Cryptosporidium and Colon Cancer: Cause or Consequence?
Abstract
1. Introduction
2. Infection, an Important Cause of Cancer
- The periods between primary infection and malignant transformation are frequently very long [7].
- Even if the majority of the infectious agents associated to human cancers are ubiquitous and common in the human population, only a small proportion of infected individuals develops cancer.
- Some infections are linked to cancer development as associated risk factors [8].
- Infectious agents act mainly as indirect oncogenes, without persistence of their genes within the respective cancer host cells. The most common indirect infectious carcinogens are agents causing immunosuppression, such as Human Immunodeficiency Virus (HIV) leading to Kaposi’s sarcoma, or inflammation caused by the bacteria Helicobater pylori, the trematode Schistosoma hematobium and the Hepatitis C and B viruses [7].
- The main mechanisms by which infectious agents promote cancer are not necessarily involving direct mutagenesis, but instead are due to the complex interactions between hosts and pathogens [8].
- An infectious agent may trigger the initial events of oncogenesis while being absent in the final tumor [7].
- Pathogens associated with cancer are directing pathogen-driven processes leading to cell transformation. However, many non-oncogenic pathogens can also regulate these processes, indicating that other factors must be involved [8].
- In the cases of viruses, oncogenesis can occur through the persistence of the viral genome in a latent form in an infected host cell, either without replication or through integration of the viral genome into a host-cell chromosome [8].
- Koch’s postulates for proving a causal connection between a particular infectious agent and a disease cannot be applied to many human diseases as it would be unethical to experimentally infect humans with a potentially lethal infectious agent [8].
- Existing diagnostic tools may not be sensitive enough to link infectious agents with cancer development or testing may occur too long after the exposure [9].
3. The Special Case of Cryptosporidium: A Public Health Issue
4. Cryptosporidium and Cancer: A Growing Body of Evidence
4.1. Clinical Studies in Humans
4.2. Natural or Experimental Infection in Animals
4.3. In Vitro Models
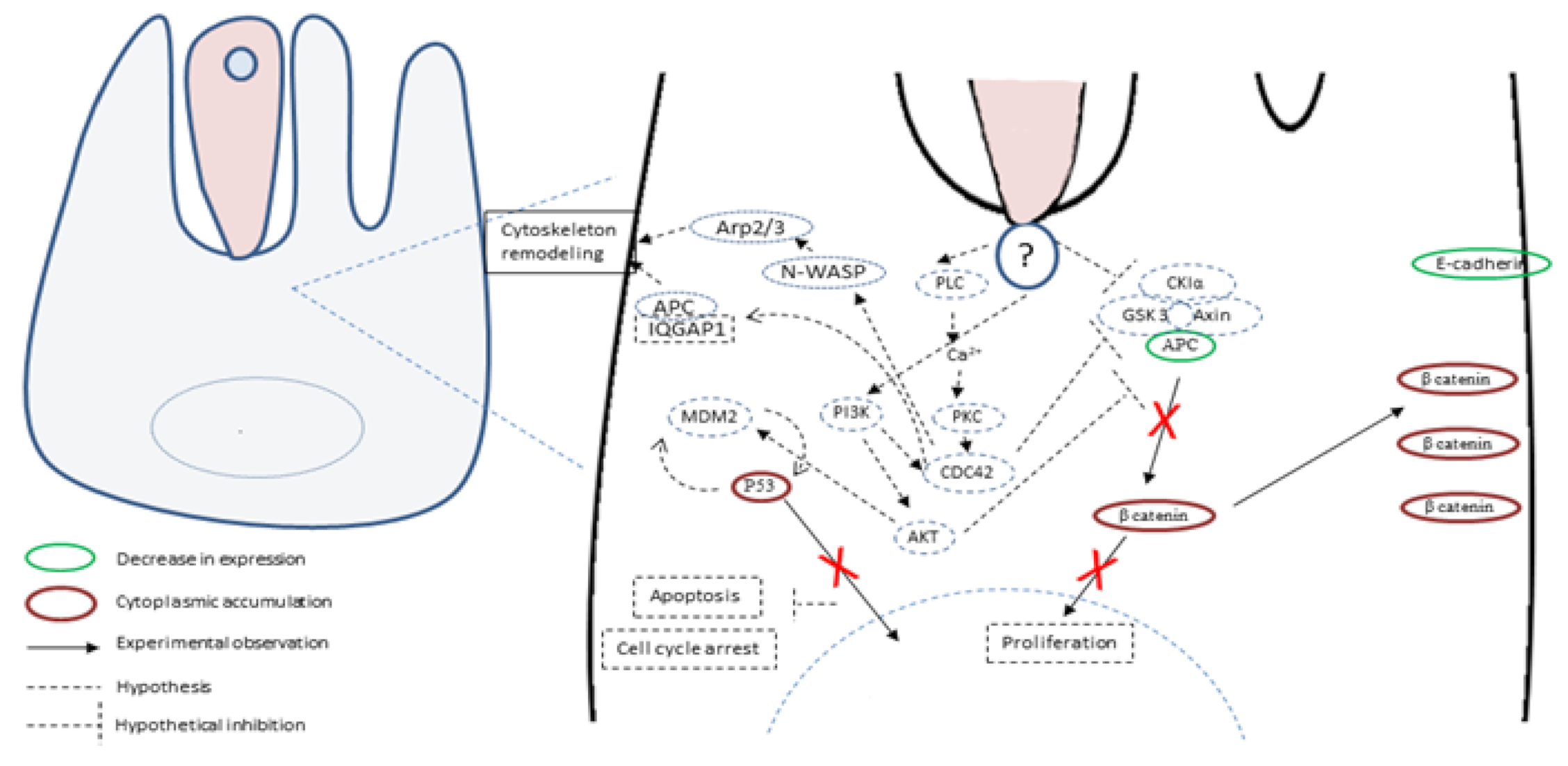
5. Hypotheses about Molecules and Mechanisms Involved in the Induction of Tumorigenesis by Cryptosporidium
6. Conclusions and Future Directions
7. Search Strategy and Selection Criteria
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.; Certad, G.; Weitzman, J.B. Parasites et cancer: Existe-t-il un lien? Méd./Sci. 2016, 32, 867–873. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whiteman, D.C.; Wilson, L.F. The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiol. 2016, 44, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Benamrouz, S.; Conseil, V.; Creusy, C.; Calderon, E.; Dei-Cas, E.; Certad, G. Parasites and malignancies, a review, with emphasis on digestive cancer induced by Cryptosporidium parvum (Alveolata: Apicomplexa). Parasite 2012, 19, 101–115. [Google Scholar] [CrossRef]
- Bañuls, A.L.; Thomas, F.; Renaud, F. Of parasites and men. Infect. Genet. Evol. 2013, 20, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Zur Hausen, H. The search for infectious causes of human cancers: Where and why. Virology 2009, 392, 1–10. [Google Scholar] [CrossRef]
- Dalton-Griffin, L.; Kellam, P. Infectious causes of cancer and their detection. J. Biol. 2009, 8, 67. [Google Scholar] [CrossRef]
- O’Connor, S.M.; Taylor, C.E.; Hughes, J.M. Emerging Infectious Determinants of Chronic Diseases. Emerg. Infect. Dis. 2006, 12, 1051–1057. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Biological Agents, Volume 100 B, a Review of Human Carcinogens Lyon, France. 2012. Available online: http://monographs.iarc.fr (accessed on 1 August 2020).
- Ewald, P.W.; Swain Ewald, H.A. Infection and cancer in multicellular organisms. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140224. [Google Scholar] [CrossRef]
- Stark, J.R.; Judson, G.; Alderete, J.F.; Mundodi, V.; Kucknoor, A.S.; Giovannucci, E.L.; Platz, E.A.; Sutcliffe, S.; Fall, K.; Kurth, T.; et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians’ Health Study. J. Natl. Cancer Inst. 2009, 101, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, W.; Wang, H.; Wang, Y.; Li, J.; Wu, X. Trichomonas vaginalis infection-associated risk of cervical cancer: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Dubey, M.L.; Malla, N. Association of parasitic infections and cancers. Indian J. Med. Microbiol. 2005, 23, 74–79. [Google Scholar] [PubMed]
- Marsolier, J.; Perichon, M.; DeBarry, J.D.; Villoutreix, B.O.; Chluba, J.; Lopez, T.; Garrido, C.; Zhou, X.Z.; Lu, K.P.; Fritsch, L.; et al. Theileria parasites secrete a prolyl isomerase to maintain host leukocyte transformation. Nature 2015, 520, 378–382. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Deroubaix, S.; Feldhahn, N.; Oliveira, T.Y.; Callen, E.; Wang, Q.; Jankovic, M.; Silva, I.T.; Rommel, P.C.; Bosque, D.; et al. Plasmodium infection promotes genomic instability and AID-dependent B cell lymphoma. Cell 2015, 162, 727–737. [Google Scholar] [CrossRef]
- Checkley, W.; White, A.C.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect. Dis. 2015, 15, 85–94. [Google Scholar] [CrossRef]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Putignani, L.; Menichella, D. Global distribution, public health and clinical impact of the protozoan pathogen Cryptosporidium. Interdiscip. Perspect. Infect. Dis. 2010, 2010, 753512. [Google Scholar] [CrossRef]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef]
- Bhalchandra, S.; Cardenas, D.; Ward, H.D. Recent breakthroughs and ongoing limitations in Cryptosporidium research. F1000Research 2018, 7, 1380. [Google Scholar] [CrossRef]
- Lilja, M.; Widerström, M.; Lindh, J. Persisting post-infection symptoms 2 years after a large waterborne outbreak of Cryptosporidium hominis in northern Sweden. BMC Res. Notes 2018, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Gibson, A.R.; Striepen, B. Cryptosporidium. Curr. Biol. 2018, 28, R193–R194. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Troeger, C.; Rao, P.C.; Blacker, B.F.; Brown, A.; Brewer, T.G.; Colombara, D.V.; De Hostos, E.L.; Engmann, C.; Guerrant, R.L.; et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: A meta-analyses study. Lancet Glob. Health 2018, 6, e758–e768. [Google Scholar] [CrossRef]
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Widerström, M.; Schönning, C.; Lilja, M.; Lebbad, M.; Ljung, T.; Allestam, G.; Ferm, M.; Björkholm, B.; Hansen, A.; Hiltula, J.; et al. Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg. Infect. Dis. 2014, 20, 581–589. [Google Scholar]
- Corso, P.S.; Kramer, M.H.; Blair, K.A.; Addiss, D.G.; Davis, J.P.; Haddix, A.C. Cost of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg. Infect. Dis. 2003, 9, 426–431. [Google Scholar] [CrossRef]
- Mac Kenzie, W.R.; Hoxie, N.J.; Proctor, M.E.; Gradus, M.S.; Blair, K.A.; Peterson, D.E.; Kazmierczak, J.J.; Addiss, D.G.; Fox, K.R.; Rose, J.B.; et al. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 1994, 331, 161–167. [Google Scholar] [CrossRef]
- Baldursson, S.; Karanis, P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks—An update 2004–2010. Water Res. 2011, 45, 6603–6614. [Google Scholar] [CrossRef]
- Efstratiou, A.; Ongerth, J.E.; Karanis, P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks—An update 2011–2016. Water Res. 2017, 114, 14–22. [Google Scholar] [CrossRef]
- CDC. Crypto Outbreaks Linked to Swimming Have Doubled since 2014. 2017. Available online: https://www.cdc.gov/media/releases/2017/p0518-Cryptosporidium-outbreaks.html (accessed on 1 August 2020).
- Izquierdo, J.; Antúnez, I.; Calderón, M.T.; Pérez Giraldo, C.; Muñoz Sanz, A. Diarrhea caused by Cryptosporidium and colonic neoplasia]. Rev. Clínica Española 1988, 182, 393–394. [Google Scholar]
- Souza, L.D.R.D.; Rodrigues, M.A.M.; Morceli, J.; Kemp, R.; Mendes, R.P. Cryptosporidiosis of the biliary tract mimicking pancreatic cancer in an AIDS patient. Rev. Soc. Bras. Med. Trop. 2004, 37, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Shebl, F.M.; Engels, E.A.; Goedert, J.J. Opportunistic intestinal infections and risk of colorectal cancer among people with AIDS. AIDS Res. Hum. Retrovir. 2012, 28, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.R.; Levy, J.; Facchetti, F.; Notarangelo, L.; Ochs, H.D.; Etzioni, A.; Bonnefoy, J.Y.; Cosyns, M.; Weinberg, A. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J. Immunol. 1997, 158, 977–983. [Google Scholar]
- Stephens, J.; Cosyns, M.; Jones, M.; Hayward, A.R. Liver and bile duct pathology following Cryptosporidium parvum infection of immunodeficient mice. Hepatology 1999, 30, 27–35. [Google Scholar] [CrossRef]
- Leven, E.A.; Maffucci, P.; Ochs, H.D.; Scholl, P.R.; Buckley, R.H.; Fuleihan, R.L.; Geha, R.S.; Cunningham, C.K.; Bonilla, F.A.; Conley, M.E.; et al. Hyper IgM syndrome: A report from the USIDNET registry. J. Clin. Immunol. 2016, 36, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Chapel, H.; Chapman, R.W.; Collier, J.D. Cholangiocarcinoma complicating secondary sclerosing cholangitis from cryptosporidiosis in an adult patient with CD40 ligand deficiency: Case report and review of the literature. Int. Arch. Allergy Immunol. 2012, 159, 204–208. [Google Scholar] [CrossRef]
- Osman, M.; Benamrouz, S.; Guyot, K.; Baydoun, M.; Frealle, E.; Chabe, M.; Gantois, N.; Delaire, B.; Goffard, A.; Aoun, A.; et al. High association of Cryptosporidium spp. infection with colon adenocarcinoma in Lebanese patients. PLoS ONE 2017, 12, e0189422. [Google Scholar] [CrossRef]
- Baydoun, M.; Vanneste, S.B.; Creusy, C.; Guyot, K.; Gantois, N.; Chabe, M.; Delaire, B.; Mouray, A.; Baydoun, A.; Forzy, G.; et al. Three-dimensional (3D) culture of adult murine colon as an in vitro model of cryptosporidiosis: Proof of concept. Sci. Rep. 2017, 7, 17288. [Google Scholar] [CrossRef]
- Sulzyc-Bielicka, V.; Kuźna-Grygiel, W.; Kołodziejczyk, L.; Bielicki, D.; Kładny, J.; Stepień-Korzonek, M.; Telatyńska-Smieszek, B. Cryptosporidiosis in patients with colorectal cancer. J. Parasitol. 2007, 93, 722–724. [Google Scholar] [CrossRef]
- Sulżyc-Bielicka, V.; Kołodziejczyk, L.; Jaczewska, S.; Bielicki, D.; Kładny, J.; Safranow, K. Prevalence of Cryptosporidium sp. in patients with colorectal cancer. Pol. Przegl. Chir. 2012, 84, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Sulżyc-Bielicka, V.; Kołodziejczyk, L.; Jaczewska, S.; Bielicki, D.; Safranow, K.; Bielicki, P.; Kładny, J.; Rogowski, W. Colorectal cancer and Cryptosporidium spp. infection. PLoS ONE 2018, 13, e0195834. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, Ż.; Kváč, M.; Karpiński, P.; Hendrich, A.B.; Sąsiadek, M.M.; Leszczyński, P.; Sak, B.; McEvoy, J.; Kicia, M. The First Evidence of Cryptosporidium meleagridis infection in a colon adenocarcinoma from an immunocompetent patient. Front. Cell. Infect. Microbiol. 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yu, X.; Zhang, H.; Cui, L.; Li, X.; Zhang, X.; Gong, P.; Li, J.; Li, Z.; Wang, X.; et al. Prevalence and genotyping of Cryptosporidium parvum in gastrointestinal cancer patients. J. Cancer 2020, 11, 3334–3339. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Hanson, D.L.; Sullivan, P.S.; Novak, R.M.; Moorman, A.C.; Tong, T.C.; Holmberg, S.D.; Brooks, J.T. Adult and adolescent spectrum of disease project and HIV outpatient study investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann. Intern. Med. 2008, 148, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R.; Nichols, G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin. Microbiol. Rev. 2002, 15, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, N.; Gorgani-Firouzjaee, T.; Ghaffari, S.; Bayani, M.; Ghaffari, T.; Chehrazi, M. Association between Cryptosporidium infection and cancer: A systematic review and meta-analysis. Parasitol. Int. 2020, 74, 101979. [Google Scholar] [CrossRef]
- Uhl, E.W.; Jacobson, E.; Bartick, T.E.; Micinilio, J.; Schimdt, R. Aural-pharyngeal polyps associated with Cryptosporidium infection in three iguanas (Iguana iguana). Vet. Pathol. 2001, 38, 239–242. [Google Scholar]
- Fitzgerald, S.D.; Moisan, P.G.; Bennett, R. Aural polyp associated with cryptosporidiosis in an iguana (Iguana Iguana ). J. Vet. Diagn. Investig. 1998, 10, 179–180. [Google Scholar] [CrossRef]
- Nakagun, S.; Horiuchi, N.; Sugimoto, M.; Tomikawa, S.; Watanabe, K.; Kobayashi, Y. Proventriculitis associated with Cryptosporidium baileyi in a snowy owl (Bubo scandiacus) and its epidemiological investigation. J. Parasitol. 2017, 103, 451–457. [Google Scholar] [CrossRef]
- Terrell, S.P.; Uhl, E.W.; Funk, R.S. Proliferative enteritis in leopard geckos (Eublepharis macularius) associated with Cryptosporidium sp. infection. J. Zoo Wildl. Med. 2003, 34, 69–75. [Google Scholar] [PubMed]
- Certad, G.; Ngouanesavanh, T.; Guyot, K.; Gantois, N.; Chassat, T.; Mouray, A.; Fleurisse, L.; Pinon, A.; Cailliez, J.C.; Dei-Cas, E.; et al. Cryptosporidium parvum, a potential cause of colic adenocarcinoma. Infect. Agent Cancer 2007, 2, 22. [Google Scholar] [CrossRef]
- Benamrouz, S.; Guyot, K.; Gazzola, S.; Mouray, A.; Chassat, T.; Delaire, B.; Chabé, M.; Gosset, P.; Viscogliosi, E.; Dei-Cas, E.; et al. Cryptosporidium parvum infection in SCID mice infected with only one oocyst: qPCR assessment of parasite replication in tissues and development of digestive cancer. PLoS ONE 2012, 7, e51232. [Google Scholar] [CrossRef]
- Certad, G.; Creusy, C.; Guyot, K.; Mouray, A.; Chassat, T.; Delaire, B.; Pinon, A.; Sitja-Bobadilla, A.; Alvarez-Pellitero, P.; Praet, M.; et al. Fulminant cryptosporidiosis associated with digestive adenocarcinoma in SCID mice infected with Cryptosporidium parvum TUM1 strain. Int. J. Parasitol. 2010, 40, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Certad, G.; Benamrouz, S.; Guyot, K.; Mouray, A.; Chassat, T.; Flament, N.; Delhaes, L.; Coiteux, V.; Delaire, B.; Praet, M.; et al. Fulminant cryptosporidiosis after near-drowning: A human Cryptosporidium parvum strain implicated in invasive gastrointestinal adenocarcinoma and cholangiocarcinoma in an experimental model. Appl. Environ. Microbiol. 2012, 78, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Audebert, C.; Bonardi, F.; Caboche, S.; Guyot, K.; Touzet, H.; Merlin, S.; Gantois, N.; Creusy, C.; Meloni, D.; Mouray, A.; et al. Genetic basis for virulence differences of various Cryptosporidium parvum carcinogenic isolates. Sci. Rep. 2020, 10, 7316. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.G.; Harba, N.M.; Afifi, A.F.; Elnaidany, N.F. Assessment of Cryptosporidium parvum infection in immunocompetent and immunocompromised mice and its role in triggering intestinal dysplasia. Int. J. Infect. Dis. 2013, 17, e593–e600. [Google Scholar] [CrossRef]
- Mostafa, N.E.; Abdel Hamed, E.F.; Fawzy, E.M.; Zalat, R.S.; Rashed, H.E.; Mohamed, S.Y. The new trend in the treatment of experimental cryptosporidiosis and the resulting intestinal dysplasia. Color. Cancer 2018, 7, CRC06. [Google Scholar] [CrossRef]
- Certad, G.; Creusy, C.; Ngouanesavanh, T.; Guyot, K.; Gantois, N.; Mouray, A.; Chassat, T.; Flament, N.; Fleurisse, L.; Pinon, A.; et al. Development of Cryptosporidium parvum-induced gastrointestinal neoplasia in severe combined immunodeficiency (SCID) mice: Severity of lesions is correlated with infection intensity. Am. J. Trop. Med. Hyg. 2010, 82, 257–265. [Google Scholar] [CrossRef]
- O’Hara, S.P.; Chen, X.M. The cell biology of Cryptosporidium infection. Microbes Infect. 2011, 13, 721–730. [Google Scholar] [CrossRef]
- Certad, G.; Viscogliosi, E.; Chabé, M.; Cacciò, S.M. Pathogenic Mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017, 33, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Weydig, C.; Wessler, S. Targeting focal adhesions: Helicobacter pylori-host communication in cell migration. Cell Commun. Signal. 2008, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Shostak, K.; Chariot, A. EGFR and NF-κB: Partners in cancer. Trends Mol. Med. 2015, 21, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gu, H.; Lan, Z.; Lei, Q.; Wang, W.; Ruan, J.; Yu, M.; Lin, J.; Cui, Q. Downregulation of cyclooxygenase-1 stimulates mitochondrial apoptosis through the NF-κB signaling pathway in colorectal cancer cells. Oncol. Rep. 2018, 41, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.L.; Fan, X.C.; Li, Y.H.; Yuan, Y.J.; Yin, Y.L.; Wang, X.T.; Zhang, L.X.; Zhao, G.H. Expression profiles of mRNA and lncRNA in HCT-8 cells infected with Cryptosporidium parvum IId subtype. Front. Microbiol. 2018, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Saraf, S.; Verma, A.; Panda, K.; Jain, S.K. Novel targeting approaches and signaling pathways of colorectal cancer: An insight. World J. Gastroenterol. 2018, 24, 4428–4435. [Google Scholar] [CrossRef]
- Benamrouz, S.; Conseil, V.; Chabé, M.; Praet, M.; Audebert, C.; Blervaque, R.; Guyot, K.; Gazzola, S.; Mouray, A.; Chassat, T.; et al. Cryptosporidium parvum-induced ileo-caecal adenocarcinoma and Wnt signaling in a mouse model. Dis. Model. Mech. 2014, 7, 693–700. [Google Scholar] [CrossRef]
- Yamaoka, Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 629–641. [Google Scholar] [CrossRef]
- Xu, P.; Widmer, G.; Wang, Y.; Ozaki, L.S.; Alves, J.M.; Serrano, M.G.; Puiu, D.; Manque, P.; Akiyoshi, D.; Mackey, A.J.; et al. The genome of Cryptosporidium hominis. Nature 2004, 431, 1107–1112. [Google Scholar] [CrossRef]
- Puiu, D.; Enomoto, S.; Buck, G.A.; Abrahamsen, M.S.; Kissinger, J.C. CryptoDB: The Cryptosporidium genome resource. Nucleic Acids Res. 2004, 32, D329–D331. [Google Scholar] [CrossRef]
- Hadfield, S.J.; Pachebat, J.A.; Swain, M.T.; Robinson, G.; Cameron, S.J.; Alexander, J.; Hegarty, M.J.; Elwin, K.; Chalmers, R.M. Generation of whole genome sequences of new Cryptosporidium hominis and Cryptosporidium parvum isolates directly from stool samples. BMC Genom. 2015, 16, 650. [Google Scholar] [CrossRef] [PubMed]



| Type of Cancer | Type of Study | Geographic Localization | Clinical Sample:Laboratory Method | n (%) | p-Value | Immuno-Suppression | Reference |
|---|---|---|---|---|---|---|---|
| Colonic adenocarcinoma | Case report | Spain | Not reported | 1 | NA a | No | [33] |
| Pancreatic cancer | Case report | Brazil | Tissues/Microscopical observation | 1 | NA a | HIV/AIDS | [34] |
| Colorectal cancer(Adenocarcinoma) | Data matching between HIV/AIDS and cancer registry databases in 16 U.S. states | United States | Tissues/Microscopical observation | 3/269 (1%) | 0.70 | HIV/AIDS | [35] |
| Colorectalsquamous cell carcinoma | Data matching between HIV/AIDS and cancer registry databases in 16 U.S. states | United States | Tissues/Microscopical observation | 1/8 (12.5%) | 0.02 b | HIV/AIDS | [35] |
| Uncommon colorectal cancers) | Data matching between HIV/AIDS and cancer registry databases in 16 U.S. states | United States | Tissues/Microscopical observation | 3/43 (7%) | 0.04 b | HIV/AIDS | [35] |
| Bile duct carcinoma | Case reports | United States | Tissues/Microscopical observation | Not reported | NA a | X linked immunodeficiency with hyper-lgM | [36] |
| Hepatoma | Analysis of the USIDNET Registry | United States | Not reported | 1/145(1%) | NA a | X-linked hyper-IgMsyndrome in children | [38] |
| Cholangiocarcinoma | Case report | United Kingdom | Stool samples,Coprological analysis | 1 | NA a | CD40L deficiency | [39] |
| Colonic adenocarcinoma | Case-control | Lebanon | DNA from biopsies, PCR | 15/72 (21%) | 0.003 b | No | [42] |
| Colonic adenocarcinoma | Cases | Poland | Stool samples, coprology and ELISA | 4/55(18%) | NA a | No | [43] |
| Colonic adenocarcinoma | Cases | Poland | Stool samples,ELISA | 10/87(12%) | NA a | No | [44] |
| Colonic adenocarcinoma | Case-control | Poland | Stool samples, coprology analysis and ELISA | 14/108 b(13%) | 0.015 b | No | [45] |
| Colonic adenocarcinoma | Cases | Poland | DNA from stools, PCR | 1/145(1%) | NA a | No | [46] |
| Colonic adenocarcinoma | Case-control | China | DNA from stools, PCR | 20/116 (17.24%) | <0.001 b | No | [46] |
| Gastric | Case-control | China | DNA from stools, PCR | 2/51 (4%) | 0.121 | No | [46] |
| Esophageal | Case-control | China | DNA from stools, PCR | 1/16 (6.25%) | 0.029 b | No | [46] |
| Liver | Case-control | China | DNA from stools, PCR | 1/7 (14.29%) | <0.001 b | No | [46] |
| Small Intestine | Case-control | China | DNA from stools, PCR | 2/5 (40%) | <0.001 b | NO | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawant, M.; Baydoun, M.; Creusy, C.; Chabé, M.; Viscogliosi, E.; Certad, G.; Benamrouz-Vanneste, S. Cryptosporidium and Colon Cancer: Cause or Consequence? Microorganisms 2020, 8, 1665. https://doi.org/10.3390/microorganisms8111665
Sawant M, Baydoun M, Creusy C, Chabé M, Viscogliosi E, Certad G, Benamrouz-Vanneste S. Cryptosporidium and Colon Cancer: Cause or Consequence? Microorganisms. 2020; 8(11):1665. https://doi.org/10.3390/microorganisms8111665
Chicago/Turabian StyleSawant, Manasi, Martha Baydoun, Colette Creusy, Magali Chabé, Eric Viscogliosi, Gabriela Certad, and Sadia Benamrouz-Vanneste. 2020. "Cryptosporidium and Colon Cancer: Cause or Consequence?" Microorganisms 8, no. 11: 1665. https://doi.org/10.3390/microorganisms8111665
APA StyleSawant, M., Baydoun, M., Creusy, C., Chabé, M., Viscogliosi, E., Certad, G., & Benamrouz-Vanneste, S. (2020). Cryptosporidium and Colon Cancer: Cause or Consequence? Microorganisms, 8(11), 1665. https://doi.org/10.3390/microorganisms8111665







