1. Introduction
Bioleaching refers to the solubilization of target metals by microorganisms from materials such as minerals and wastes [
1,
2,
3,
4]. Considering the relatively low environmental and operating cost of bioleaching, numerous studies have explored the use of various microorganisms for biosolubilization [
5]. Redoxolysis, acidolysis, and complexolysis are the main mechanisms to drive bioleaching. Chemolithoautotrophs such as
Acidithiobacillus (
A.)
ferrooxidans and
A. thiooxidans have been used to solubilize base metals, especially copper [
6,
7,
8]. These species oxidize ferrous iron to ferric iron and/or reduced sulfur compounds to sulfuric acid, which leach metals from minerals and wastes via redoxolysis and acidolysis, respectively [
6,
9,
10]. Heterothophic fungi, such as
Aspergillus niger and
Penicillium simplicissimum have also been employed for bioleaching metals from electronic waste (e-waste) [
1,
11,
12]. Fungal bioleaching occurs through acidolysis and complexolysis, facilitated by the production of organic acids such as citric, oxalic and gluconic acid [
6]. Some fungi have additionally been shown to oxidize gold [
13].
Recently, the application of iodide oxidizing bacteria (IOB) for bioleaching gold was proposed [
10] and demonstrated for gold-containing sulfide ore [
14]. A heterotrophic bacterium “
Pseudomonas iodooxidans” has been reported to oxidize I
− to I
2 with hydrogen peroxide (H
2O
2) as an electron acceptor (Reaction 1) [
15,
16,
17,
18]:
Another strain,
Roseovarius (
R.) sp., can oxidise I
− to I
2 with oxygen as the electron acceptor (Reaction 2) [
18,
19,
20,
21]:
Iodide (I
−) reacts chemically with iodine (I
2) to form in triiodide (I
3−) according to Reaction (3):
Gold can be solubilised according to reactions (4) and (5):
E-waste generation is rapidly increasing globally [
22]. The composition of e-waste is very heterogenous, and includes iron, non-ferrous metals, plastics and other constituents (e.g., rubber, concrete and ceramics) [
22]. In terms of metallic components, e-waste contains precious metals (Ag, Au and Pt), base metals (Al, Co, Cu, Ni, Zn and Fe) and others (e.g., Be, Cd, Cr, Hg, Pb, Sb, Sn and Ti). If improperly managed, e-waste can cause toxicity to humans and the environment. On the other hand, e-waste contains high amounts of precious metals, the extraction and recovery of which is warranted. Bioleaching of e-waste has been explored as an alternative to chemical leaching, especially using cyanide forming microorganisms [
23,
24,
25]. However, due to the high toxicity of cyanide, more environmentally friendly biolixiviants should be explored. To the best of our knowledge, the use of IOB for bioleaching gold from e-waste and sulfidic gold ore concentrate has not been previously explored. Therefore, the objectives of this study were (1) to evaluate the ability of IOB,
R. tolerans and
R. mucosus, to bioleach gold from e-waste and sulfidic gold ore concentrate, and (2) to evaluate the effect of various leaching approaches (one-step, two-step and spent medium bioleaching) on gold extraction.
2. Materials and Methods
2.1. Preparation and Analysis Of Sulfide Ore Concentrate and e-Waste
Sulfidic gold ore concentrate was sourced from a gold mine in Western Australia and had a particle size with a P80 (i.e., 80% of mass passing) of 120 µm. The gold ore concentrate was sterilized by autoclaving before leaching experiments (121 °C for 20 min). Shredded e-waste (high-grade printed circuit boards) was sourced from Total Green Recycling in Perth, Western Australia. The shredded e-waste was pulverized with Essa® LM5 mill (FMSmidth, Denmark) at Nagrom, Western Australia.
2.2. Iodide Oxidising Bacteria (IOB) Culture Conditions
The IOB species used in this study,
R. tolerans DSM 11457
T and
R. mucosus DSM 17069
T, were selected based on previous report by [
14] and ordered from the German Collection of Microorganisms and Cell Cultures GmbH (DSMZ, Braunschweig, Germany). Both strains were cultured in 37.4 g·L
−1 of Difco™ Marine Broth 2216 that contained (g·L
−1): 5 peptone, 1 yeast extract, 0.1 C
6H
5FeO
7, 19.45 NaCl, 5.9 MgCl
2, 3.24 MgSO
4, 1.8 CaCl
2, 0.55 KCl, 0.16 NaHCO
3, 0.08 KBr and (mg·L
−1): 34 SrCl
3, 22 H
3BO
3, 4 NaSiO
3, 2.4 NaF, 1.6 NH
4NO
3 and 8 Na
2HPO
4. The cultures were incubated under aerobic condition at 100 rpm and 30 °C for two days. The initial cell number for the bioleaching experiments was adjusted to 3 × 10
6 cell·mL
−1 according to previous study [
14].
2.3. Bioleaching Experiments
The culture medium for gold bioleaching by IOB contained 18.7 g·L−1 of Difco™ Marine Broth 2216 and 10.9 g·L−1 of potassium iodide (KI) (pH 7.2). A 50 mL liquid volume was used in a 250 mL flask for leaching experiments. The culture medium was sterilized at 121 °C for 20 min. Thereafter, 10% of inoculum (5 mL) was added into 45 mL of culture medium followed by 0.5 g of milled e-waste or gold ore concentrate for a one-step bioleaching at 1% pulp density. For two-step bioleaching, the 50 mL culture was incubated for 3 days before adding 0.5 g e-waste or gold ore concentrate for 1% pulp density. For spent medium bioleaching, cells from a 3-day culture were removed by 0.2 μm syringe filtration (Millex, Merck Millipore, Ireland). Thereafter, 50 mL of filtrate was transferred to a new 250 mL flask and 0.5 g e-waste or gold ore concentrate was added to achieve 1% pulp density. Spent medium condition was used to study the ability of metabolites from Roseovarius spp. to leach gold. The chemical ore and e-waste controls had only ore concentrate or e-waste in the medium, respectively, without bacterial inoculum. The medium control had medium only without ore concentrate, e-waste or bacteria. The biological bacteria control had the medium inoculated with bacteria, but no ore concentrate or e-waste. Each condition was tested in triplicate. All leaching experiments were conducted for 10 days at 30 °C, in a shaker (Innova44, Eppendorf, Enfield, CT, USA) at 100 rpm. Samples were taken at days 0, 5 and 10 for the analysis of soluble gold, pH, redox potential, triiodide concentration and bacterial cell numbers.
2.4. Analytical Methods
The elemental compositions of the gold ore concentrate and e-waste were analysed at LabWest, Western Australia. Platinum group metals were determined by a PGM fire assay followed by aqua regia digestion and analysis by inductively coupled plasma optical emission spectrometry (ICP-OES). Other metals were analysed by microwave assisted digestion with a mixture of HF, HCl and HNO3, followed by analysis using a combination of ICP-OES and ICP mass spectrometry (MS).
For analysing soluble gold, 4 mL aliquots of samples filtered through 0.2 μm syringe filters were acidified with 0.1 mL of 7 M nitric acid and 7.5 mL of 10% hydrochloric acid and diluted with 3.4 mL ultrapure water to a final volume of 15 mL. Soluble gold concentrations were measured by ICP-MS at The Commonwealth Scientific and Industrial Research Organisation (CSIRO) Mineral Resources Waterford laboratory, Western Australia. The pH and redox potential of filtrate solution were measured at room temperature using TPS smart CHEM-Lab meter with a TPS pH probe (EPBUFN-121207) and a TPS redox probe (EOREFN-121262) with Ag/AgCl reference.
Triiodide concentration was measured using a spectrometric method described by [
26]. Triiodide standards were prepared by dissolving potassium iodide (KI) and iodine to obtain 100 mM iodide and 7.88 mM iodine solutions, respectively. Then, triiodide was formed by mixing iodine solution and potassium iodide solution (Reaction 3). The standard was prepared with 0.5 mM of potassium iodide and 0, 0.005, 0.01, 0.02, 0.04, 0.06, 0.08, and 0.10 mM of iodine. The absorbance was measured at 351 nm using a microplate reader (Varioskan
TM LUX, Thermo Scientific, Vantaa, Uusimaa, Finland). Numbers of suspended cells were counted by phase contrast microscopy (Leica DM4000 B, Germany) using Helber bacterial counting chamber.
2.5. Calculations and Statistical Analysis
The gold leaching yields were calculated using Equations (6)–(10). The mass of the dissolved gold collected during sampling (
MRi, mg) was estimated from Equation (6):
where
Ci is the dissolved gold concentration in the sample (mg·L
−1) and
SVi is the subsample volume (L). Cumulative mass of dissolved gold removed in samples (CMR, mg) was calculated using Equation (7):
The mass of gold dissolved in the remaining leachate in the flask (SM, mg) was estimated using Equation (8):
where
C is the dissolved gold concentration in the leachate (mg·L
−1) and
V is the remaining volume of leachate in the flask (L). The total mass of dissolved gold (TL, mg) was calculated using Equation (9):
The leaching yield (Y, %) was calculated using Equation (10):
where
Original gold mass refers to the mass of gold (mg) in the ore concentrate or e-waste used for leaching. Averages and standard deviations of gold leaching yields were determined for each time point.
Two- and one-way analyses of variance (ANOVA) were used to determine the statistical significance of the differences detected between bioleaching conditions. Statistical tests were conducted in Microsoft Excel 365 ProPlus and p-values < 0.05 were considered significant.
4. Discussion
The application of IOB for bioleaching gold has been previously proposed as a sustainable alternative to cyanide [
10] and, the use of IOB isolated from iodide-rich brine waters has been explored for leaching gold from gold-containing ore [
14,
21]. This study evaluated the ability of IOB sourced from a commercial culture collection,
R. tolerans DSM 11457
T and
R. mucosus DSM 17069
T, to bioleach gold from e-waste and sulfidic gold ore concentrate.
R. tolerans DSM 11457
T was originally isolated from a hypersaline lake in East Antarctica [
27] whereas
R. mucosus DSM 17069
T was isolated from a marine dinoflagellate [
28]. Neither of the strains was described as an IOB in the original species descriptions, but were selected for this study based on other reports on the members of
Roseovarius genus being able to oxidize iodide [
14,
18,
21]. While previous studies have evaluated the use of strains of
Roseovarius genus to leach gold from ore using one-step leaching, this study explored gold bioleaching with
R. tolerans DSM 11457
T and
R. mucosus DSM 17069
T from gold ore concentrate and e-waste using one-step, two-step and spent medium bioleaching. Both species were able to bioleach gold from ore concentrate, and two-step bioleaching resulted in the highest gold yields, followed by spent medium leaching and one-step leaching (
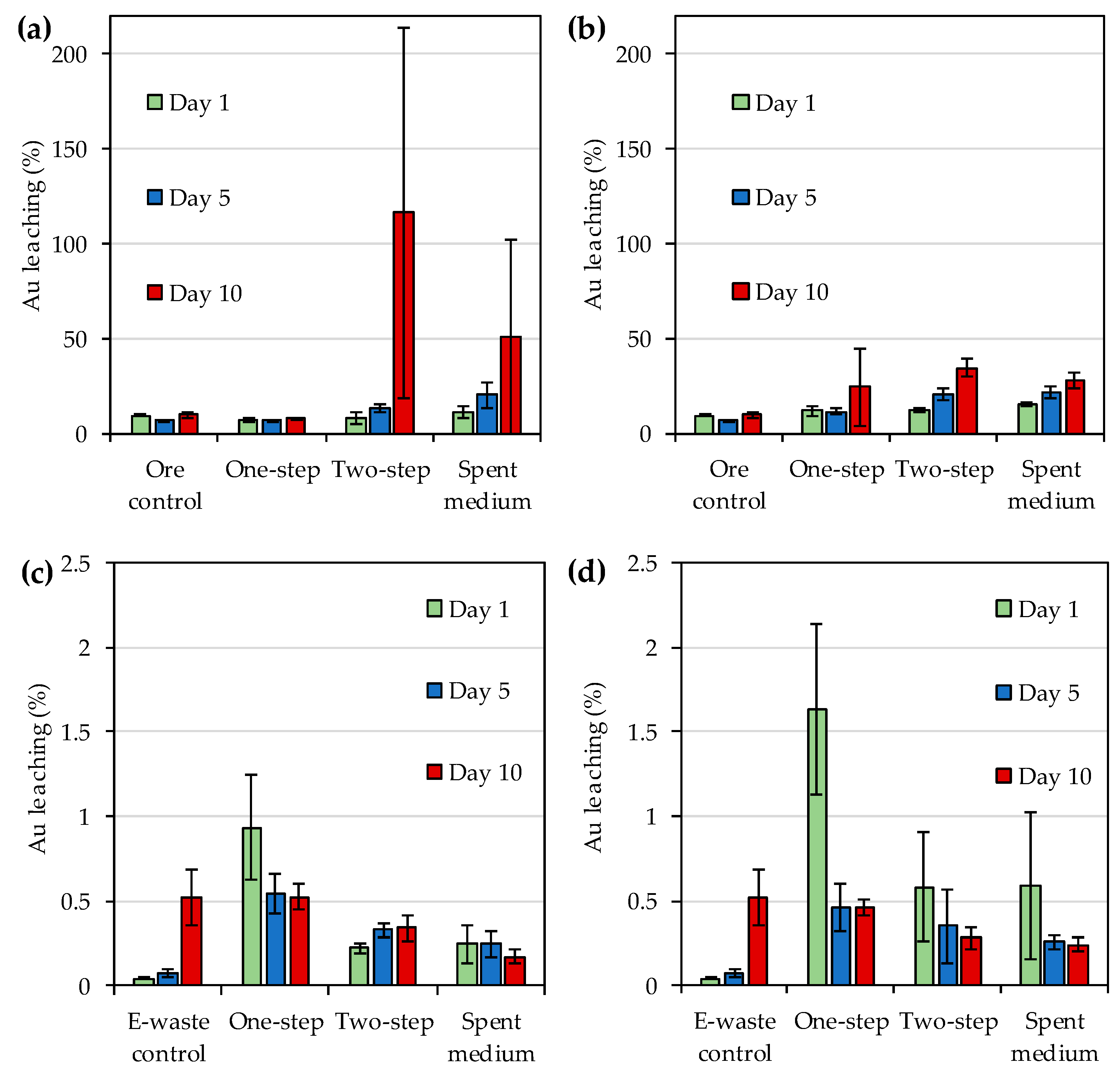
Figure 1a,b). However, the yields were much lower for e-waste with both strains as compared to the yields obtained for the gold ore concentrate (
Figure 1). While bioleaching yields generally increased over time for ore concentrate, declining trend in yields was recorded for e-waste during the experiment. A comparison of maximum bioleaching yields achieved in this study and selected previous studies is shown in
Table 7. The maximum gold yield detected for
R. tolerans with gold concentrate in this study was similar to that reported by Khaing et al. [
14,
21] for other
R. tolerans strains leaching sulfide ore. However, the maximum yield obtained with
R. mucosus for ore concentrate was lower than that previously reported for strains of
Roseovarius genus. The bioleaching yields for e-waste remained lower for both IOB strains than previously reported for e.g., organic acid producing
Aspergillus niger [
29] and cyanide producing
Chromobacterium violaceum [
25].
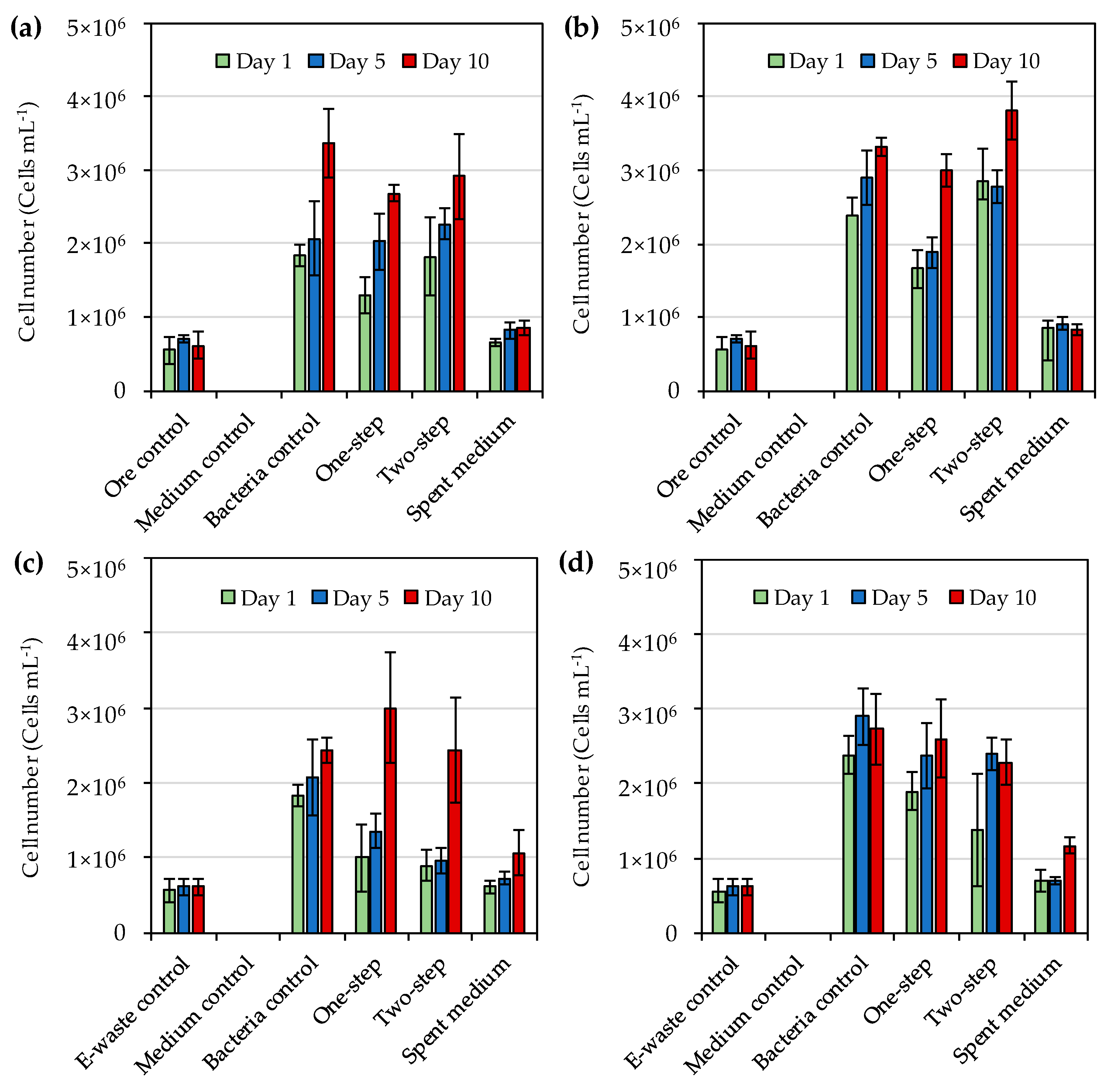
In this study, the initial cell number at the start of bioleaching was set as 3 × 10
6 cell·mL
−1 and the cell numbers remained in the same order of magnitude during the 10-day study. On the contrary, Khaing et al. [
14] reported the cell numbers of a strain from
Roseovarius genus to increase from 3 × 10
6 cell·mL
−1 to 5 × 10
8 cell·mL
−1 during the bioleaching of gold from sulfide ore. In another study, Khaing et al. [
21] reported that initial cell numbers of IOB had no effect on gold dissolution from sulfide ore when the initial cell numbers were 1 × 10
4 cell·mL
−1 to 1 × 10
6 cell·mL
−1 and during the 10-day incubation the cell numbers increased from 1 × 10
4 cell·mL
−1 to 4.2 × 10
7 cell·mL
−1. Therefore, the initial cell numbers used in the present study were likely not the limiting factor in the gold bioleaching, but some toxic components of the ore concentrate and e-waste used for bioleaching may have inhibited bacterial growth [
30]. Hence, pre-treatment of the materials to remove possible inhibitory compounds should be explored in future studies. Some microbial cells were also detected in spent medium flasks. It may be possible that some native microorganisms were present in the e-waste and ore concentrate, and survived autoclaving as similar cell numbers as observed in spent medium flasks were also found in ore and e-waste control flasks.
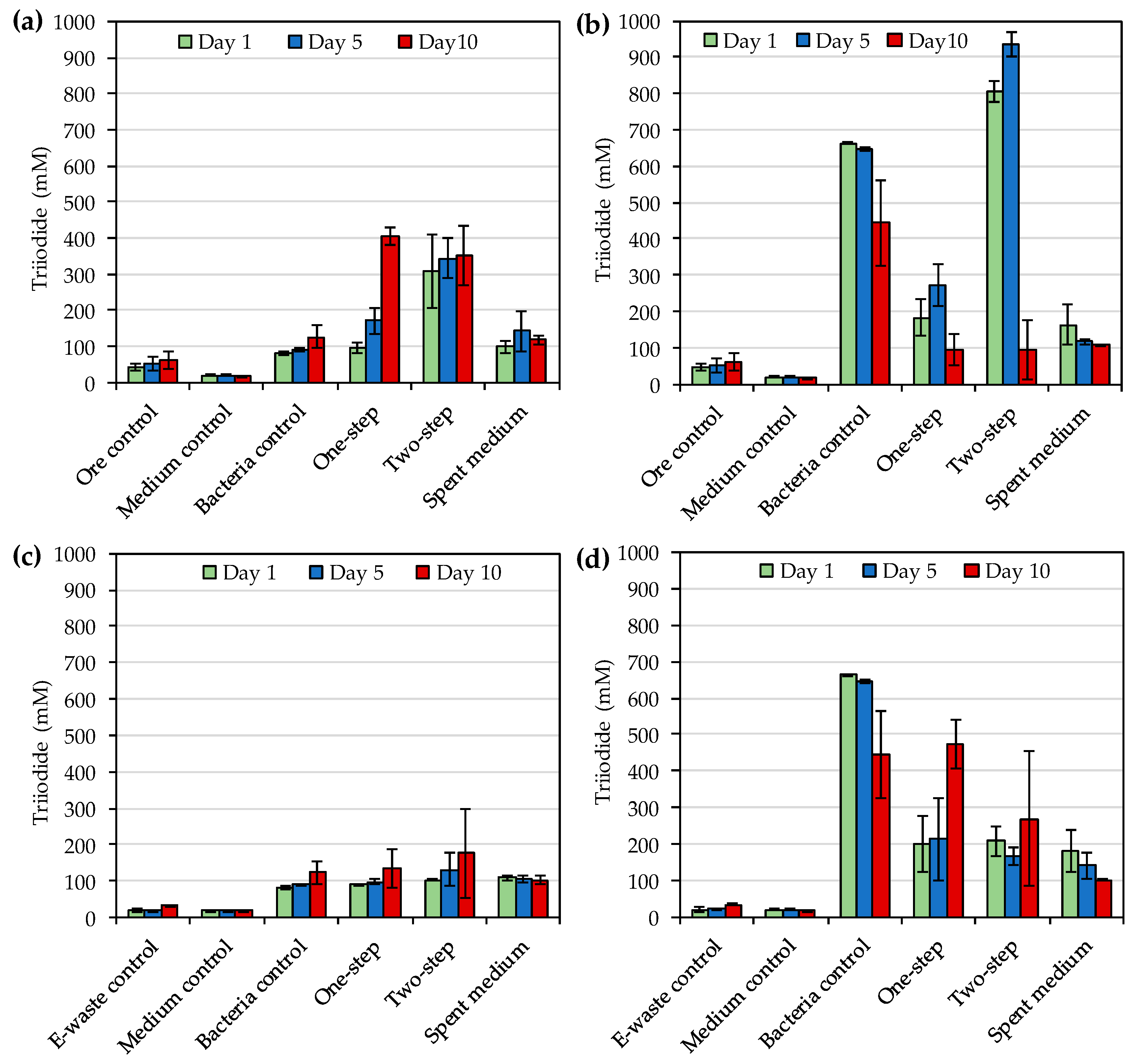
The highest concentrations of triiodide during the bioleaching of gold ore concentrate with
R. tolerans and
R. mucosus were 406 mg·L
−1 and 934 mg·L
−1, respectively and the highest triiodide concentrations with e-waste were 176 mg·L
−1 and 472 mg·L
−1 for
R. tolerans and
R. mucosus, respectively. These concentrations were notably higher than the concentrations (up to 240 mg·L
−1) reported by Khaing et al. [
14] for other strains of
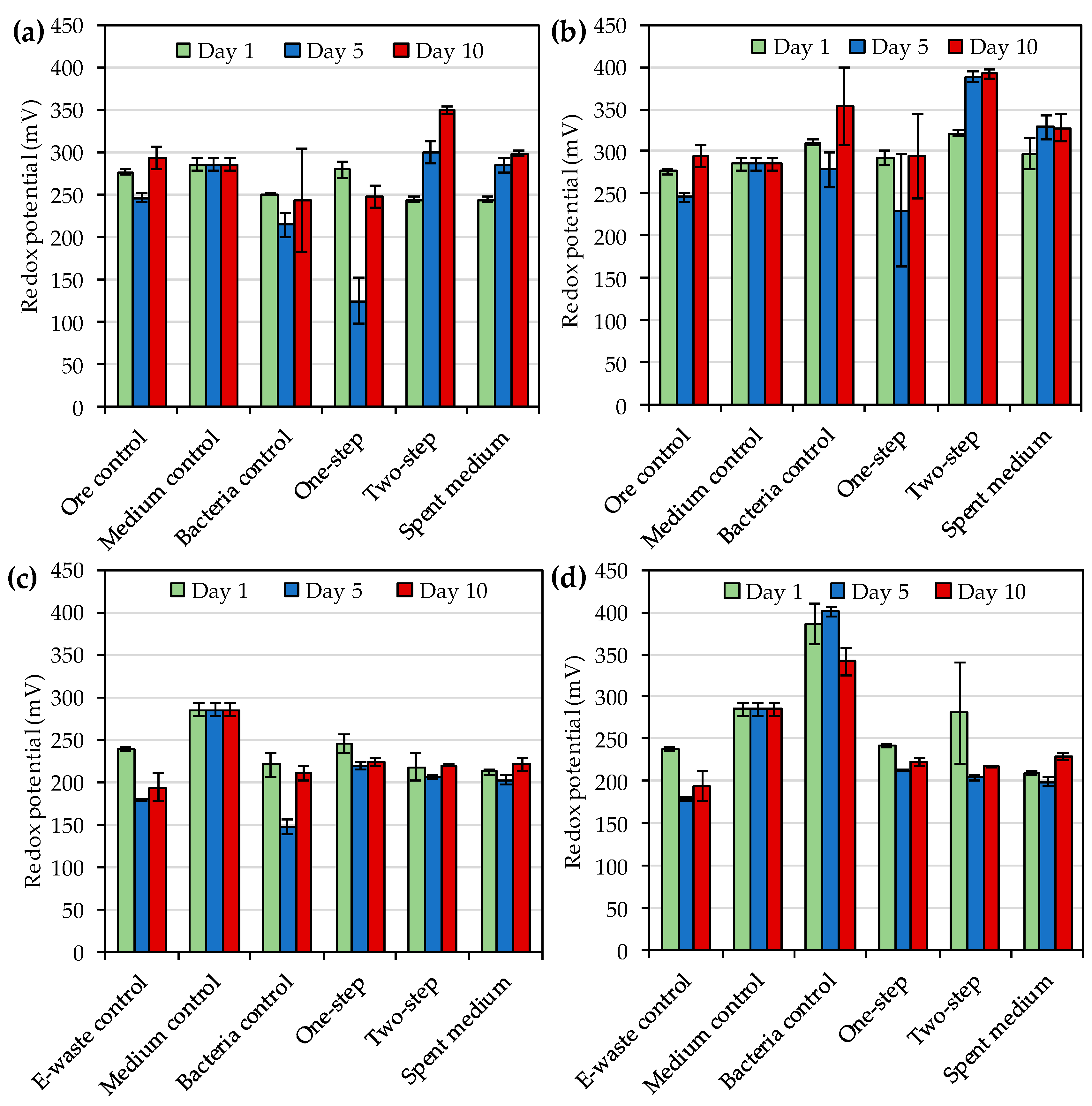
Roseovarius genus. During the ore concentrate bioleaching, redox potentials varied from 124 to 350 mV and from 230 to 392 mV depending on the leached material, leaching method and sampling time for
R. tolerans and
R. mucosus, respectively. For comparison, the redox potentials reported by Khaing et al. [
14,
21] during sulfidic ore bioleaching with strains of
Roseovarius genus were notably higher, 472–547 mV. According to Baghalha [
31] AuI
2− complex is stable at redox potentials from 500 to 700 mV (standard hydrogen electrode), which is approximately 300–500 mV vs. Ag/AgCl. The low redox potentials detected especially during e-waste bioleaching (<300 mV vs. Ag/AgCl) may explain the modest Au leaching yields in the present study. Moreover, the reduction in leaching yield over time in the presence of e-waste indicated the instability of the leached gold under the conditions used. Therefore, future studies should explore the optimization of the bioleaching e.g., by pre-treating the e-waste and ore concentrate with biooxidation to remove oxidant consuming materials before iodide bioleaching.
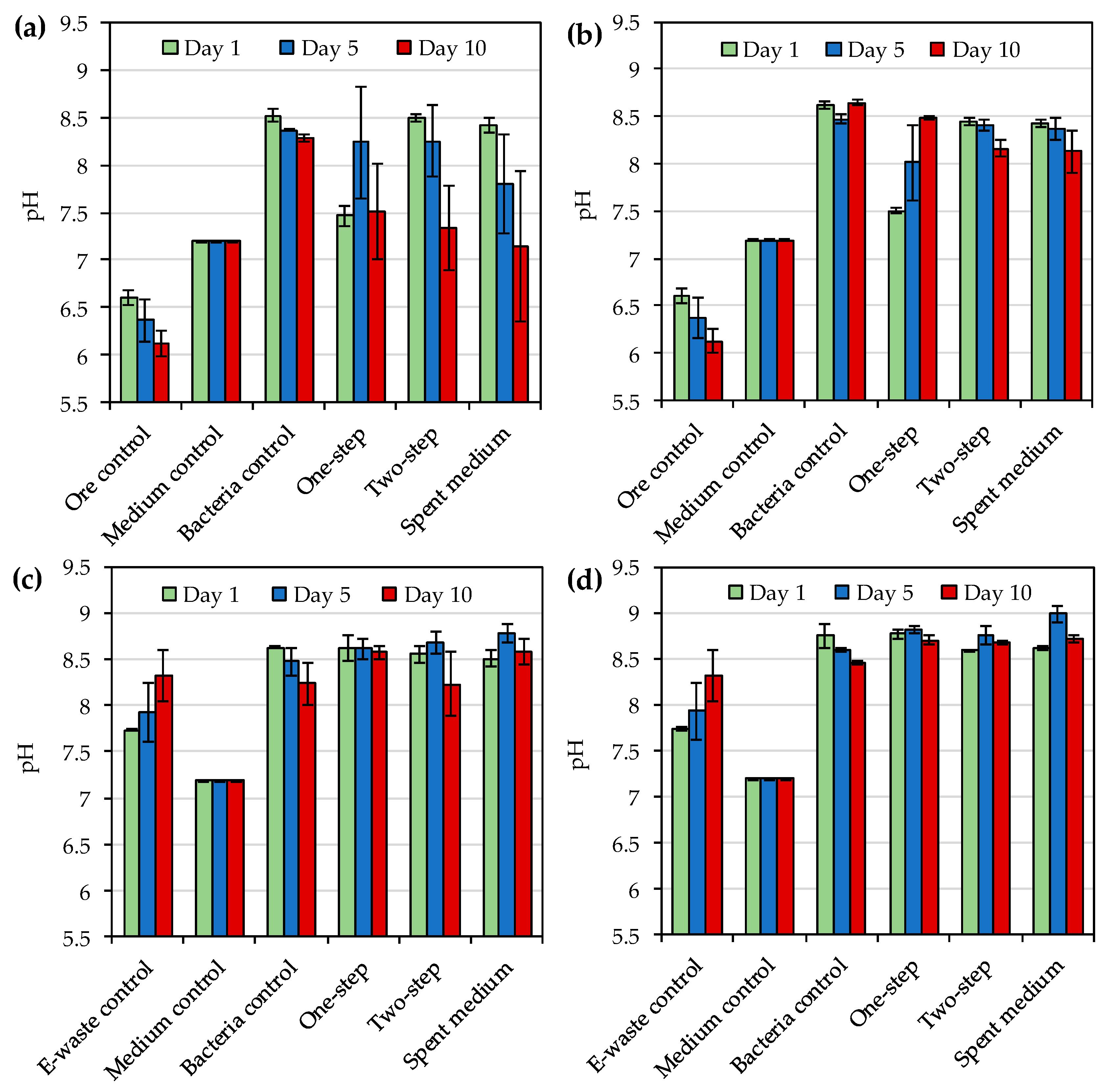
One advantage of iodide leaching over cyanide leaching is the stability of AuI
2− complex over a wide pH range of 0–13 [
31]. In the present study pH varied from 7.1 to 8.8 and from 7.5 to 9.0 depending on the leached material, leaching method and sampling time for
R. tolerans and
R. mucosus, respectively. These were similar to the pH values (pH 7.7–8.8) reported by Khaing et al. [
14] during the bioleaching of gold ore with other strains of
Roseovarius genus. Therefore, pH was unlikely to have a major impact on gold leaching in the present study
In conclusion, this study showed the ability of two culture collection strains, R. tolerans DSM 11457T and R. mucosus DSM 17069T, to bioleach gold from e-waste and sulfidic gold ore concentrate. While the leaching yields from ore concentrate were promising, the yields from e-waste remained low. Limiting factors for bioleaching were likely inhibition of bacterial growth and low redox potential caused by some constituents of the ore concentrate and e-waste used for the bioleaching. Therefore, future studies should explore the pre-treatment of the ore concentrate and e-waste to remove inhibitory and oxidant consuming compounds before bioleaching with IOB to optimize leaching yields. Moreover, the use of IOB could be explored for extracting gold from refractory gold ores to determine whether a prior oxidation step is required before iodine-based gold bioleaching.