Diversity and Composition of the Skin, Blood and Gut Microbiome in Rosacea—A Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
3. Results
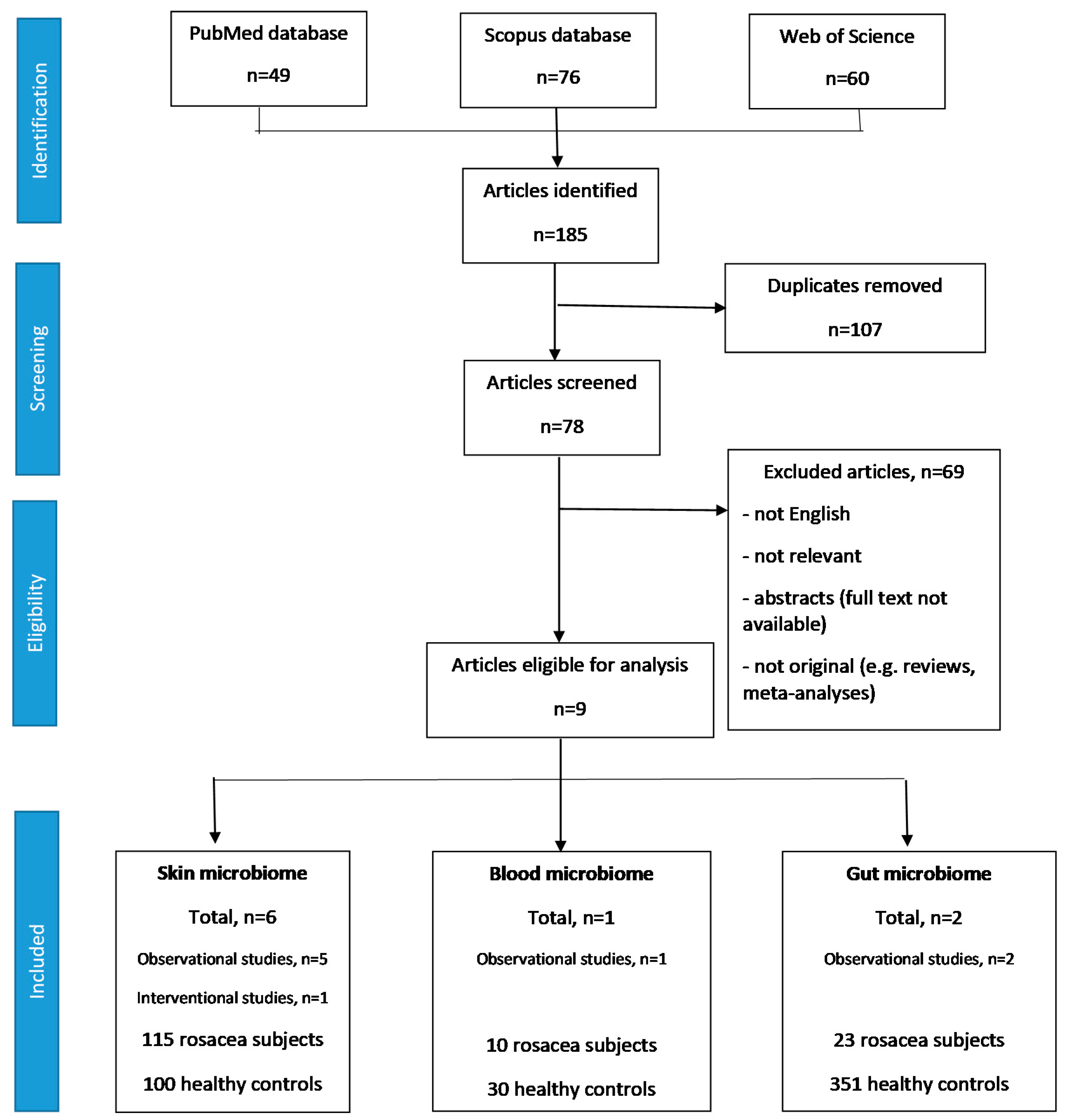
3.1. Search Results
3.2. Skin Microbiome in Rosacea
3.2.1. Study Characteristics
3.2.2. Skin Microbiome α- and β-Diversity in Rosacea
3.2.3. Composition of the Skin Microbiome in Rosacea
3.2.4. Impact of Antibiotic Treatment on the Skin Microbiome Composition
3.3. Blood Microbiome in Rosacea
3.3.1. Study Characteristics
3.3.2. Blood Microbiome α- and β-Diversity in Rosacea
3.3.3. Composition of the Blood Microbiome in Rosacea
3.4. Gut Microbiome in Rosacea
3.4.1. Study Characteristics
3.4.2. Gut Microbiome α- and β-Diversity in Rosacea
3.4.3. Composition of the Gut Microbiome in Rosacea
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sinikumpu, S.P.P.; Jokelainen, J.J.; Haarala, A.K.K.; Keränen, M.H.H.; Keinänen-Kiukaanniemi, S.S.; Huilaja, L. The high prevalence of skin disease in adults aged 70 or older. J. Am. Geriatr. Soc. 2020. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, B.B.; Deng, Y.Y.; Shi, W.W.; Jian, D.D.; Liu, F.F.; Huang, Y.Y.; Tang, Y.Y.; Zhao, Z.Z.; Huang, X.X.; et al. Epidemiological features of rosacea in Changsha, China: A population-based, cross-sectional study. J. Dermatol. 2020, 47, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Shaller, M.M.; Almeida, L.M.C.C.; Bewley, A.A.; Cribier, B.B.; Del Rosso, J.J.; Dlova, N.C.C.; Gallo, R.L.L.; Granstein, R.D.D.; Kautz, G.G.; Mannis, M.J.J.; et al. Recommendations for rosacea diagnosis, classification and management: Update from the global ROSacea Consensus 2019 panel. Br. J. Dermatol. 2020, 182, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, J.K. Rosacea: Pathophysiology and treatment. Arch. Dermatol. 1994, 130, 359–362. [Google Scholar] [CrossRef]
- Powell, F.C. What’s going on in rosacea? J. Eur. Acad. Dermatol. Venereol. 2000, 14, 351–352. [Google Scholar] [CrossRef]
- Whitfeld, M.M.; Gunasingam, N.N.; Leow, L.J.J.; Shirato, K.K.; Preda, V. Staphylococcus epidermidis: A possible role in the pustules of rosacea. J. Am. Acad. Dermatol. 2011, 64, 49–52. [Google Scholar] [CrossRef]
- Ursell, L.K.K.; Metcalf, J.L.L.; Parfrey, L.W.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. 1), S38–S44. [Google Scholar] [CrossRef]
- Pothmann, A.A.; Illing, T.T.; Wiegand, C.C.; Hartmann, A.A.A.; Elsner, P. The microbiome and atopic dermatitis: A review. Am. J. Clin. Dermatol. 2019, 20, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.B.; Byun, E.J.J.; Kim, H.S. Potential role of the microbiome in acne: A comprehensive review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.D.; Issa, N.N.; Afifi, L.L.; Jeon, C.C.; Chang, H.W.W.; Liao, W. The role of the skin and gut microbiome in psoriatic disease. Curr. Dermatol. Rep. 2017, 6, 94–103. [Google Scholar] [CrossRef]
- Hispan, P.P.; Murcia, O.O.; Gonzalez-Villanueva, I.I.; Frances, R.R.; Gimenez, P.P.; Riquelme, J.J.; Betlloch, I.I.; Pascual, J.C. Identification of bacterial DNA in the peripheral blood of patients with active hidradenitis suppurativa. Arch. Dermatol. Res. 2020, 312, 159–163. [Google Scholar] [CrossRef]
- Murillo, N.N.; Aubert, J.J.; Raoult, D. Microbiota of Demodex mites from rosacea patients and controls. Microb. Pathog. 2014, 71–72, 37–40. [Google Scholar] [CrossRef]
- Zaidi, A.K.K.; Spaunhurst, K.K.; Sprockett, D.D.; Thomason, Y.Y.; Mann, M.W.W.; Fu, P.P.; Ammons, C.C.; Gerstenblith, M.M.; Tuttle, M.S.S.; Popkin, D.L. Characterization of the facial microbiome in twins discordant for rosacea. Exp. Dermatol. 2018, 27, 295–298. [Google Scholar] [CrossRef]
- Rainer, B.M.M.; Thompson, K.G.G.; Antonescu, C.C.; Florea, L.L.; Mongodin, E.F.F.; Bui, J.J.; Fischer, A.H.H.; Pasieka, H.B.B.; Garza, L.A.A.; Kang, S.S.; et al. Characterization and analysis of the skin microbiota in rosacea: A case-control study. Am. J. Clin. Dermatol. 2020, 21, 139–147. [Google Scholar] [CrossRef]
- Thompson, K.G.G.; Rainer, B.M.M.; Antonescu, C.C.; Florea, L.L.; Mongodin, E.F.F.; Kang, S.S.; Chien, A.L. Comparison of the skin microbiota in acne and rosacea. Exp. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.R.; Farhat, M.M.; Na, J.J.; Li, R.R.; Wu, Y. Bacterial and fungal microbiome characterization in patients with rosacea and healthy controls. Br. J. Dermatol. 2020. [Google Scholar] [CrossRef]
- Woo, Y.R.R.; Lee, S.H.H.; Cho, S.H.H.; Lee, J.D.D.; Kim, H.S. Characterization and analysis of the skin microbiota in rosacea: Impact of systemic antibiotics. J. Clin. Med. 2020, 9, 185. [Google Scholar] [CrossRef]
- Yun, Y.Y.; Kim, H.N.N.; Chang, Y.Y.; Lee, Y.Y.; Ryu, S.S.; Shin, H.H.; Kim, W.S.S.; Kim, H.L.L.; Nam, J.H. Characterization of the blood microbiota in Korean females with rosacea. Dermatology 2019, 235, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.H.; Yun, Y.Y.; Kim, H.S.S.; Kim, H.N.N.; Jung, H.J.J.; Chang, Y.Y.; Ryu, S.; Shin, H.; Kim, H.L.; Kim, W.S. Rosacea and its association with enteral microbiota in Korean females. Exp. Dermatol. 2018, 27, 37–42. [Google Scholar] [CrossRef]
- Chen, Y.J.; Lee, W.H.; Ho, H.J.; Tseng, C.H.; Wu, C.Y. An altered fecal microbial profiling in rosacea patients compared to matched controls. J. Formos. Med. Assoc. 2020. [Google Scholar] [CrossRef]
- Barnard, E.; Shi, B.; Kang, D.; Craft, N.; Li, H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci. Rep. 2016, 6, 39491. [Google Scholar] [CrossRef] [PubMed]
- Jahns, A.C.; Lundskog, B.; Dahlberg, I.; Tamayo, N.C.; McDowell, A.; Patrick, S.; Alexeyev, O.A. No link between rosacea and Propionibacterium acnes. APMIS 2012, 120, 922–925. [Google Scholar] [CrossRef]
- Myles, I.A.; Earland, N.J.; Anderson, E.D.; Moore, I.N.; Kieh, M.D.; Williams, K.W.; Saleem, A.; Fontecilla, N.M.; Welch, P.A.; Darnell, D.A.; et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018, 3, e120608. [Google Scholar] [CrossRef]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Mantaring, J.B.V., 3rd; Yen, E.; Recto, M.S.T.; Sison, O.T.; Alejandria, M.M. Comparative effectiveness of probiotic strains for the treatment of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol. 2020. [Google Scholar] [CrossRef]
- Egeberg, A.; Weinstock, L.B.; Thyssen, E.P.; Gislason, G.H.; Thyssen, J.P. Rosacea and gastrointestinal disorders: A population-based cohort study. Br. J. Dermatol. 2017, 176, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Sato, N.; Ogihara, T.; Morita, K.; Ogawa, M.; Okayasu, I. Fusobacterium varium localized in the colonic mucosa of patients with ulcerative colitis stimulates species-specific antibody. J. Gastroenterol. Hepatol. 2002, 17, 849–853. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabarnero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Chilton, P.M.; Embry, C.A.; Mitchell, T.C. Effects of differences in Lipid A structure on TLR4 pro-inflammatory signaling and inflammasome activation. Front. Immunol. 2012, 3, 154. [Google Scholar] [CrossRef]
- Meisel, J.S.; Hannigan, G.D.; Tyldsley, A.S.; SanMiguel, A.J.; Hodkinson, B.P.; Zheng, Q.; Grice, E.A. Skin microbiome surveys are strongly influenced by experimental design. J. Investig. Dermatol. 2016, 136, 947–956. [Google Scholar] [CrossRef]
| Study/Country | Rosacea | Type of Rosacea | Control Group | Control | Remarks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age (Mean ± SD) (Years) | Females (%) | ETR (%) | PPR (%) | Other (%) | Number | Age (Mean ± SD) (Years) | Females (%) | |||||||
| Skin microbiome | |||||||||||||||
| Murillo et al., 2014 [12] */Germany | 30 | 50.86 ± 11.2 (ETR) 52.82 ± 13.08 (PPR) | N/A | 15 (50.0) | 15 (50.0) | - | Healthy volunteers | 17 | 52.82 ± 13.08 | N/A | Age- and sex-matched controls | ||||
| Zaidi et al., 2018 [13]/USA | 18 | 37.83 ± 10.62 | 17 (94.4) | N/A | N/A | N/A | Healthy twins | 42 | 36.36 ± 17.27 | 37 (88.1) | Twins discordant for rosacea | ||||
| Rainer et al., 2020 [14] **/USA | 19 | 48.5 ± 12.6 | 14 (73.7) | 11 (57.9) | 6 (31.6) | 2 (10.5) (ETR/PPR overlap) | Healthy volunteers | 19 | N/A | N/A | Age-, sex-, and race-matched controls | ||||
| Thompson et al., 2020 [15] **/USA | 19 | 48.5 ± 12.6 | 14 (73.7) | 11 (57.9) | 6 (31.6) | 2 (10.5) (ETR/PPR overlap) | Acne subjects | 8 | N/A | 7 (87.5) | |||||
| Wang et al., 2020 [16]/China | 36 | N/A | N/A | 21 (58.3) | 15 (41.7) | - | Healthy volunteers | 22 | N/A | N/A | Age- and sex-matched controls | ||||
| Woo et al., 2020 [17]/South Korea | 12 | N/A | 11 (91.7) | 12 (100.0) | - | - | Same group after taking oral antibiotics | 12 | N/A | 11 (91.7) | |||||
| Blood microbiome | |||||||||||||||
| Yun et al., 2019 [18]/South Korea | 10 | N/A | 10 (100.0) | N/A | N/A | N/A | Healthy volunteers | 30 | N/A | 30 (100.0) | Age-, sex- and BMI-matched controls | ||||
| Gut microbiome | |||||||||||||||
| Nam et al., 2018 [19]/South Korea | 12 | 42.58 ± 7.98 | 12 (100) | 6 (50.0) | 2 (16.7) | 4 (33.3) | Healthy controls | 251 | 43.02 ± 8.23 | 251 (100) | Age- and sex-matched controls | ||||
| Chen et al. 2020 [20]/Taiwan | 11 | 49.9 ± 11.3 | 10 (90.9) | 4 (36.3) | 7 (63.7) | - | Healthy controls | 110 | 50.6 ± 10.2 | 100 (90.9) | Age- and sex- matched controls | ||||
| Study | Sample | Sample Transportation and Storage Until Analysis | DNA Extraction | Microbiota Analysis Technique | Sequencing Target | Sequencing Platform | Data Analysis Platform | Reference Sequences Database |
|---|---|---|---|---|---|---|---|---|
| Skin microbiome | ||||||||
| Murillo et al., 2014 [12] * | Standardized skin surface biopsies on the malar crease | stored at −80 °C | QIAmp DNA Mini kit | 16S rRNA gene sequencing | - | Real-time PCR | ChromasPro | BLASTn nucleotide collection database |
| Zaidi et al., 2018 [13] | Sebutape strips from bilateral malar cheeks | N/A | MO-BIO PowerSoil DNA Isolation Kit | 16S rRNA gene sequencing | V3-V4 | Illumina MiSeq | QIIME | Greengenes database |
| Rainer et al., 2020 [14] ** | Skin swabs of the nose and bilateral cheeks | Sample tube containing Amies medium, stored at −80 °C | Zymo fecal DNA kit | Bacterial 16S rRNA gene sequencing | V3-V4 | Illumina MiSeq platform | QIIME1/MetaStats 2.0 | Greengenes database |
| Thompson et al., 2020 [15] ** | Skin swabs of the nose and bilateral cheeks | Sample tube containing Amies medium, stored at −80 °C | Zymo fecal DNA kit | Bacterial 16S r RNA gene sequencing | V3-V4 | Illumina MiSeq platform | QIIME1/MetaStats 2.0 | Greengenes database |
| Wang et al., 2020 [16] | Skin swabs from bilateral cheeks | N/A | Qiagen DNA extraction kit | ITS1 and 16S rRNA gene sequencing | N/A | Illumina HiSeq 2500 platform | QIIME 1.7.0 | N/A |
| Woo et al., 2020 [17] | Skin swabs of the nose and bilateral cheeks | N/A | ZR Fecal DNA MiniPrep | 16S rRNA gene sequencing | V3-V4 | Illumina HiSeq platform | CD-HIT-OUT analysis program QIIME v1.9 | BLASTN v2.4.0 National Center for Biotechnology Information 16S |
| Blood microbiome | ||||||||
| Yun et al., 2019 [18] | Whole blood collected by peripheral vein puncture | Stored at −4 °C | G-DEX IIb Genomic DNA Extraction Kit for Blood | 16S rRNA gene sequencing | V3-V4 | Illumina MiSeq platform | QIIME2 | GreenGenes database |
| Gut microbiome | ||||||||
| Nam et al., 2018 [19] | stool | N/A | MO-BIO PowerSoil DNA Isolation Kit | 16S rRNA gene sequencing | V3-V4 | Illumina MiSeq platform | QIIME 1.9 | Greengenes 13_8 database |
| Chen et al., 2020 [20] | stool | Transferred by using cooler bags, stored at −20 °C | Qiagen DNA isolation kit | Bacterial 16S rRNA gene sequencing | V3-V4 | Illumina MiSeq 2000 platform | USEARCH | Greengenes 13_5 database |
| Study | α-Diversity | β-Diversity |
|---|---|---|
| Skin microbiome | ||
| Zaidi et al., 2018 [13] | -No significant difference between monozygotic twin pairs with and without rosacea -Negative association with the severity of rosacea | -No distinct segregation between rosacea subjects and healthy controls -Greater weighted UniFrac distance between siblings in which one has rosacea than between siblings with rosacea and siblings without rosacea (not statistically significant) -monozygotic twins have more similar facial microbiome than dizygotic twins |
| Rainer et al., 2020 [14] | -Mean microbial α-diversity (total and within individual rosacea subtypes) higher in rosacea subjects than in controls, but the difference was not significant | -No significant difference (total and with regards to individual rosacea subtypes) |
| Thompson et al., 2020 [15] | -Significantly decreased skin microbial diversity in rosacea subjects, compared to acne patients | -Significant difference between rosacea patients and acne patients |
| Wang et al., 2020 [16] | -Bacterial microbiome: increased bacterial diversity in PPR, compared with controls -Fungal microbiome: no significant difference between rosacea subjects and healthy controls | -Bacterial microbiome: overlap between ETR and PPR, and incomplete separation from healthy controls -Fungal microbiome: no significant differences between rosacea subjects and controls |
| Woo et al., 2020 [17] | -no significant difference before and after treatment -no significant difference with age (≤60 versus >60) and rosacea severity (IGA3 versus IGA4) | -mild clustering of samples by patient and minimal clustering of samples by treatment |
| Blood microbiome | ||
| Yun et al., 2019 [18] | -no significant difference (Shannon Index, observed OTUs) -marginally significant difference (Faith’s phylogenetic diversity) | -significant difference (weighted and unweighted UniFrac) -partially separate clustering of the blood microbiota from rosacea subjects and controls (weighted UniFrac) |
| Gut microbiome | ||
| Nam et al., 2018 [19] | -No significant difference | -Significant difference |
| Chen et al., 2020 [20] | -Significantly decreased fecal microbial richness (number of observed OTUs and Chao 1) -No significant difference (Shannon Index) | -Significant difference |
| Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|
| Actinobacteria ↑ [15] a ↓ [12] ** [16] */** | Actinobacteria | Corynebacteriales | Corynebacteriaceae | Corynebacterium | Corynebacterium kroppenstedtii ↑ [14] ** |
| Actinomycetales | Propionibacteriaceae | Cutibacterium ↓ [16] */** | Cutibacterium acnes ↓ [14] ↑ [15] a | ||
| Cutibacterium granulosum ↓ [14] ** | |||||
| Gordoniaceae | Gordonia ↑ [13] | ||||
| Actinomycetaceae | Actinomyces | Actinomyces europaeus ↑ [14] ** | |||
| Bacteroidetes | Bacteroidetes | Bacteroidales | Prevotellaceae | Prevotella | Prevotella tannerae ↑ [14] ** |
| Prevotella intermedia ↑ [14] ** | |||||
| Porphyromonadaceae | Dysgonomonas | Dysgonomonas gadei ↓ [14] ** | |||
| Porphyromonas | Porphyromonas endodontalis ↓ [14] * | ||||
| Flavobacteriia | Flavobacteriales | Flavobacteriaceae | Chryseobacterium ↑ [13] | ||
| Wautersiella ↑ [13] | |||||
| Proteobacteria ↓ [15] a ↑ [12] ** | Alphaproteobacteria | Rhizobiales | Xanthobacteraceae | Azorhizobium | Azorhizobium doebereinerae ↓ [14] */** |
| Brucellaeceae | Ochrobactrum | Ochrobactrum grignonense ↑ [12] */** | |||
| Bartonellaceae | Bartonella ↑ [12] * | ||||
| Rhodospirillales | Acetobacteraceae | Roseomonas | Roseomonas mucosa ↓ [14] * | ||
| Betaproteobacteria | Burkholderiales | Oxalobacteraceae | Duganella | Duganella zoogloeoides ↑ [12] * | |
| Gammaproteobacteria | Alteromonadales | Shewanellaceae | Shewanella | Shewanella algae ↓ [14] */** | |
| Enterobacterales | Morganellaceae | Providencia | Providencia stuartii ↓ [14] */** | ||
| Yersiniaceae | Serratia | Serratia marcescens ↑ [15] a | |||
| Enterobacteriaceae | Escherichia ↑ [12] ** | ||||
| Pseudomonadales | Moraxellaceae | Acinetobacter | Acinetobacter pittii ↑ [12] ** | ||
| Pasteurellales | Pasteurellaceae | Haemophilus ↑ [12] * | |||
| Epsilonproteobacteria | Campylobacterales | Campylobacteraceae | Campylobacter | Campylobacter ureolyticus ↑ [14] ** | |
| Firmicutes ↑ [12] ** [16] */** | Bacilli | Bacillales | Bacillaceae | Anoxybacillus | Anoxybacillus kestanbolensis ↓ [14] ** |
| Geobacillus ↓ [13] | |||||
| Staphylococcaceae | Staphylococcus ↑ [16] */**(NS) | Staphylococcus hominis ↑ [12]*/** | |||
| Lactobacillales | Streptococcaceae | Streptococcus ↑ [16] ** | Streptococcus oralis ↑ [12] */** | ||
| Streptococcus pneumoniae ↑ [12] */** | |||||
| Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | Ruminococcus gnavus ↓ [14] * | |
| Lachnospiraceae | Blautia ↑ [13] |
| Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|
| Proteobacteria | Alphaproteobacteria | Sphingomonadales | Sphingomonadaceae | Sphingobium ↑ [18] |
| Rhodobacterales | Rhodobacteraceae | Paracoccus ↑ [18] | ||
| Rhodovulum ↑ [18] | ||||
| Gammaproteobacteria | Chromatiales | Chromatiaceae ↑ [18] | Rheinheimera ↑ [18] | |
| Alteromonadales | Alteromonadaceae | Marinobacter ↑ [18] | ||
| Enterobacterales | Enterobacteriaceae | Citrobacter ↑ [18] | ||
| Fusobacteria | Fusobacteriia | Fusobacteriales | Fusobacteriaceae ↑ [18] | Fusobacterium ↑ [18] |
| Firmicutes | Clostridia | Clostridiales | Tissierellaceae | Tissierellaceae family unknown genus ↑ [18] |
| Clostridiaceae | Clostridiaceae family unknown genus ↑ [18] | |||
| Verrucomicrobia | Spartobacteria | Cthnoniobacterales | Chtoniobacteraceae | Chtoniobacteraceae family unknown genus ↑ [18] |
| Euryarchaeota | Methanobacteria | Methanobacteriales | Methanobacteriaceae | Methanobacterium ↑ [18] |
| Armatimonadetes | Armatimonadia | Armatimonadales | Armatimonadaceae | Armatimonadaceae family unknown genus ↓ [18] |
| Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides ↑ [20] |
| Prevotellaceae | Prevotella ↓ [20] | |||
| CF231 ↑ [20] | ||||
| Fusobacteria | Fusobacteriia | Fusobacteriales | Fusobacteriaceae | Fusobacterium ↑ [20] |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Sutterellaceae | Sutterella ↓ [20] |
| Gammaproteobacteria | Pasteurellales | Pasteurellaceae | Haemophilus ↓ [20] | |
| Enterobacterales | Enterobacteriaceae | Citrobacter ↓ [20] [19] | ||
| Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio ↓ [19] | |
| Chlamydiae | Chlamydiae | Chlamydiales | Rhabdochlamydiaceae | Rhabdochlamydia ↑ [20] |
| Actinobacteria | Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium ↑ [20] |
| Coriobacteriales | Coriobacteriaceae | Slackia ↓ [19] | ||
| Firmicutes | Clostridia | Clostridiales | Clostridiaceae | Sarcina ↑ [20] |
| Clostridium ↓ [20] | ||||
| Ruminococcaceae | Ruminococcus ↑ [20] | |||
| Lachnospiraceae | Roseburia ↓ [20] | |||
| Peptococcaceae | Peptococcaceae family unknown genus ↓ [19] | |||
| Bacilli | Lactobacillales | Lactobacillaceae | Lactobacillus ↓ [20] | |
| Lactobacillales order unknown family unknown genus ↑ [19] | ||||
| Negativicutes | Selenomonadales | Veillonellaceae | Megasphaera ↓ [20] ↑ [19] | |
| Acidaminococcaceae | Acidaminococcus ↓ [20] ↑ [19] | |||
| Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | Coprobacillus ↓ [20] | |
| Euryarchaeota | Methanobacteria | Methanobacteriales | Methanobacteriaceae | Methanobrevibacter ↓ [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutka, K.; Żychowska, M.; Reich, A. Diversity and Composition of the Skin, Blood and Gut Microbiome in Rosacea—A Systematic Review of the Literature. Microorganisms 2020, 8, 1756. https://doi.org/10.3390/microorganisms8111756
Tutka K, Żychowska M, Reich A. Diversity and Composition of the Skin, Blood and Gut Microbiome in Rosacea—A Systematic Review of the Literature. Microorganisms. 2020; 8(11):1756. https://doi.org/10.3390/microorganisms8111756
Chicago/Turabian StyleTutka, Klaudia, Magdalena Żychowska, and Adam Reich. 2020. "Diversity and Composition of the Skin, Blood and Gut Microbiome in Rosacea—A Systematic Review of the Literature" Microorganisms 8, no. 11: 1756. https://doi.org/10.3390/microorganisms8111756
APA StyleTutka, K., Żychowska, M., & Reich, A. (2020). Diversity and Composition of the Skin, Blood and Gut Microbiome in Rosacea—A Systematic Review of the Literature. Microorganisms, 8(11), 1756. https://doi.org/10.3390/microorganisms8111756






