittet, D. Antimicrobial resistance: A global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob. Resist. Infect. Control. 2013, 2, 31. [Google Scholar] [CrossRef]
- Gutierrez-Gutierrez, B.; Rodriguez-Bano, J. Current options for the treatment of infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae in different groups of patients. Clin. Microbiol. Infect. 2019, 25, 932–942. [Google Scholar] [CrossRef]
- Falagas, M.E.; Tansarli, G.S.; Karageorgopoulos, D.E.; Vardakas, K.Z. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg. Infect. Dis. 2014, 20, 1170–1175. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States, CDC. 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 27 October 2020).
- Brusselaers, N.; Vogelaers, D.; Blot, S. The rising problem of antimicrobial resistance in the intensive care unit. Ann. Intensive Care 2011, 1, 47. [Google Scholar] [CrossRef]
- Grimaldi, D.; Aissaoui, N.; Blonz, G.; Carbutti, G.; Courcelle, R.; Gaudry, S.; Gaultier, A.; D’Hondt, A.; Higny, J.; Horlait, G.; et al. Characteristics and outcomes of acute respiratory distress syndrome related to COVID-19 in Belgian and French intensive care units according to antiviral strategies: The COVADIS multicentre observational study. Ann. Intensive Care 2020, 10, 131. [Google Scholar] [CrossRef]
- Bogossian, E.G.; Attanasio, L.; Creteur, J.; Grimaldi, D.; Schuind, S.; Taccone, F.S. The impact of extra-cerebral infection after subarachnoid hemorrhage: A single center cohort study. World Neurosurg. 2020. [Google Scholar] [CrossRef]
- Witiw, C.D.; Ibrahim, G.M.; Fallah, A.; Macdonald, R.L. Early predictors of prolonged stay in a critical care unit following aneurysmal subarachnoid hemorrhage. Neurocritical Care 2013, 18, 291–297. [Google Scholar] [CrossRef]
- Alaraj, A.; Hussein, A.E.; Esfahani, D.R.; Amin-Hanjani, S.; Aletich, V.A.; Charbel, F.T. Reducing length of stay in aneurysmal subarachnoid hemorrhage: A three year institutional experience. J. Clin. Neurosci. 2017, 42, 66–70. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 90; 2019. Available online: https://eucast.org/clinical_breakpoints/ (accessed on 1 September 2019).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- De Waele, J.J.; Boelens, J.; Leroux-Roels, I. Multidrug-resistant bacteria in ICU: Fact or myth. Curr. Opin. Anaesthesiol. 2020, 33, 156–161. [Google Scholar] [CrossRef]
- De Bus, L.; Denys, W.; Catteeuw, J.; Gadeyne, B.; Vermeulen, K.; Boelens, J.; Claeys, G.; De Waele, J.J.; Decruyenaere, J.; Depuydt, P.O. Impact of de-escalation of beta-lactam antibiotics on the emergence of antibiotic resistance in ICU patients: A retrospective observational study. Intensive Care Med. 2016, 42, 1029–1039. [Google Scholar] [CrossRef]
- Kerneis, S.; Lucet, J.C. Controlling the Diffusion of Multidrug-Resistant Organisms in Intensive Care Units. Semin. Respir. Crit. Care Med. 2019, 40, 558–568. [Google Scholar] [CrossRef]
- Grundmann, H.; Hahn, A.; Ehrenstein, B.; Geiger, K.; Just, H.; Daschner, F.D. Detection of cross-transmission of multiresistant Gram-negative bacilli and Staphylococcus aureus in adult intensive care units by routine typing of clinical isolates. Clin. Microbiol. Infect. 1999, 5, 355–363. [Google Scholar] [CrossRef][Green Version]
- Chetchotisakd, P.; Phelps, C.L.; Hartstein, A.I. Assessment of bacterial cross-transmission as a cause of infections in patients in intensive care units. Clin. Infect. Dis. 1994, 18, 929–937. [Google Scholar] [CrossRef]
- Boyce, J.M.; Pittet, D.; Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am. J. Infect. Control. 2002, 30, S1–S46. [Google Scholar] [CrossRef]
- Osterholm, M.T. Preparing for the next pandemic. N. Engl. J. Med. 2005, 352, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Nieuwlaat, R.; Mbuagbaw, L.; Mertz, D.; Burrows, L.; Bowdish, D.M.E.; Moja, L.; Wright, G.D.; Schunemann, H.J. COVID-19 and Antimicrobial Resistance: Parallel and Interacting Health Emergencies. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. COVID-19, superinfections and antimicrobial development: What can we expect? Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Sturdy, A.; Basarab, M.; Cotter, M.; Hager, K.; Shakespeare, D.; Shah, N.; Randall, P.; Spray, D.; Arnold, A. Severe COVID-19 and Healthcare Associated Infections on the ICU: Time to Remember the Basics? J. Hosp. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.K. The Novel Coronavirus COVID-19 Outbreak: Global Implications for Antimicrobial Resistance. Front. Microbiol. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Chatzopoulou, M.; Reynolds, L. Role of antimicrobial restrictions in bacterial resistance control: A systematic literature review. J. Hosp. Infect. 2020, 104, 125–136. [Google Scholar] [CrossRef]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Burn, E.; You, S.C.; Sena, A.G.; Kostka, K.; Abedtash, H.; Abrahao, M.T.F.; Alberga, A.; Alghoul, H.; Alser, O.; Alshammari, T.M.; et al. An international characterisation of patients hospitalised with COVID-19 and a comparison with those previously hospitalised with influenza. medRxiv 2020. [Google Scholar] [CrossRef]
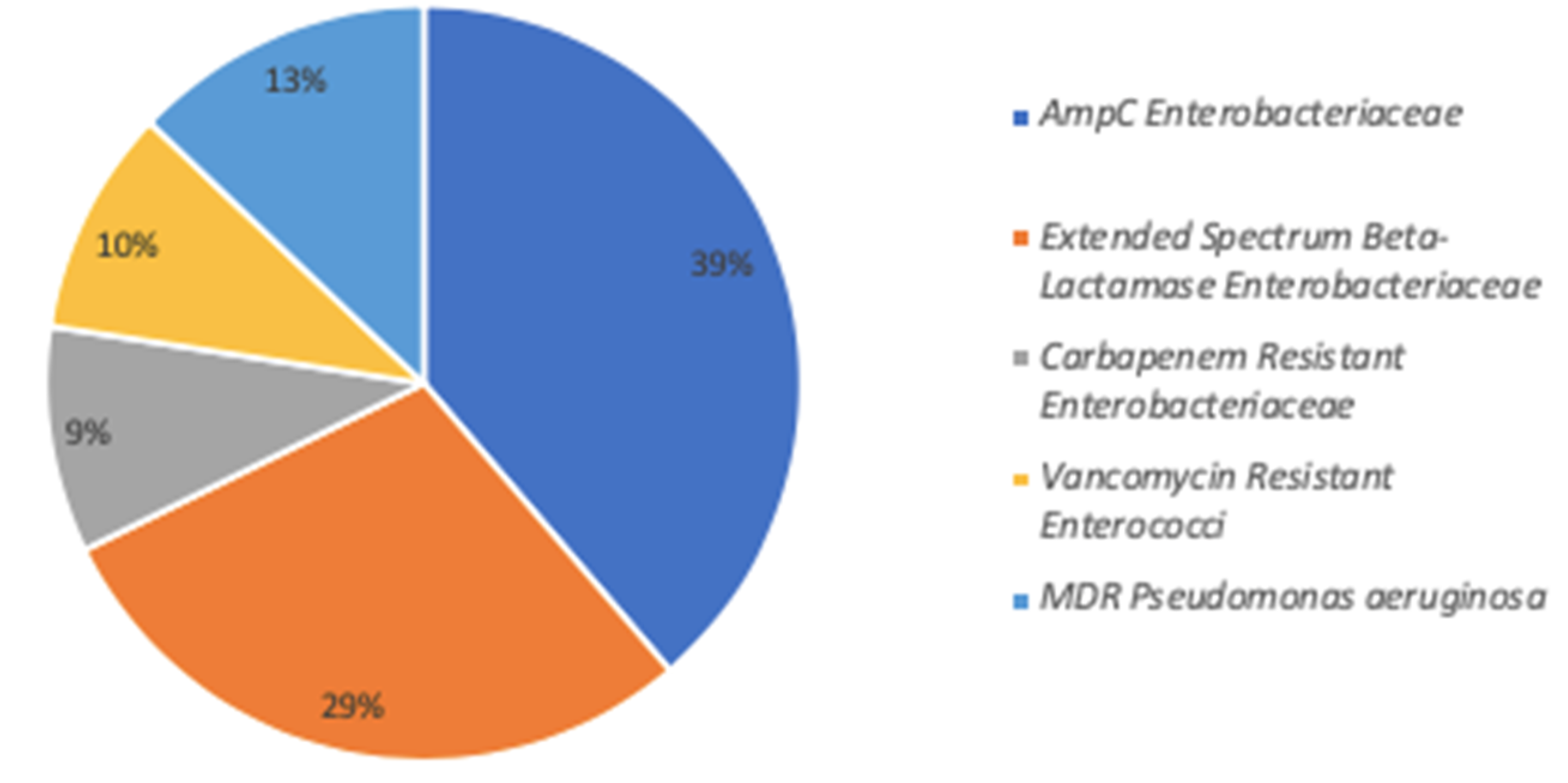

| All Patients (N = 72) | MDRB- (N = 48) | MDRB+ (N = 24) | p Value ** | |
|---|---|---|---|---|
| Age; years, mean (±SD) | 61 (±14) | 62 (±15) | 61 (±9) | 0.84 |
| Male gender, n (%) | 47 (65) | 30 (63) | 17 (71) | 0.60 |
| Charlson comorbidity index, median (IQR) | 1(0–3) | 1 (0–3) | 1 (0–4) | 0.77 |
| Hospitalization in the last 6 months, n (%) | 11 (15) | 9 (19) | 2 (8) | 0.32 |
| Patients MDRB+ at admission, n (%) | 5 (7) | 4 (8) | 1 (4) | 0.66 |
| SAPS 3 score, median (IQR) | 56 (47–69) | 56 (47–70) | 57 (47–65) | 0.95 |
| SOFA sore, median (IQR) | 6 (3–9) | 6 (3–10) | 7 (5–9) | 0.39 |
| ICU stay | ||||
| Central venous catheter *, n (%) | 68 (94) | 45 (93) | 23 (96) | 0.99 |
| Urinary tract catheter *, n (%) | 65 (90) | 43 (90) | 22 (92) | 0.99 |
| Mechanical ventilation *, n (%) | 40 (56) | 24 (50) | 16 (67) | 0.22 |
| Length of MV *; days, median (IQR) | 3 (0–12) | 1 (0–11) | 7 (0–15) | 0.27 |
| Vasopressor use, n (%) | 50 (69) | 28 (58) | 22 (92) | 0.006 |
| Renal replacement therapy, n (%) | 17 (24) | 10 (21) | 7 (29) | 0.56 |
| ECMO, n (%) | 11 (15) | 7 (15) | 4 (17) | 0.99 |
| Corticosteroid therapy | 7 (10) | 4 (8) | 3 (13) | 0.57 |
| Surgery *, n (%) | 10 (14) | 8 (17) | 2 (8) | 0.48 |
| Antimicrobial therapy *, n (%) | 56 (78) | 34 (71) | 22 (92) | 0.05 |
| Length of Antimicrobial therapy *; days, median (IQR) | 5 (2–7) | 4 (0–7) | 6 (4–7) | 0.09 |
| Length of exposure *; days, median (IQR) | 9 (4–18) | 5 (2–18) | 12 (8–18) | 0.02 |
| Admission to double bed ICU room *, n (%) | 8 (11) | 4 (8) | 4 (17) | 0.43 |
| Outcome | ||||
| ICU LOS; days, median (IQR) | 11 (3–28) | 6 (2–18) | 28 (17–32) | <0.001 |
| ICU LOS of survivors; days, median (IQR) | 7 (2–28) | 4 (2–9) | 26 (24–28) | 0.001 |
| Hospital LOS; days, median (IQR) | 24 (12–45) | 19 (11–34) | 39 (21–61) | 0.005 |
| Hospital LOS of survivors; days, median (IQR) | 33 (16–52) | 20 (11–39) | 53 (33–69) | 0.002 |
| ICU mortality, n (%) | 22 (31) | 16 (33) | 6 (25) | 0.59 |
| Hospital mortality, n (%) | 25 (35) | 19 (40) | 6 (25) | 0.30 |
| COVID-19 N = 72 | Control N = 72 | p-Value # | |
|---|---|---|---|
| Age; years, mean (±SD) | 61 (±14) | 53 (±16) | <0.001 |
| Male gender, n (%) | 47 (65) | 32 (44) | 0.02 |
| Charlson comorbidity index, median (IQR) | 1 (0–3) | 1 (0–3) | 0.68 |
| Hospitalization in the previous 6 months, n (%) | 11 (15) | 3 (4) | 0.02 |
| Transfer from another hospital ** | 20 (28) | 0 (0) | <0.001 |
| MDRB+ at admission, n (%) *** | 5 (7) | 4 (6) | 0.99 |
| SAPS 3 score, median (IQR) | 56 (47–69) | 33 (27–39) | <0.001 |
| SOFA sore, median (IQR) | 6 (3–9) | 5 (1–10) | 0.08 |
| Lymphocyte count; G/L, median (IQR) **** | 1.07 (0.73–1.74) | 1.05 (0.77–1.56) | 0.90 |
| ICU stay | |||
| Central venous catheter *, n (%) | 68 (94) | 44 (61) | <0.001 |
| Urinary tract catheter *, n (%) | 65 (90) | 41 (57) | <0.001 |
| Mechanical ventilation, n(%) | 52 (72) | 52 (72) | 1.0 |
| Length of MV *; days, median (IQR) | 3 (0–12) | 1 (0–10) | 0.55 |
| Vasopressor, n (%) | 50 (69) | 44 (61) | 0.38 |
| Renal replacement therapy, n (%) | 17 (24) | 1 (1) | <0.001 |
| Antimicrobial therapy *, n (%) | 56 (78) | 37 (51) | 0.002 |
| Length of Antimicrobial therapy * days, median (IQR) | 5 (2–7) | 1 (0–5) | <0.001 |
| Length of exposure; days, median (IQR) | 9 (4–18) | 10 (4–22) | 0.67 |
| Outcome | |||
| MDRB acquisition during ICU stay, n (%) | 24 (33) | 16 (22) | 0.19 |
| ICU LOS; days, median (IQR) | 11 (3–28) | 11 (3–26) | 0.86 |
| ICU LOS of survivors; days, median (IQR) | 7 (2–28) | 10 (3–27) | 0.98 |
| ICU mortality, n (%) | 22 (31) | 16 (22) | 0.26 |
| Univariable sHR (CI 95%) | Multivariable sHR (CI 95%) | |
|---|---|---|
| SOFA score | 0.97 (0.89–1.05) | |
| Mechanical ventilation | 0.58 (0.30–1.11) | 0.61 (0.3–1.26) |
| Central venous catheter | 0.54 (0.26–1.13) | 0.66 (0.27–1.62) |
| Urinary tract catheter | 0.98 (0.41–2.33) | |
| Antimicrobial therapy | 1.32 (0.48–3.61) | |
| Admission to COVID-19 ICUs | 1.62 (0.88–2.99) | 1.71 (0.93–3.12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogossian, E.G.; Taccone, F.S.; Izzi, A.; Yin, N.; Garufi, A.; Hublet, S.; Njimi, H.; Ego, A.; Gorham, J.; Byl, B.; et al. The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study. Microorganisms 2020, 8, 1821. https://doi.org/10.3390/microorganisms8111821
Bogossian EG, Taccone FS, Izzi A, Yin N, Garufi A, Hublet S, Njimi H, Ego A, Gorham J, Byl B, et al. The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study. Microorganisms. 2020; 8(11):1821. https://doi.org/10.3390/microorganisms8111821
Chicago/Turabian StyleBogossian, Elisa G., Fabio S. Taccone, Antonio Izzi, Nicolas Yin, Alessandra Garufi, Stephane Hublet, Hassane Njimi, Amedee Ego, Julie Gorham, Baudouin Byl, and et al. 2020. "The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study" Microorganisms 8, no. 11: 1821. https://doi.org/10.3390/microorganisms8111821
APA StyleBogossian, E. G., Taccone, F. S., Izzi, A., Yin, N., Garufi, A., Hublet, S., Njimi, H., Ego, A., Gorham, J., Byl, B., Brasseur, A., Hites, M., Vincent, J.-L., Creteur, J., & Grimaldi, D. (2020). The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study. Microorganisms, 8(11), 1821. https://doi.org/10.3390/microorganisms8111821





