Cryptosporidium parvum Subverts Antimicrobial Activity of CRAMP by Reducing Its Expression in Neonatal Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite
2.2. Mouse Models and Ethic Statements
2.3. mICcl2 Cell Line Culture and Viability
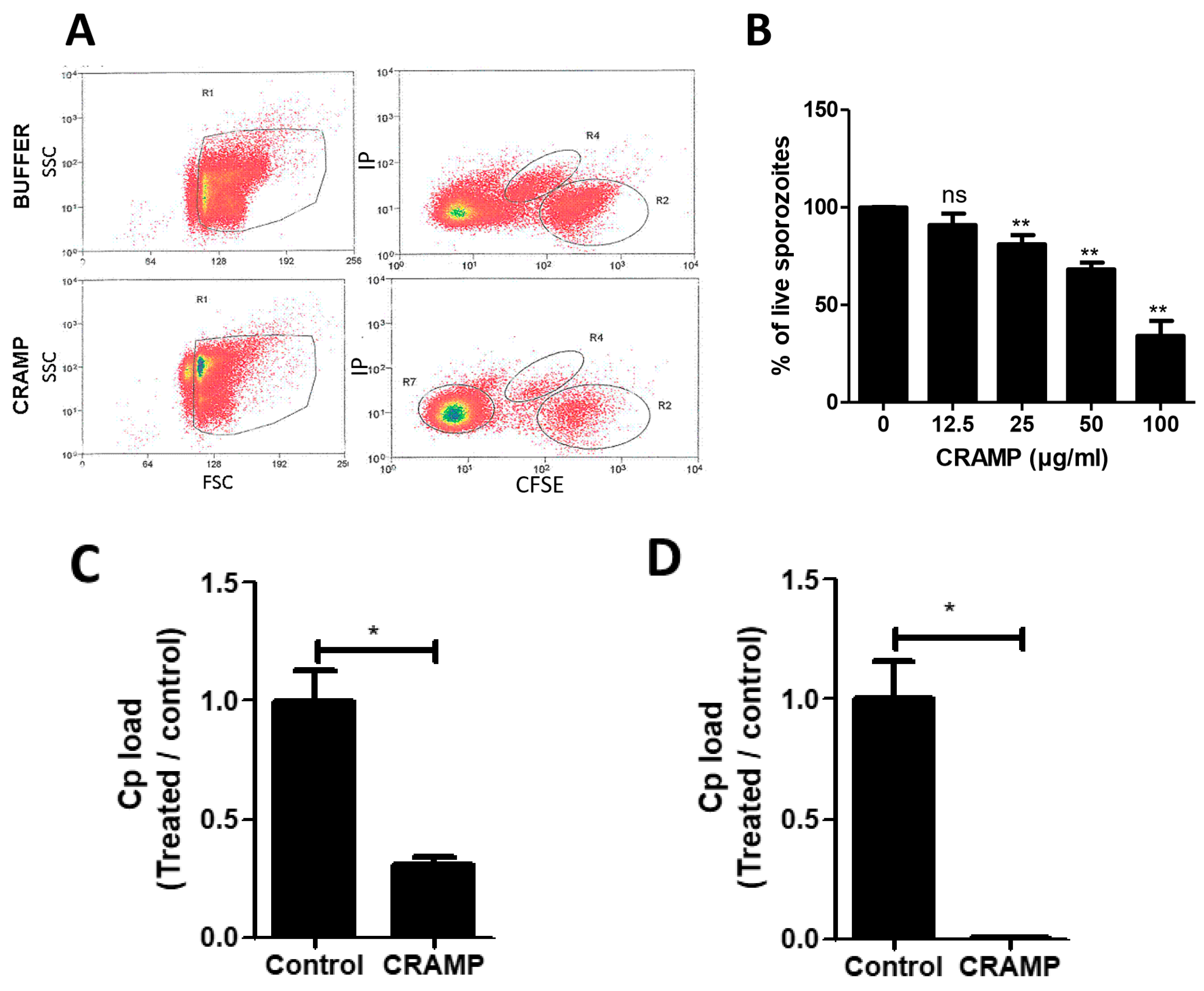
2.4. Test of Sporozoite Viability
2.5. Isolation of Intestinal Epithelial Cells (IEC)
2.6. RNA Extraction and qRT-PCR
2.7. Lamina propria Cell Preparation and Flow Cytometry
3. Results
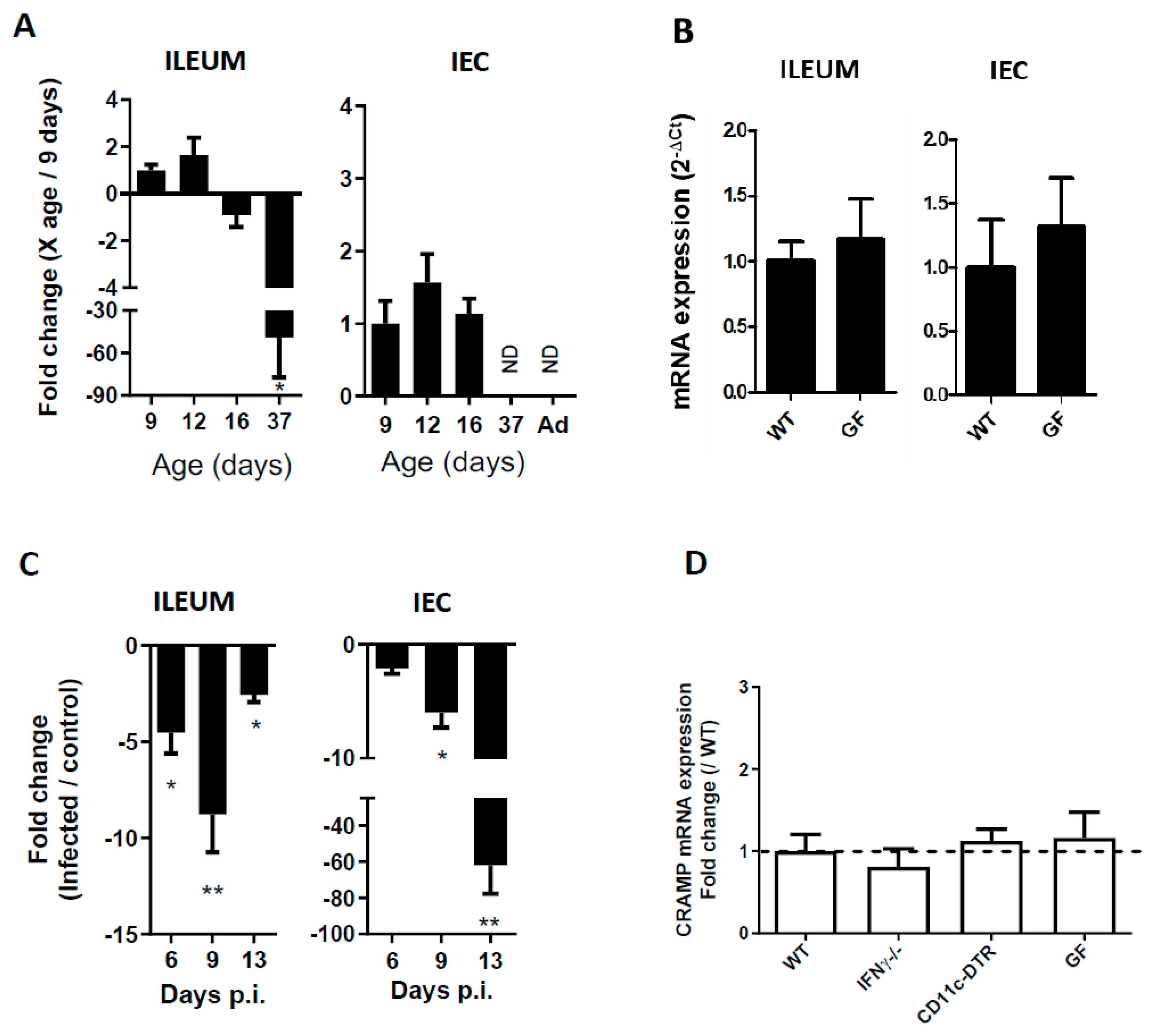
3.1. CRAMP Expression in Neonatal Mice Is Reduced during C. parvum Infection
3.2. The Reduced Expression of CRAMP Is Independent of IFNγ, Dendritic Cells and the Microbiota
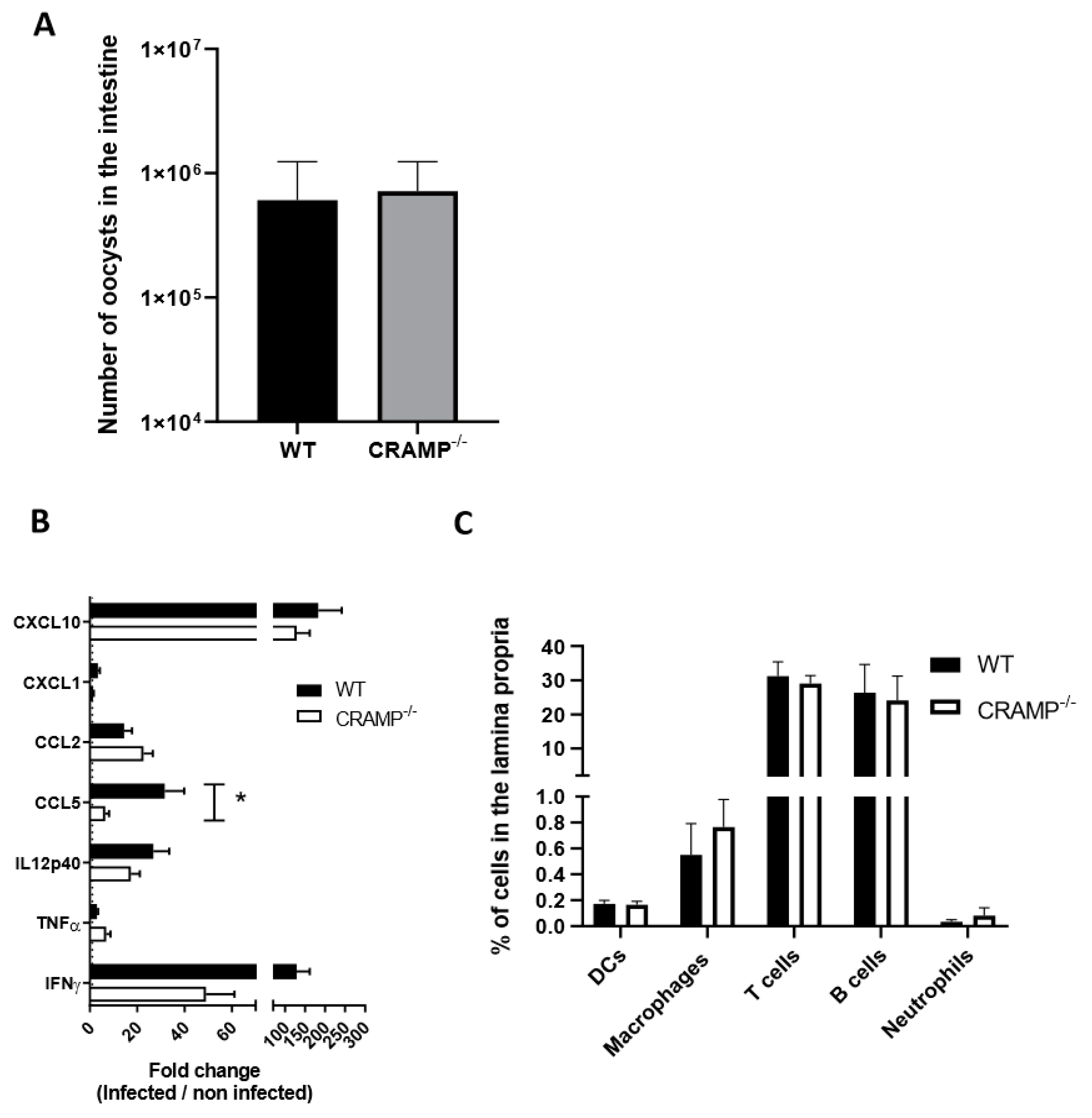
3.3. Depletion of CRAMP in Neonatal Mice Does Not Worsen C. parvum Infection
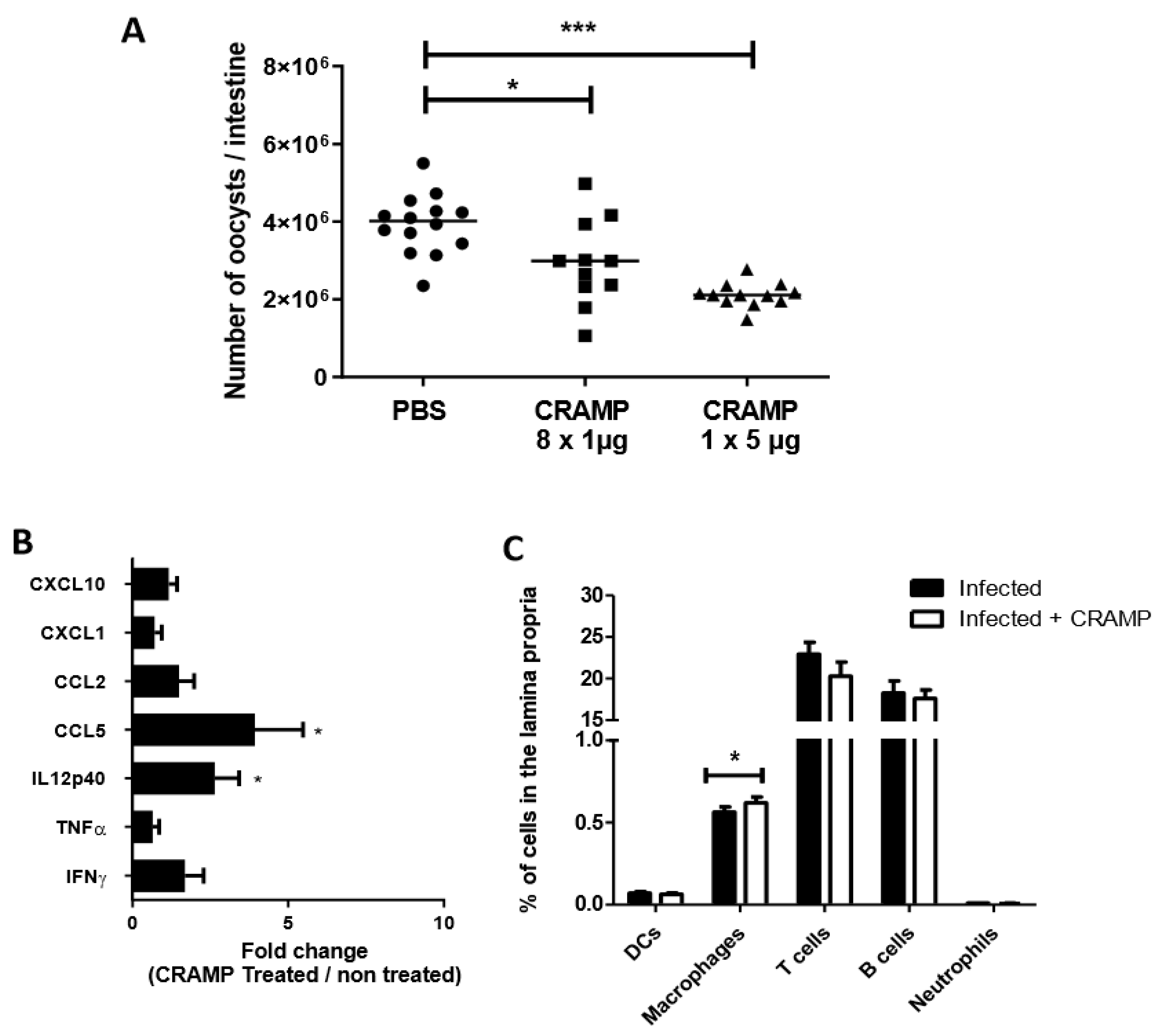
3.4. Oral Administrations of Recombinant CRAMP to C. parvum Infected Neonatal Mice Significantly Reduce Parasite Load
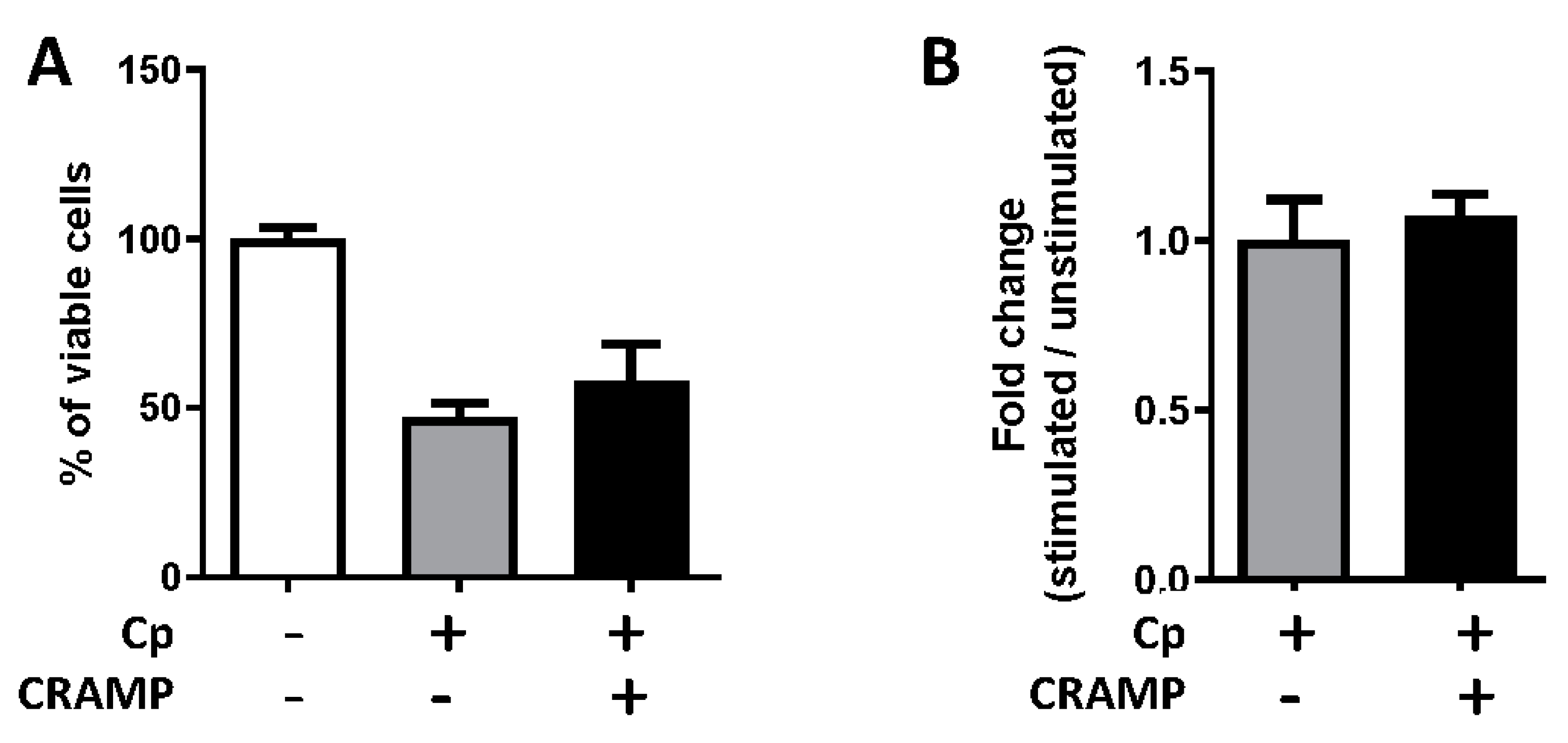
3.5. The Stimulation of Enterocytes by CRAMP Does Not Change Their Permissiveness to C. parvum
3.6. CRAMP Displays Antimicrobial Activity against C. parvum Sporozoites
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Striepen, B. Parasitic infections: Time to tackle cryptosporidiosis. Nature 2013, 503, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.; Hamilton, C.A.; Hope, J.C.; Katzer, F.; Mabbott, N.A.; Morrison, L.J.; Innes, E.A. Bovine cryptosporidiosis: Impact, host-parasite interaction and control strategies. Vet. Res. 2017, 48, 42. [Google Scholar] [CrossRef] [PubMed]
- Ojcius, D.M.; Perfettini, J.L.; Bonnin, A.; Laurent, F. Caspase-dependent apoptosis during infection with Cryptosporidium parvum. Microbes Infect. 1999, 1, 1163–1168. [Google Scholar] [CrossRef]
- McCole, D.F.; Eckmann, L.; Laurent, F.; Kagnoff, M.F. Intestinal epithelial cell apoptosis following Cryptosporidium parvum infection. Infect. Immun. 2000, 68, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Laurent, F.; Eckmann, L.; Savidge, T.C.; Morgan, G.; Theodos, C.; Naciri, M.; Kagnoff, M.F. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect. Immun. 1997, 65, 5067–5073. [Google Scholar] [CrossRef]
- Lacroix-Lamande, S.; Mancassola, R.; Naciri, M.; Laurent, F. Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection. Infect. Immun. 2002, 70, 2090–2099. [Google Scholar] [CrossRef]
- Lantier, L.; Lacroix-Lamande, S.; Potiron, L.; Metton, C.; Drouet, F.; Guesdon, W.; Gnahoui-David, A.; Le Vern, Y.; Deriaud, E.; Fenis, A.; et al. Intestinal CD103+ dendritic cells are key players in the innate immune control of Cryptosporidium parvum infection in neonatal mice. PLoS Pathog. 2013, 9, e1003801. [Google Scholar] [CrossRef]
- Zaalouk, T.K.; Bajaj-Elliott, M.; George, J.T.; McDonald, V. Differential regulation of beta-defensin gene expression during Cryptosporidium parvum infection. Infect. Immun. 2004, 72, 2772–2779. [Google Scholar] [CrossRef]
- Carryn, S.; Schaefer, D.A.; Imboden, M.; Homan, E.J.; Bremel, R.D.; Riggs, M.W. Phospholipases and cationic peptides inhibit Cryptosporidium parvum sporozoite infectivity by parasiticidal and non-parasiticidal mechanisms. J. Parasitol. 2012, 98, 199–204. [Google Scholar] [CrossRef]
- Guesdon, W.; Auray, G.; Pezier, T.; Bussiere, F.I.; Drouet, F.; Le Vern, Y.; Marquis, M.; Potiron, L.; Rabot, S.; Bruneau, A.; et al. CCL20 Displays Antimicrobial Activity Against Cryptosporidium parvum, but Its Expression Is Reduced During Infection in the Intestine of Neonatal Mice. J. Infect. Dis. 2015, 212, 1332–1340. [Google Scholar] [CrossRef]
- van Harten, R.M.; van Woudenbergh, E.; van Dijk, A.; Haagsman, H.P. Cathelicidins: Immunomodulatory Antimicrobials. Vaccines 2018, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 1998, 95, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Saiman, L.; Tabibi, S.; Starner, T.D.; San Gabriel, P.; Winokur, P.L.; Jia, H.P.; McCray, P.B., Jr.; Tack, B.F. Cathelicidin peptides inhibit multiply antibiotic-resistant pathogens from patients with cystic fibrosis. Antimicrob. Agents Chemother. 2001, 45, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.S.; Cornicelli, M.D.; Kovach, M.A.; Newstead, M.W.; Zeng, X.; Kumar, A.; Gao, N.; Yoon, S.G.; Gallo, R.L.; Standiford, T.J. Flagellin stimulates protective lung mucosal immunity: Role of cathelicidin-related antimicrobial peptide. J. Immunol. 2010, 185, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, D.M.; Davidson, D.J.; Hancock, R.E. Immunomodulatory properties of defensins and cathelicidins. Curr. Top. Microbiol. Immunol. 2006, 306, 27–66. [Google Scholar] [PubMed]
- Whelehan, C.J.; Barry-Reidy, A.; Meade, K.G.; Eckersall, P.D.; Chapwanya, A.; Narciandi, F.; Lloyd, A.T.; O’Farrelly, C. Characterisation and expression profile of the bovine cathelicidin gene repertoire in mammary tissue. BMC Genom. 2014, 15, 128. [Google Scholar] [CrossRef]
- Menard, S.; Forster, V.; Lotz, M.; Gutle, D.; Duerr, C.U.; Gallo, R.L.; Henriques-Normark, B.; Putsep, K.; Andersson, M.; Glocker, E.O.; et al. Developmental switch of intestinal antimicrobial peptide expression. J. Exp. Med. 2008, 205, 183–193. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Del Prete, M.S.; Skerlavaj, B.; Circo, R.; Zanetti, M.; Scalise, G. In vitro effect on Cryptosporidium parvum of short-term exposure to cathelicidin peptides. J. Antimicrob. Chemother. 2003, 51, 843–847. [Google Scholar] [CrossRef][Green Version]
- Lacroix, S.; Mancassola, R.; Naciri, M.; Laurent, F. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: Role of tumor necrosis factor alpha in protection. Infect. Immun. 2001, 69, 1635–1642. [Google Scholar] [CrossRef]
- Bens, M.; Bogdanova, A.; Cluzeaud, F.; Miquerol, L.; Kerneis, S.; Kraehenbuhl, J.P.; Kahn, A.; Pringault, E.; Vandewalle, A. Transimmortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am. J. Physiol. 1996, 270, C1666–C1674. [Google Scholar] [CrossRef] [PubMed]
- Cobo, E.R.; He, C.; Hirata, K.; Hwang, G.; Tran, U.; Eckmann, L.; Gallo, R.L.; Reed, S.L. Entamoeba histolytica induces intestinal cathelicidins but is resistant to cathelicidin-mediated killing. Infect. Immun. 2012, 80, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chu, X.; Liu, C.; Huang, W.; Yao, Y.; Xia, Y.; Sun, P.; Long, Q.; Feng, X.; Li, K.; et al. Exogenous murine antimicrobial peptide CRAMP significantly exacerbates Ovalbumin-induced airway inflammation but ameliorates oxazolone-induced intestinal colitis in BALB/c mice. Hum. Vaccines Immunother. 2018, 14, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Fachi, J.L.; Felipe, J.S.; Pral, L.P.; da Silva, B.K.; Correa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Camara, N.O.S.; de Sales, E.S.E.L.; et al. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019, 27, 750–761.e757. [Google Scholar] [CrossRef] [PubMed]
- Theodos, C.M.; Sullivan, K.L.; Griffiths, J.K.; Tzipori, S. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: The extent of gamma interferon modulation determines the outcome of infection. Infect. Immun. 1997, 65, 4761–4769. [Google Scholar] [CrossRef] [PubMed]
- Pollok, R.C.; Farthing, M.J.; Bajaj-Elliott, M.; Sanderson, I.R.; McDonald, V. Interferon gamma induces enterocyte resistance against infection by the intracellular pathogen Cryptosporidium parvum. Gastroenterology 2001, 120, 99–107. [Google Scholar] [CrossRef]
- de Sablet, T.; Potiron, L.; Marquis, M.; Bussiere, F.I.; Lacroix-Lamande, S.; Laurent, F. Cryptosporidium parvum increases intestinal permeability through interaction with epithelial cells and IL-1beta and TNFalpha released by inflammatory monocytes. Cell. Microbiol. 2016, 18, 1871–1880. [Google Scholar] [CrossRef]
- Potiron, L.; Lacroix-Lamande, S.; Marquis, M.; Levern, Y.; Fort, G.; Franceschini, I.; Laurent, F. Batf3-Dependent Intestinal Dendritic Cells Play a Critical Role in the Control of Cryptosporidium parvum Infection. J. Infect. Dis. 2019, 219, 925–935. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–324. [Google Scholar] [CrossRef]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Pan, L.L.; Zhou, Q.; Zhao, C.; Xie, Y.; Wu, C.; Meng, X.; Gu, H.; Xu, J.; Zhou, L.; et al. Cathelicidin-related antimicrobial peptide protects against myocardial ischemia/reperfusion injury. BMC Med. 2019, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, M.; Ortsater, H.; Lundeberg, E.; Juntti-Berggren, L.; Chen, Y.Q.; Haeggstrom, J.Z.; Gudmundsson, G.H.; Diana, J.; Agerberth, B. Cathelicidins positively regulate pancreatic beta-cell functions. FASEB J. 2016, 30, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Crauwels, P.; Bank, E.; Walber, B.; Wenzel, U.A.; Agerberth, B.; Chanyalew, M.; Abebe, M.; Konig, R.; Ritter, U.; Reiling, N.; et al. Cathelicidin Contributes to the Restriction of Leishmania in Human Host Macrophages. Front. Immunol. 2019, 10, 2697. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.; Heinhuis, B.; Gomes, R.S.; Damen, M.S.; Real, F.; Mortara, R.A.; Keating, S.T.; Dinarello, C.A.; Joosten, L.A.; Ribeiro-Dias, F. Cytokines and microbicidal molecules regulated by IL-32 in THP-1-derived human macrophages infected with New World Leishmania species. PLoS Negl. Trop. Dis. 2017, 11, e0005413. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Liu, X.; Derrick, S.C.; Yang, A.; Tian, J.; Kolibab, K.; Kumar, S.; Morris, S.L. Molecular analysis of non-specific protection against murine malaria induced by BCG vaccination. PLoS ONE 2013, 8, e66115. [Google Scholar] [CrossRef]
- Bandeira, I.C.J.; Bandeira-Lima, D.; Mello, C.P.; Pereira, T.P.; De Menezes, R.; Sampaio, T.L.; Falcao, C.B.; Radis-Baptista, G.; Martins, A.M.C. Antichagasic effect of crotalicidin, a cathelicidin-like vipericidin, found in Crotalus durissus terrificus rattlesnake’s venom gland. Parasitology 2018, 145, 1059–1064. [Google Scholar] [CrossRef]
- Coorens, M.; Scheenstra, M.R.; Veldhuizen, E.J.; Haagsman, H.P. Interspecies cathelicidin comparison reveals divergence in antimicrobial activity, TLR modulation, chemokine induction and regulation of phagocytosis. Sci. Rep. 2017, 7, 40874. [Google Scholar] [CrossRef]
- Hu, G.; Gong, A.Y.; Roth, A.L.; Huang, B.Q.; Ward, H.D.; Zhu, G.; Larusso, N.F.; Hanson, N.D.; Chen, X.M. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013, 9, e1003261. [Google Scholar] [CrossRef]
- Hornef, M.W.; Torow, N. ‘Layered immunity’ and the ‘neonatal window of opportunity’—Timed succession of non-redundant phases to establish mucosal host-microbial homeostasis after birth. Immunology 2020, 159, 15–25. [Google Scholar] [CrossRef]
- Kai-Larsen, Y.; Bergsson, G.; Gudmundsson, G.H.; Printz, G.; Jornvall, H.; Marchini, G.; Agerberth, B. Antimicrobial components of the neonatal gut affected upon colonization. Pediatr. Res. 2007, 61, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Ming, Z.; Gong, A.Y.; Wang, Y.; Zhang, X.T.; Li, M.; Dolata, C.E.; Chen, X.M. Trans-suppression of defense DEFB1 gene in intestinal epithelial cells following Cryptosporidium parvum infection is associated with host delivery of parasite Cdg7_FLc_1000 RNA. Parasitol. Res. 2018, 117, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, B.; Regnault, B.; Guo, J.; Zhang, Z.; Stanley, S.L., Jr.; Sansonetti, P.J.; Pedron, T. Virulent Shigella flexneri subverts the host innate immune response through manipulation of antimicrobial peptide gene expression. J. Exp. Med. 2008, 205, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Heilborn, J.D.; Nilsson, M.F.; Kratz, G.; Weber, G.; Sorensen, O.; Borregaard, N.; Stahle-Backdahl, M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Investig. Dermatol. 2003, 120, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Agier, J.; Efenberger, M.; Brzezinska-Blaszczyk, E. Cathelicidin impact on inflammatory cells. Cent. Eur. J. Immunol. 2015, 40, 225–235. [Google Scholar] [CrossRef] [PubMed]
- van der Does, A.M.; Beekhuizen, H.; Ravensbergen, B.; Vos, T.; Ottenhoff, T.H.; van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; Nibbering, P.H. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J. Immunol. 2010, 185, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer Sequences | Reverse Primer Sequences |
|---|---|---|
| CCL2 | 5′-TGCTACTCATTCACCAGCAAGAT-3′ | 5′-GTGGTTGTGGAAAAGGTAGTGG-3′ |
| CCL5 | 5′-TCTCTGCAGCTGCCCTCACC-3′ | 5′-TCTTGAACCCACTTCTTCTC-3′ |
| CRAMP | 5′-CCCAAGTCTGTGAGGTTCCG-3′ | 5′-AGGCAGGCCTACTACTCTGG-3′ |
| CXCL1 | 5′-CGCTCGCTTCTCTGTGCAGC-3′ | 5′-GTGGCTATGACTTCGGTTTGG-3′ |
| CXCL10 | 5′-CACGTGTTGAGATCATTGCCA-3′ | 5′-GCGTGGCTTCACTCCAGTTA-3′ |
| IFNγ | 5′-TCTTCTTGGATATCTGGAGGAA-3′ | 5′-AGCTCATTGAATGCTTGGCGCTG-3′ |
| IL-12p40 | 5′-CTCACATCTGCTGCTCCACAA-3′ | 5′-GACGCCATTCCACATGTCACT-3′ |
| PPIA | 5′-GTCTCCTTCGAGCTGTTTGC-3′ | 5′-GATGCCAGGACCTGTATGCT-3′ |
| TBP | 5′-CAGCCTTCCACCTTATGCTC-3′ | 5′-TTGCTGCTGCTGTCTTTGTT-3′ |
| TNFα | 5′-ATGAGCACAGAAAGCATGATC-3′ | 5′-TACAGCCTTGTCACTCGAATT-3′ |
| Cp18S | 5′-CCGATAACGAACGAGACTCTGG-3′ | 5′-TAGAGATTGGAGGTTGTTCCT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guesdon, W.; Pezier, T.; Menard, S.; Nicolosi, A.; Le Vern, Y.; Silvestre, A.; Diana, J.; Laurent, F.; Lacroix-Lamandé, S. Cryptosporidium parvum Subverts Antimicrobial Activity of CRAMP by Reducing Its Expression in Neonatal Mice. Microorganisms 2020, 8, 1635. https://doi.org/10.3390/microorganisms8111635
Guesdon W, Pezier T, Menard S, Nicolosi A, Le Vern Y, Silvestre A, Diana J, Laurent F, Lacroix-Lamandé S. Cryptosporidium parvum Subverts Antimicrobial Activity of CRAMP by Reducing Its Expression in Neonatal Mice. Microorganisms. 2020; 8(11):1635. https://doi.org/10.3390/microorganisms8111635
Chicago/Turabian StyleGuesdon, William, Tiffany Pezier, Sandrine Menard, Alessandra Nicolosi, Yves Le Vern, Anne Silvestre, Julien Diana, Fabrice Laurent, and Sonia Lacroix-Lamandé. 2020. "Cryptosporidium parvum Subverts Antimicrobial Activity of CRAMP by Reducing Its Expression in Neonatal Mice" Microorganisms 8, no. 11: 1635. https://doi.org/10.3390/microorganisms8111635
APA StyleGuesdon, W., Pezier, T., Menard, S., Nicolosi, A., Le Vern, Y., Silvestre, A., Diana, J., Laurent, F., & Lacroix-Lamandé, S. (2020). Cryptosporidium parvum Subverts Antimicrobial Activity of CRAMP by Reducing Its Expression in Neonatal Mice. Microorganisms, 8(11), 1635. https://doi.org/10.3390/microorganisms8111635






