Arbo-Score: A Rapid Score for Early Identification of Patients with Imported Arbovirosis Caused by Dengue, Chikungunya and Zika Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Inclusion Criteria
- (1)
- presentation to the service no later than 2 weeks from the first day of fever
- (2)
- being tested for DENV, and/or CHIKV, and/or ZIKV
2.2. Definitions
2.3. Variables and Statistical Approach
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, K.S.; Chakravarti, A. Simultaneous detection of IgM antibodies against dengue and chikungunya: Coinfection or cross-reactivity? J. Fam. Med. Prim. Care 2019, 8, 2420–2423. [Google Scholar] [CrossRef]
- Patterson, J.; Sammon, M.; Garg, M. Dengue, Zika and Chikungunya: Emerging arboviruses in the New World. West J. Emerg. Med. 2016, 17, 671–679. [Google Scholar] [CrossRef]
- World Health Organization/Department of Control of Neglected Tropical Diseases and TDR. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-154787-1. [Google Scholar]
- Graham, B.S.; Repik, P.M.; Yactayo, S. Chikungunya in the Americas: Recommendations and conclusions. J. Infect. Dis. 2016, 214, 510–513. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.L.R.; Bezerra, J.M.T.; Said, R.F.C.; Gama Neto, A.N.D.; Cotrim, E.C.; Mendez, D.; Amâncio, F.F.; Carneiro, M. Emergence of chikungunya and Zika in a municipality endemic to dengue, Santa Luzia, MG, Brazil, 2015–2017. Revista Sociedade Brasileira Medicina Tropical 2019, 52, e20180347. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, C.; Remoli, M.E.; Rizzo, C.; Benedetti, E.; Fiorentini, C.; Bella, A.; Argentini, C.; Farchi, F.; Castilletti, C.; Capobianchi, M.R.; et al. Imported arboviral infections in Italy, July 2014–October 2015: A national reference laboratory report. BMC Infect. Dis. 2017, 17, 216. [Google Scholar] [CrossRef]
- Añez, G.; Rios, M. Dengue in the United States of America: A worsening scenario? BioMed Res. Int. 2013, 2013, 678645. [Google Scholar] [CrossRef]
- Succo, T.; Leparc Goffart, I.; Ferré, J.B.; Roiz, D.; Broche, B.; Maquart, M.; Noel, H.; Catelinois, O.; Entezam, F.; Caire, D.; et al. Autochthonous dengue outbreak in Nîmes, South of France, July to September 2015. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Rezza, G. Chikungunya is back in Italy: 2007–2017. J. Travel Med. 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease prevention and Control. Autochthonous Cases of Dengue in Spain and France; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2019. [Google Scholar]
- Angelo, K.M.; Stoney, R.J.; Brun Cottan, G.; Leder, K.; Grobusch, M.P.; Hochberg, N.; Kuhn, S.; Bottieau, E.; Schlagenhauf, P.; Chen, L.; et al. Zika among international travellers presenting to GeoSentinel sites, 2012–2019: Implications for clinical practice. J. Travel Med. 2020, 27, taaa061. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Zika Virus Disease in Var department, France; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2019. [Google Scholar]
- Sabino, E.C.; Loureiro, P.; Lopes, M.E.; Capuani, L.; McClure, C.; Chowdhury, D.; Di-Lorenzo Oliveira, C.; Oliveira, L.C.; Linnen, J.M.; Lee, T.H.; et al. International component of the NHLBI recipient epidemiology and donor evaluation Study-III. transfusion-transmitted Dengue and associated clinical symptoms during the 2012 epidemic in Brazil. J. Infect. Dis. 2016, 213, 694–702. [Google Scholar] [CrossRef]
- Venturi, G.; Zammarchi, L.; Fortuna, C.; Remoli, M.E.; Benedetti, E.; Fiorentini, C.; Trotta, M.; Rizzo, C.; Mantella, A.; Rezza, G.; et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Eurosurveillance 2016, 21, 30148. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Sexual Transmission of Dengue in Spain; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2019. [Google Scholar]
- Lagi, F.; Zammarchi, L.; Strohmeyer, M.; Bartalesi, F.; Mantella, A.; Meli, M.; Blanc, P.; Tacconi, D.; Farese, A.; Zanelli, G.; et al. Imported dengue fever in Tuscany, Italy, in the period 2006 to 2012. J. Travel Med. 2014, 21, 340–343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zammarchi, L.; Spinicci, M.; Bartoloni, A. Zika Virus: A review from the virus basics to proposed management strategies. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016056. [Google Scholar] [CrossRef]
- Paixão, E.S.; Rodrigues, L.C.; Costa, M.D.C.N.; Itaparica, M.; Barreto, F.; Gérardin, P.; Teixeira, M.G. Chikungunya chronic disease: A systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 301–316. [Google Scholar] [CrossRef]
- Viennet, E.; Ritchie, S.A.; Williams, C.R.; Faddy, H.M.; Harley, D. Public health responses to and challenges for the control of Dengue transmission in high-income countries: Four case studies. PLoS Negl. Trop. Dis. 2016, 10, e0004943. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.P.; Montalvo, T.; Bueno Marí, R.; Romero Tamarit, A.; Prats Uribe, A.; Fernández, L.; Camprubí, E.; Del Baño, L.; Peracho, V.; Figuerola, J.; et al. Zika working group in Barcelona. Imported Zika virus in a European city: How to prevent local transmission? Front. Microbiol. 2017, 8, 1319. [Google Scholar] [CrossRef]
- Gobbi, F.; Capelli, G.; Angheben, A.; Giobbia, M.; Conforto, M.; Franzetti, M.; Cattelan, A.M.; Raise, E.; Rovere, P.; Mulatti, P.; et al. Summer fever study group. Human and entomological surveillance of West Nile fever, Dengue and Chikungunya in Veneto Region, Italy, 2010–2012. BMC Infect. Dis. 2014, 14, 60. [Google Scholar] [CrossRef]
- Natrajan, M.S.; Rojas, A.; Waggoner, J.J. Beyond fever and pain: Diagnostic methods for Chikungunya virus. J. Clin. Microbiol. 2019, 57, e00350-19. [Google Scholar] [CrossRef]
- Theel, S.; Hata, D.J. Diagnostic testing for Zika virus: A postoutbreak update. J. Clin. Microbiol. 2018, 56, e01972. [Google Scholar] [CrossRef]
- Rodriguez Manzano, J.; Chia, P.Y.; Yeo, T.W.; Holmes, A.; Georgiou, P.; Yacoub, S. Improving Dengue diagnostics and management through innovative technology. Curr. Infect. Dis. Rep. 2018, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Zammarchi, L.; Colao, M.G.; Mantella, A.; Capobianco, T.; Mazzarelli, G.; Ciccone, N.; Tekle Kiros, S.; Mantengoli, E.; Rossolini, G.M.; Bartoloni, A. Evaluation of a new rapid fluorescence immunoassay for the diagnosis of dengue and Zika virus infection. J. Clin. Virol. 2019, 112, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Magurano, F.; Zammarchi, L.; Baggieri, M.; Fortuna, C.; Farese, A.; Benedetti, E.; Fiorentini, C.; Rezza, G.; Nicoletti, L.; Bartoloni, A. Chikungunya from the Caribbean: The importance of appropriate laboratory tests to confirm the diagnosis. Vector Borne Zoonotic Dis. 2015, 15, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Andries, A.C.; Duong, V.; Ngan, C.; Ong, S.; Huy, R.; Sroin, K.K.; Te, V.; Bunthin, Y.; Try, P.L.; Buchy, P. Field evaluation and impact on clinical management of a rapid diagnostic kit that detects dengue NS1, IgM and IgG. PLoS Negl. Trop. Dis 1993, 6, e1993. [Google Scholar] [CrossRef]
- Estrela, P.F.N.; Mendes, G.M.; De Oliveira, K.G.; Bailão, A.M.; Soares, C.M.A.; Assunção, N.A.; Duarte, G.R.M. Ten-minute direct detection of Zika virus in serum samples by RT-LAMP. J. Virol. Methods 2019, 271, 113675. [Google Scholar] [CrossRef]
- Hu, S.F.; Li, M.; Zhong, L.L.; Lu, S.M.; Liu, Z.X.; Pu, J.Y.; Wen, J.S.; Huang, X. Development of reverse-transcription loop-mediated isothermal amplification assay for rapid detection and differentiation of dengue virus serotypes 1-4. BMC Microbiol. 2015, 15, 265. [Google Scholar] [CrossRef]
- Italian Ministry of Health. 2020–2025 Italian National Arboviroses Prevention and Surveillance Plan. Available online: http://www.statoregioni.it/media/2371/p-1-csr-rep-n-1-15gen2020.pdf (accessed on 23 June 2020).
- Sullivan, L.M.; Massaro, J.M.; D’Agostino, R.B. Presentation of multivariate data for clinical use: The Framingham study risk score functions. Stat. Med. 2004, 23, 1631–1660. [Google Scholar] [CrossRef]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef]
- Wilson, M. Fever in returned travelers. In Travel Medicine, 2nd ed.; Keystone, J., Kozarsky, P.E., Freedman, D.O., Nothdurft, H.D., Connor, B.A., Eds.; Elsevier: Philadelphia, PA, USA, 2008; pp. 513–521. ISBN 978-0-323-03453-1. [Google Scholar]
- Lazzarini, L.; Barzon, L.; Foglia, F.; Manfrin, V.; Pacenti, M.; Pavan, G.; Rassu, M.; Capelli, G.; Montarsi, F.; Martini, S.; et al. First autochthonous dengue outbreak in Italy, August 2020. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef]
- Vermeulen, T.D.; Reimerink, J.; Reusken, C.; Giron, S.; de Vries, P.J. Autochthonous dengue in two Dutch tourists visiting Département Var, southern France, July 2020. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef]
- Mary, E.; Wilson, M.D.; Chen, L.H. Re-starting travel in the era of COVID-19: Preparing anew. J. Travel Med. 2020, 27, taaa108. [Google Scholar] [CrossRef]
- Spinicci, M.; Bartoloni, A.; Mantella, A.; Zammarchi, L.; Rossolini, G.M.; Antonelli, A. Low risk of serological cross-reactivity between dengue and COVID-19. Memórias Instituto Oswaldo Cruz 2020, 115, e200225. [Google Scholar] [CrossRef] [PubMed]
- Moulin, E.; Selby, K.; Cherpillod, P.; Kaiser, L.; Boillat Blanco, N. Simultaneous outbreaks of dengue, chikungunya and Zika virus infections: Diagnosis challenge in a returning traveller with nonspecific febrile illness. New Microbes New Infect. 2016, 11, 6–7. [Google Scholar] [CrossRef]
- Tilli, M.; Botta, A.; Bartoloni, A.; Corti, G.; Zammarchi, L. Hospitalization for Chagas disease, dengue, filariasis, leishmaniasis, schistosomiasis, strongyloidiasis, and Taenia solium taeniasis/cysticercosis, Italy, 2011–2016. Infection 2020, 48, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, G.; Trentini, F.; Poletti, P.; Baldacchino, F.A.; Montarsi, F.; Capelli, G.; Rizzoli, A.; Rosà, R.; Merler, S.; Melegaro, A. Effectiveness and economic assessment of routine larviciding for prevention of chikungunya and dengue in temperate urban settings in Europe. PLoS Negl. Trop. Dis. 2017, 11, e0005918. [Google Scholar] [CrossRef]
- Erra, E.O.; Korhonen, E.M.; Voutilainen, L.; Huhtamo, E.; Vapalahti, O.; Kantele, A. Dengue in travelers: Kinetics of viremia and NS1 antigenemia and their associations with clinical parameters. PLoS ONE 2013, 8, e65900. [Google Scholar] [CrossRef]
- Chan, M.; Johansson, M.A. The incubation periods of Dengue viruses. PLoS ONE 2012, 7, e50972. [Google Scholar] [CrossRef] [PubMed]
- Rubio, E.; Alejo, C.I.; Aylagas, C.; Camprubí, D.; Ferré, R.; Albarracín, M.R.; Gonzalo, V.; Barrachina, J.; Álvarez, M.M.J.; Valls, M.E.; et al. Diagnostic value of Platelet and Leukocyte counts in the differential diagnosis of fever in the returning traveler. Am. J. Trop. Med. Hyg. 2019, 100, 470–475. [Google Scholar] [CrossRef]
- Lee, I.K.; Liu, J.W.; Chen, Y.H.; Chen, Y.C.; Tsai, C.Y.; Huang, S.Y.; Lin, C.Y.; Huang, C.H. Development of a simple clinical risk score for early prediction of severe Dengue in adult patients. PLoS ONE 2016, 11, e0154772. [Google Scholar] [CrossRef]
- Lee, V.J.; Chow, A.; Zheng, X.; Carrasco, L.R.; Cook, A.R.; Lye, D.C.; Ng, L.C.; Leo, Y.S. Simple clinical and laboratory predictors of Chikungunya versus dengue infections in adults. PLoS Negl. Trop. Dis. 2012, 6, e1786. [Google Scholar] [CrossRef] [PubMed]
- Eckerle, I.; Briciu, V.T.; Ergönül, Ö.; Lupşe, M.; Papa, A.; Radulescu, A.; Tsiodras, S.; Tsitou, C.; Drosten, C.; Nussenblatt, V.R.; et al. Emerging souvenirs-clinical presentation of the returning traveller with imported arbovirus infections in Europe. Clin. Microbiol. Infect. 2018, 24, 240–245. [Google Scholar] [CrossRef]
| Control N = 56 (%) | Case N = 34 (%) | p-Value | |
|---|---|---|---|
| Gender | 0.115 | ||
| Male | 31 (55.4) | 13 (38.2) | |
| Female | 25 (44.6) | 21 (61.8) | |
| Age in years | 0.307 | ||
| 15–29 | 22 (39.3) | 8 (23.5) | |
| 30–49 | 21 (37.5) | 16 (47.1) | |
| 50–70 | 13 (23.2) | 10 (29.4) | |
| Region of Birth | 0.156 | ||
| Europe | 47 (83.9) | 28 (82.3) | |
| Sub-Saharan Africa | 4 (7.1) | 0 | |
| Middle South-Asia | 1 (1.8) | 2 (5.9) | |
| South-East Asia | 1 (1.8) | 0 | |
| South America | 1 (1.8) | 3 (8.8) | |
| North America | 2 (3.6) | 0 | |
| Central America | 0 | 1 (2.9) | |
| Cause of travel | 0.856 | ||
| Tourist | 49 (87.5) | 31 (91.2) | |
| Migrant | 2 (3.6) | 1 (2.9) | |
| VRF | 5 (8.9) | 2 (5.9) | |
| Days between return and onset of symptoms | |||
| (median, IQR) | 1 (0–4) | 1 (0–4) | 0.426 |
| Length of journey in days § | 15 (10–21.5) | 15 (11–21) | 0.896 |
| Days between onset of symptoms and first medical visit | |||
| (median, IQR) | 4 (2–7) | 4.5 (3–7) | 0.230 |
| Returning Continent | 0.024 | ||
| Sub-Saharan Africa | 13 (23.2) | 1 (2.9) | |
| North Africa | 1 (1.8) | 0 | |
| Middle East | 1 (1.8) | 0 | |
| Middle-South Asia | 8 (14.3) | 4 (11.8) | |
| Southeast Asia | 21 (37.5) | 10 (29.4) | |
| South America | 6 (10.7) | 6 (17.6) | |
| Central America | 6 (10.7) | 12 (35.3) | |
| Oceania | 0 | 1 (2.9) | |
| People returning from Africa | 14 (25.0) | 1 (2.9) | 0.006 |
| Myalgia | 17 (30.4) | 21 (61.8) | 0.003 |
| Rachialgia | 14 (25.0) | 13 (38.2) | 0.184 |
| Headache | 28 (50.0) | 17 (50.0) | 1.000 |
| Retro-orbital pain | 6 (10.7) | 12 (35.3) | 0.005 |
| Conjunctival hyperaemia | 2 (3.6) | 7 (20.6) | 0.009 |
| Gastrointestinal symptoms * | 19 (33.9) | 11 (32.3) | 0.878 |
| Respiratory symptoms ** | 26 (46.4) | 6 (17.6) | 0.006 |
| Disgeusia | 0 | 5 (14.7) | 0.003 |
| Rash | 8 (14.3) | 23 (67.6) | 0.000 |
| Arthritis | 0 | 4 (11.8) | 0.009 |
| Arthralgia | 13 (23.2) | 10 (29.4) | 0.513 |
| Leukocytes/mcL, median (IQR) | 6365 (4925–9310) | 3725 (2360–5340) | 0.000 |
| Leukopenia < 4000/mcL | 4 (7.1) | 20 (58.8) | 0.000 |
| Neutrophil count §§ median (IQR) | 3710 (2850–6310) § | 2146.5 (1245–3360) § | 0.000 |
| Platelets × 103/mcL, median (IQR) | 187 (148–236.5) | 145.5 (108–183) | 0.010 |
| Thrombocytopenia < 140.000/mcL | 11 (19.6) | 13 (38.2) | 0.053 |
| ALT > 60 U/L | 11 (19.6) | 12 (35.3) | 0.099 |
| ALT (U/L) median (IQR) | 33.5 (24–49) | 34.5 (23–91) | 0.516 |
| CRP > 9 mg/L §§§ | 34 (70.8) §§ | 8 (33.3) §§ | 0.002 |
| DENV N = 22 (%) | CHIKV N = 4 (%) | ZIKV N = 8 (%) | |
|---|---|---|---|
| Gender | |||
| Male | 9 (40.9) | 1 (25.0) | 3 (37.5) |
| Female | 13 (59.1) | 3 (75.0) | 5 (62.5) |
| Age in years | |||
| 15–29 | 5 (22.7) | 0 | 3 (37.5) |
| 30–49 | 10 (45.4) | 2 (50.0) | 4 (50.0) |
| 50–70 | 7 (31.8) | 2 (50.0) | 1 (12.5) |
| Continent of Birth | |||
| Europe | 19 (86.4) | 1 (25.0) | 8 (100.0) |
| Middle-South Asia | 2 (9.1) | 0 | 0 |
| South America | 0 | 3 (75.0) | 0 |
| Central America | 1 (4.5) | 0 | 0 |
| Returning continent | |||
| Sub-Saharan Africa | 1 (4.5) | 0 | 0 |
| Middle-South Asia | 4 (18.2) | 0 | 0 |
| Southeast Asia | 9 (40.9) | 0 | 1 (12.5) |
| South America | 2 (9.1) | 2 (50.0) | 2 (25.0) |
| Central America | 6 (27.3) | 2 (50.0) | 4 (50.0) |
| Oceania | 0 | 0 | 1 (12.5) |
| Myalgia | 17 (77.3) | 0 | 4 (50.0) |
| Rachialgia | 10 (45.4) | 1 (25.0) | 2 (25.0) |
| Retro-orbital pain | 8 (36.4) | 0 | 4 (50.0) |
| Conjunctival hyperaemia | 1 (4.5) | 2 (50.0) | 4 (50.0) |
| Gastrointestinal symptoms * | 9 (40.9) | 2 (50.0) | 0 |
| Respiratory symptoms ** | 4 (18.2) | 1 (25.0) | 1 (12.5) |
| Disgeusia | 5 (22.7) | 0 | 0 |
| Rash | 11 (50.0) | 4 (100.0) | 8 (100.0) |
| Arthritis | 0 | 3 (75.0) | 1 (12.5) |
| Arthralgia | 3 (13.6) | 4 (100) | 3 (37.5) |
| Leukocytes/mcL median (IQR) | 3090 (2120–3910) | 5240 (3645–6590) | 4450 (3985–7195) |
| Leukopenia < 4000/mcL | 17 (77.3) | 1 (25.0) | 2 (25.0) |
| Neutrophil count § median [IQR] | 1418 (965–2700) § | 2970 (1975–3755) § | 2540 (2146–4194) § |
| Thrombocytopenia < 140.000/mcL | 10 (45.4) | 0 | 3 (37.5) |
| Platelets × 103/mcL median (IQR) | 142 (88–169) | 349.5 (278–414.5) | 158 (137–175.5) |
| ALT > 60 U/L | 11 (50.0) | 1 (25.0) | 0 |
| ALT (U/L) median [IQR] | 60 (25–105) | 40 (19.5–65.5) | 21 (15.5–31) |
| CRP >9 mg/L §§ | 5 (29.4) §§ | 2 (66.7) §§ | 1 (25.0) |
| Variables | ORa (95% CI) | p | Regression Coefficient | Risk Score Weight |
|---|---|---|---|---|
| Rash | 23.46 (2.79–196.88) | 0.004 | 3.15 | 1 |
| Thrombocytopenia | 0.47 (0.06–3.55) | 0.463 | −0.76 | na |
| Leukopenia | 54.93 (4.56–661.57) | 0.002 | 4.01 | 2 |
| Hypertransaminasemia | 9.41 (1.23–71.66) | 0.031 | 2.24 | 1 |
| People returning from Africa | 0.04 (0.00–12.18) | 0.278 | −3.10 | na |
| Retro-orbital pain | 2.82 (0.35–22.90) | 0.331 | 1.04 | na |
| Conjunctival hyperemia | 0.80 (0.07–9.52) | 0.862 | −0.22 | na |
| Myalgia | 13.48 (1.97–92.17) | 0.008 | 2.60 | 1 |
| Respiratory symptoms | 0.10 (0.01–0.74) | 0.024 | −2.26 | −1 |
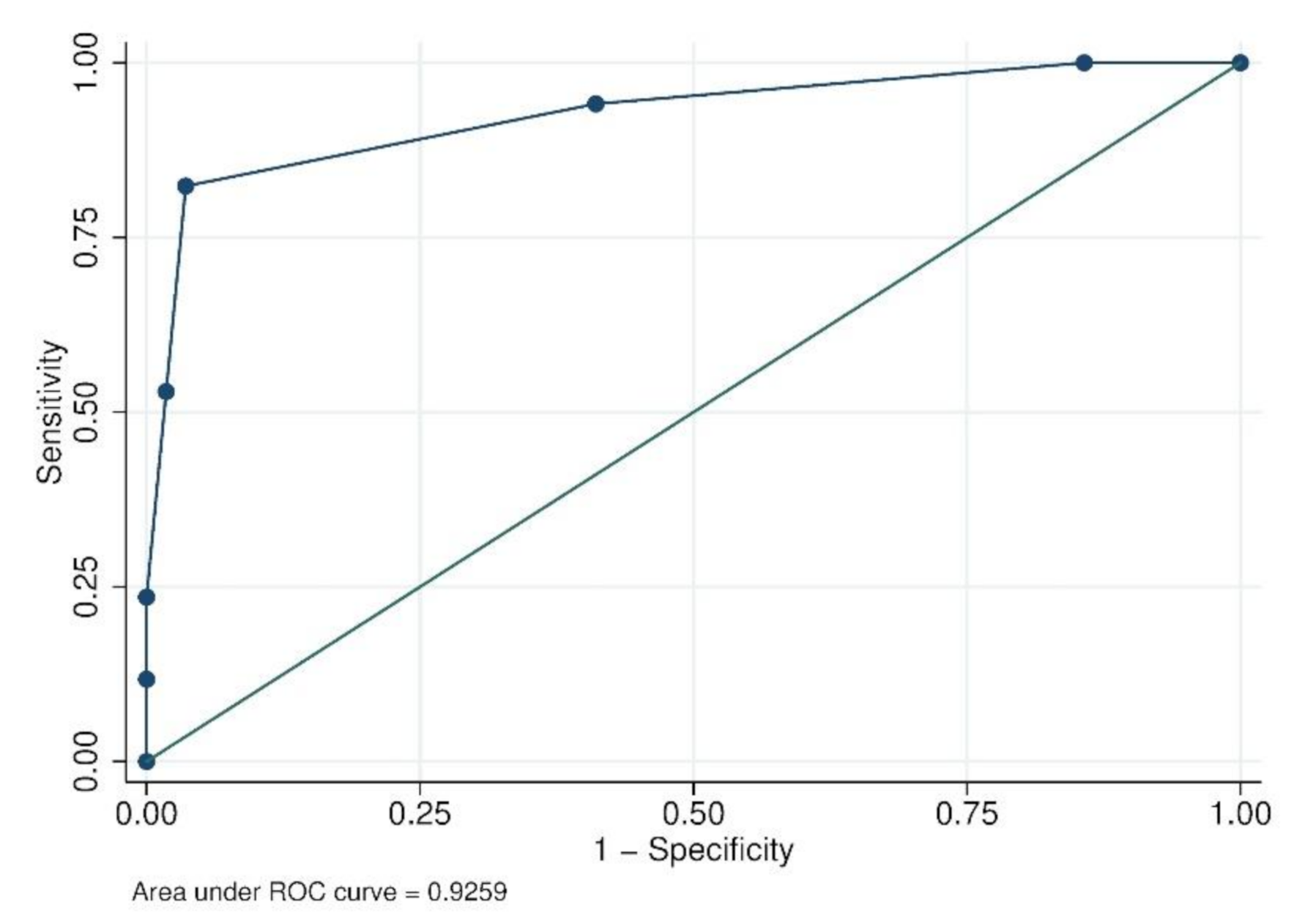
| Cut-off Point | Sensibility (%) | Specificity (%) | Youden Index |
|---|---|---|---|
| ≥−1 | 100.00 | 0.00 | 0 |
| ≥0 | 100.00 | 14.29 | 0.14 |
| ≥1 | 94.12 | 58.93 | 0.53 |
| ≥2 | 82.35 | 96.43 | 0.79 |
| ≥3 | 52.94 | 98.21 | 0.51 |
| ≥4 | 23.53 | 100.00 | 0.23 |
| ≥5 | 11.76 | 100.00 | 0.12 |
| >5 | 0 | 100.00 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellere, I.; Lagi, F.; Spinicci, M.; Mantella, A.; Mantengoli, E.; Corti, G.; Colao, M.G.; Gobbi, F.; Rossolini, G.M.; Bartoloni, A.; et al. Arbo-Score: A Rapid Score for Early Identification of Patients with Imported Arbovirosis Caused by Dengue, Chikungunya and Zika Virus. Microorganisms 2020, 8, 1731. https://doi.org/10.3390/microorganisms8111731
Vellere I, Lagi F, Spinicci M, Mantella A, Mantengoli E, Corti G, Colao MG, Gobbi F, Rossolini GM, Bartoloni A, et al. Arbo-Score: A Rapid Score for Early Identification of Patients with Imported Arbovirosis Caused by Dengue, Chikungunya and Zika Virus. Microorganisms. 2020; 8(11):1731. https://doi.org/10.3390/microorganisms8111731
Chicago/Turabian StyleVellere, Iacopo, Filippo Lagi, Michele Spinicci, Antonia Mantella, Elisabetta Mantengoli, Giampaolo Corti, Maria Grazia Colao, Federico Gobbi, Gian Maria Rossolini, Alessandro Bartoloni, and et al. 2020. "Arbo-Score: A Rapid Score for Early Identification of Patients with Imported Arbovirosis Caused by Dengue, Chikungunya and Zika Virus" Microorganisms 8, no. 11: 1731. https://doi.org/10.3390/microorganisms8111731
APA StyleVellere, I., Lagi, F., Spinicci, M., Mantella, A., Mantengoli, E., Corti, G., Colao, M. G., Gobbi, F., Rossolini, G. M., Bartoloni, A., & Zammarchi, L. (2020). Arbo-Score: A Rapid Score for Early Identification of Patients with Imported Arbovirosis Caused by Dengue, Chikungunya and Zika Virus. Microorganisms, 8(11), 1731. https://doi.org/10.3390/microorganisms8111731








