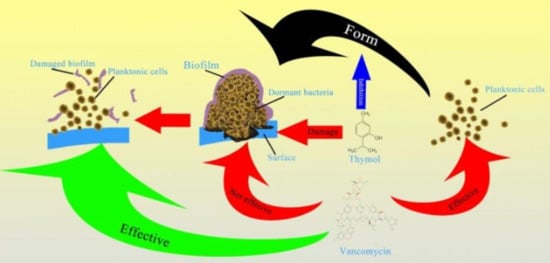
Thymol Inhibits Biofilm Formation, Eliminates Pre-Existing Biofilms, and Enhances Clearance of Methicillin-Resistant Staphylococcus aureus (MRSA) in a Mouse Peritoneal Implant Infection Model
Abstract
1. Introduction
2. Results
2.1. Effects of Thymol on Growth and the Results of Thymol-Vancomycin Checkerboard Assay
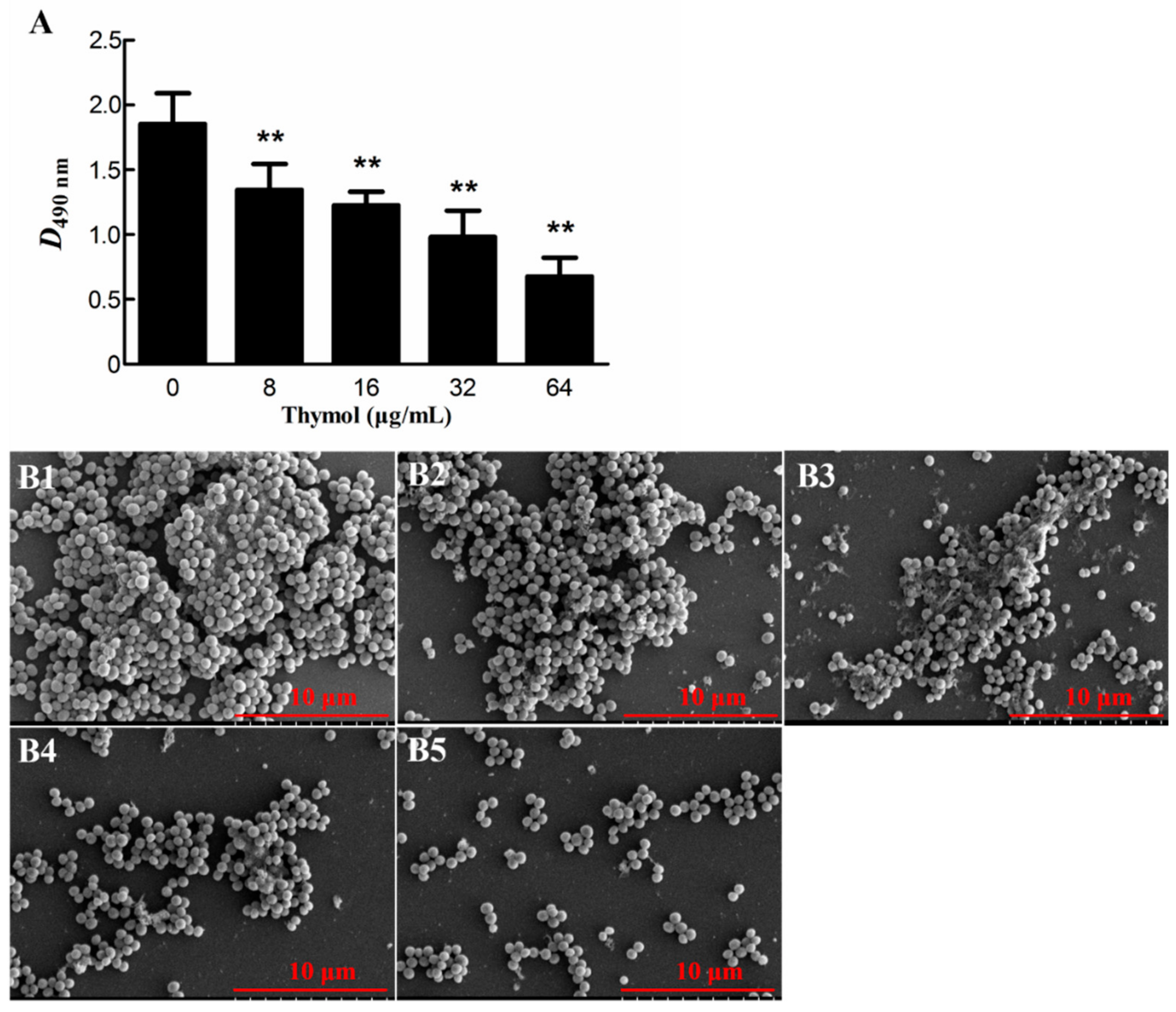
2.2. Thymol Inhibited Biofilm Formation by Strain TCH1516
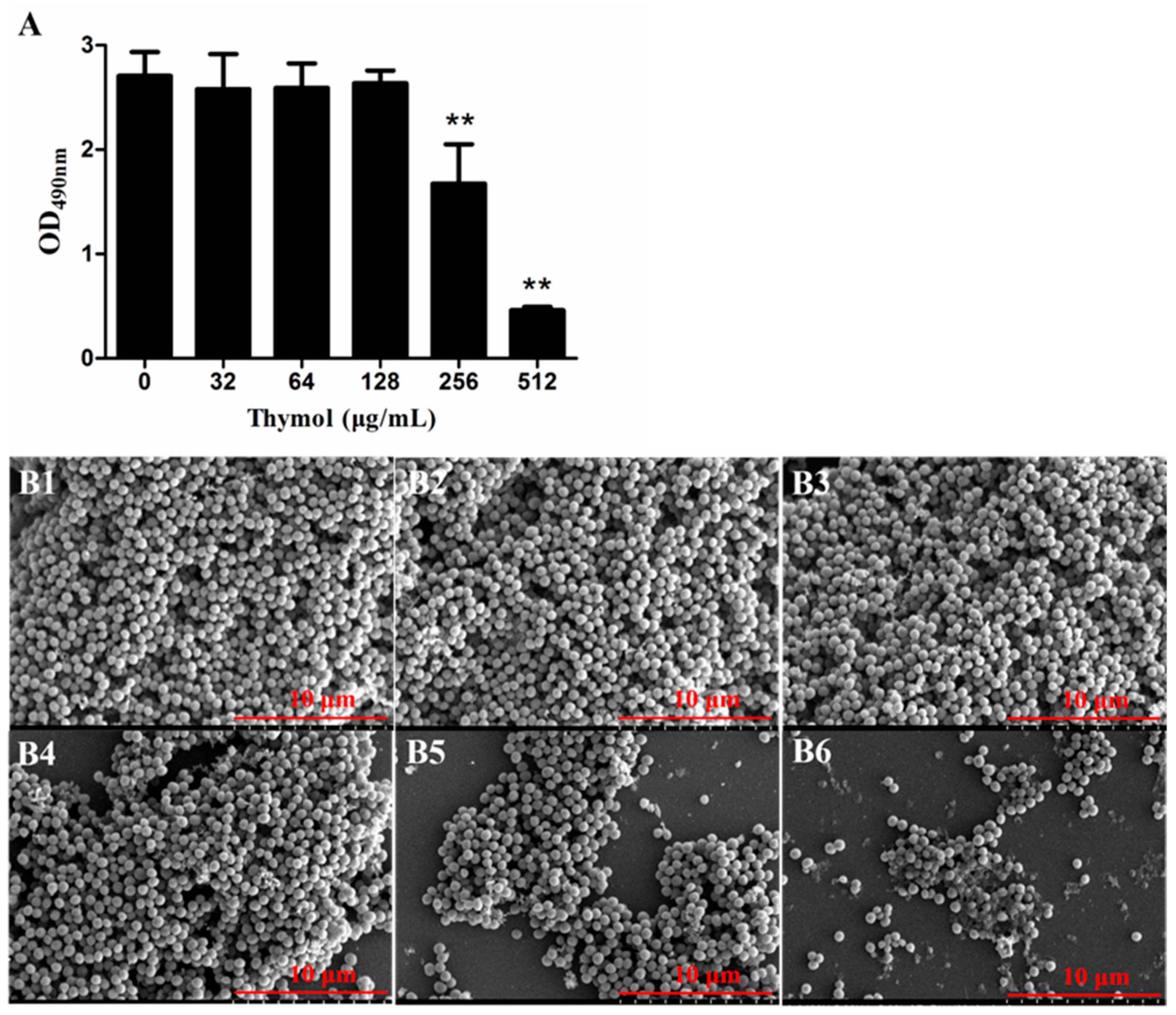
2.3. Thymol Disrupted Mature TCH1516 Biofilms
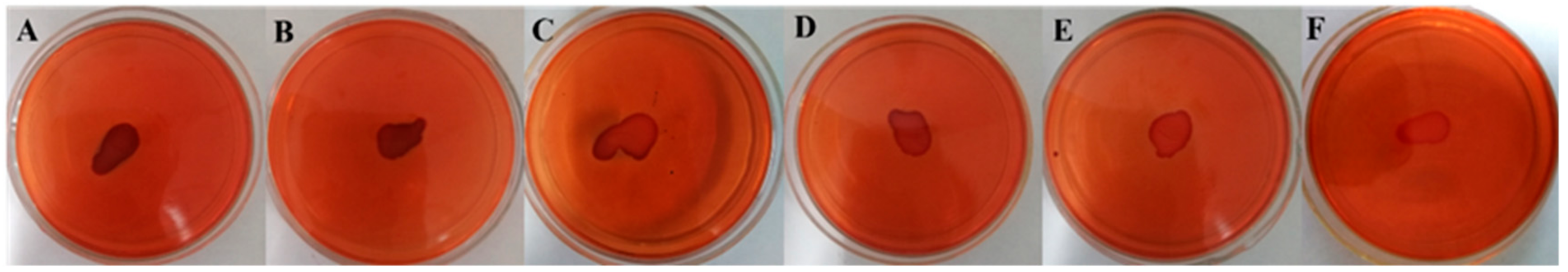
2.4. PIA Production was Inhibited by Thymol
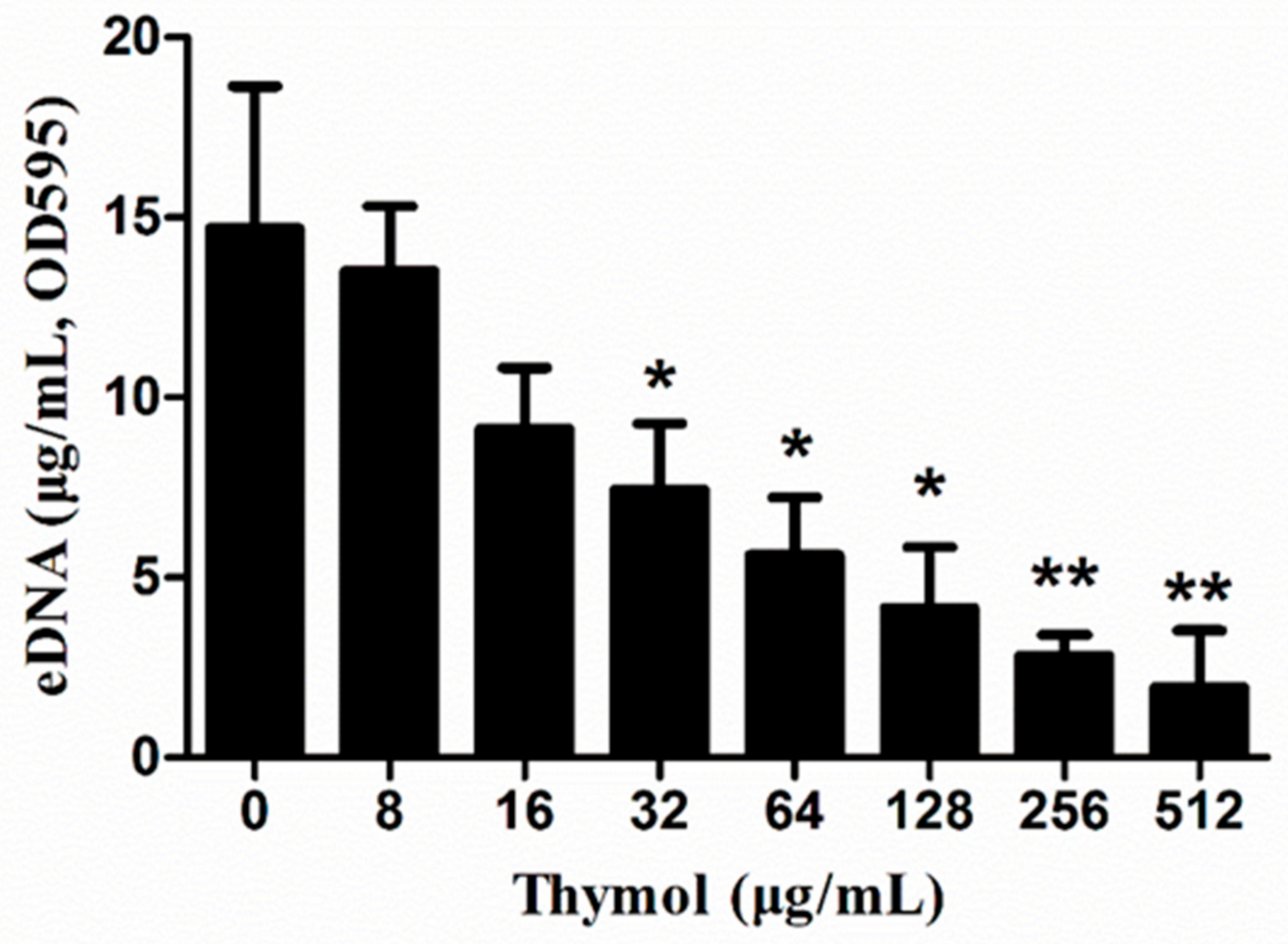
2.5. Thymol Decreased eDNA Release
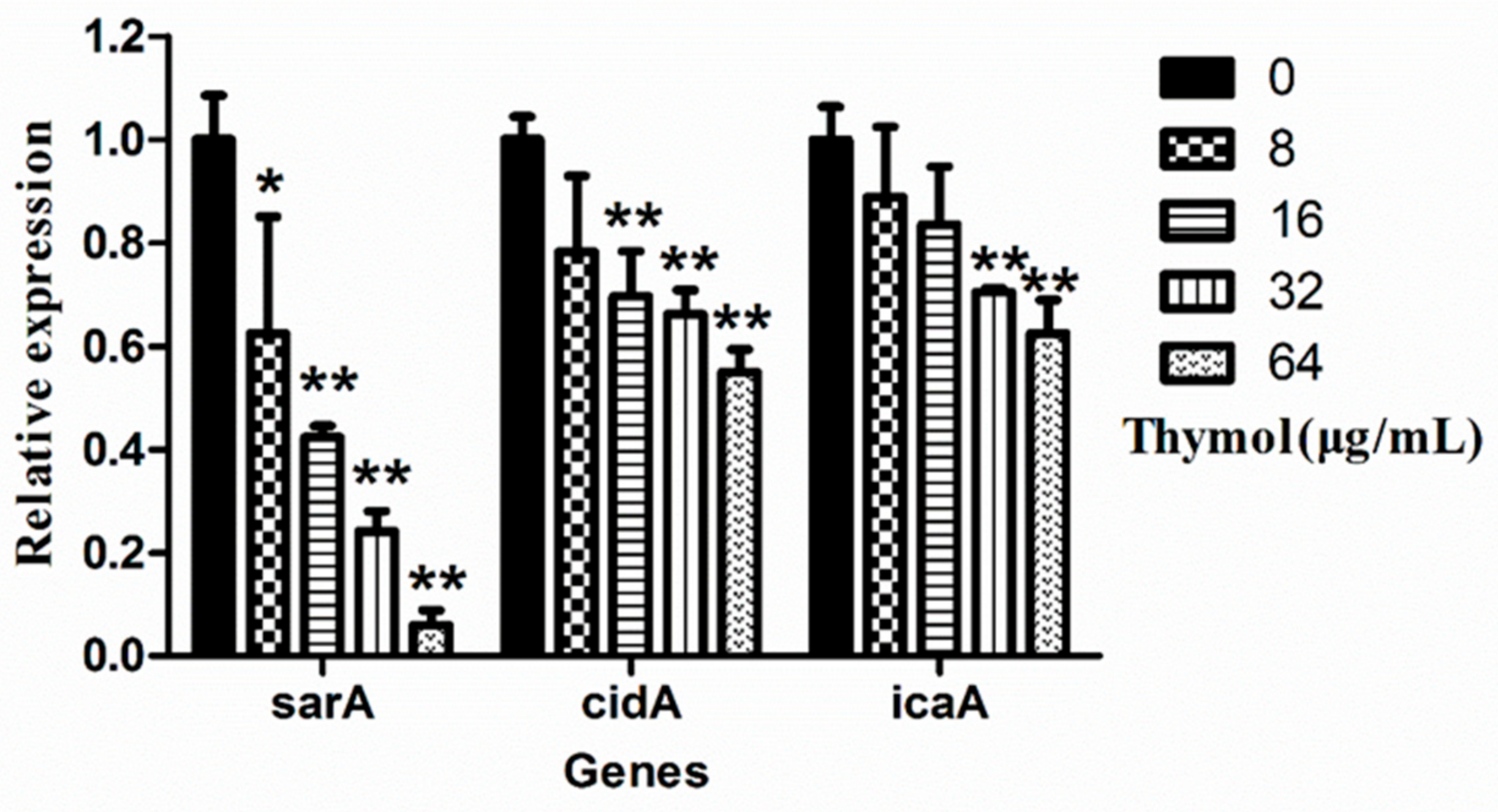
2.6. Thymol Inhibited the Transcription of Biofilm-Regulated Genes
2.7. Vancomycin Combined with Thymol Reduced Intraperitoneal Foreign-Body Biofilm Infection Caused by MRSA in Mice
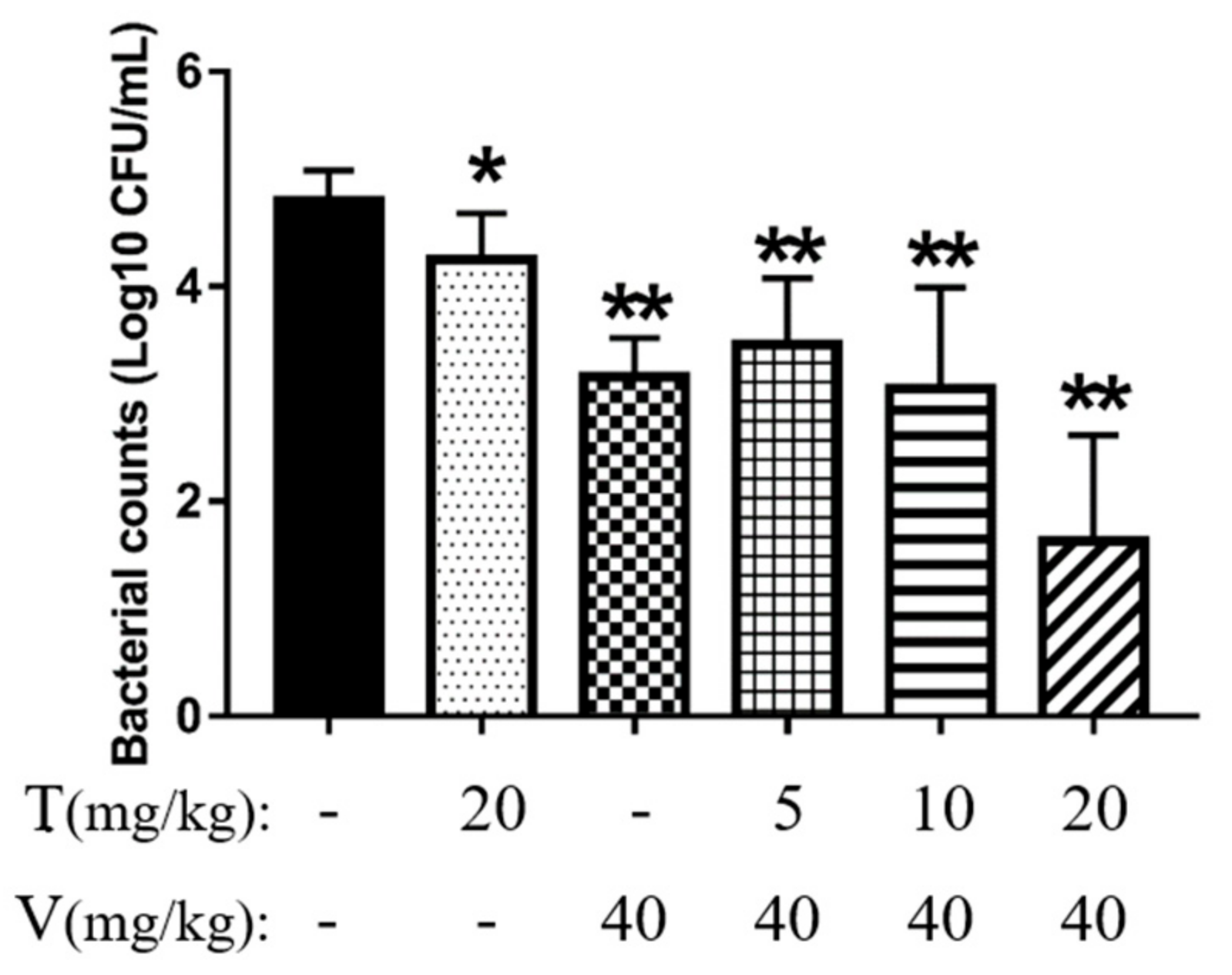
2.7.1. Vancomycin Combined with Thymol Reduced Bacterial Adhesion
2.7.2. Vancomycin in Combination with Thymol Eliminated Mature Biofilms
2.7.3. Vancomycin in Combination with Thymol Relieved the Pathological Damage Caused by MRSA Infection
2.7.4. Vancomycin in Combination with Thymol Restored the White Blood Cell (WBC) Counts in Mice
2.7.5. Vancomycin in Combination with Thymol Reduced the Levels of Interleukin 6 (IL-6) and Tumor Necrosis Factor α (TNF-α)
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Drug
4.2. Ethics Statement
4.3. Growth Curve and Checkerboard Assays
4.4. Biofilm Assessments
4.4.1. Biofilm Inhibition Assay
4.4.2. Biofilm Removal Assay
4.5. Polysaccharide Intercellular Adhesin (PIA) Assay
4.6. Determination of eDNA Release
4.7. Real-Time PCR
4.8. Effects of Thymol on MRSA Infection in a Mouse Model of Intraperitoneal Foreign-Body Infection
4.8.1. Implant Preparation
4.8.2. Establishment of a Mouse Model
4.8.3. White Blood Cell (WBC) Counts
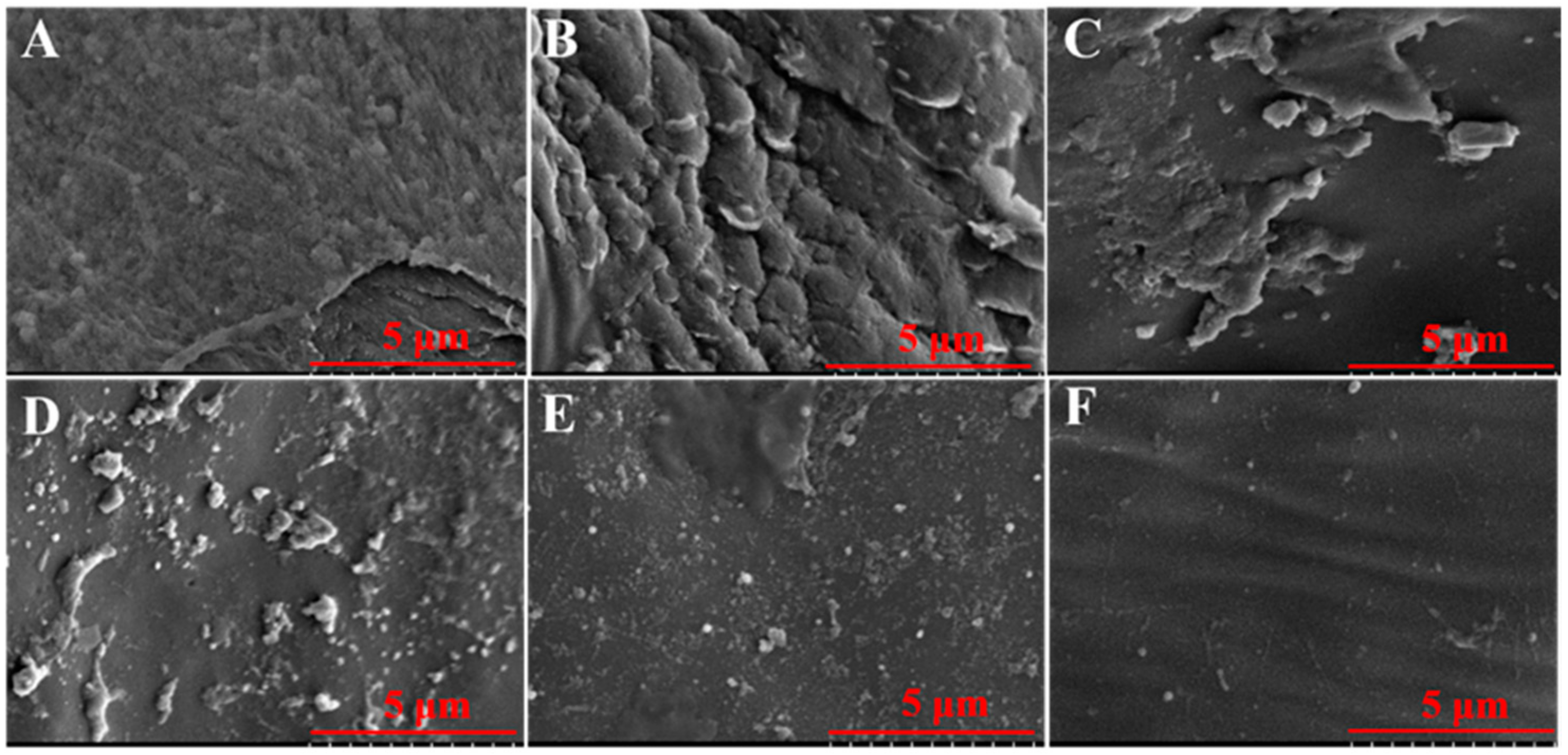
4.8.4. Colony Counting and SEM
4.8.5. Histopathological Observations
4.9. ELISA
4.10. Statistics
4.11. Data Availability
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MRSA | methicillin-resistant Staphylococcus aureus |
| PIA | polysaccharide intracellular adhesion |
| eDNA | extracellular DNA |
| SEM | scanning electron microscope |
| WBC | white blood cell |
| IL-6 | interleukin 6 |
| TNF-α | tumor necrosis factor α |
| OD | optical density |
| FICI | fractional inhibitory concentration index |
| BHI | brain heart infusion |
| LD50 | median lethal dose |
References
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Höök, M. Adhesion, invasion, and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.; Venkitanarayanan, K. Antibiofilm effect of octenidine hydrochloride on Staphylococcus aureus, MRSA and VRSA. Pathogens 2014, 3, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; McConeghy, K.W. Methicillin-resistant Staphylococcus aureus therapy: Past, present, and future. Clin. Infect. Dis. 2014, 58 (Suppl. 1), S20. [Google Scholar] [CrossRef]
- Dryden, M.; Baguneid, M.; Eckmann, C.; Corman, S.; Stephens, J.; Solem, C.; Li, J.; Charbonneau, C.; Baillon-Plot, N.; Haider, S. Complicated skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus: Epidemiology, risk factors, and presentation. Surg. Infect. 2008, 9 (Suppl. 1), s3–s10. [Google Scholar] [CrossRef] [PubMed]
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M.; et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Horswill, A.R. New approaches for treating staphylococcal biofilm infections. Ann. N. Y. Acad. Sci. 2011, 1241, 104–121. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Patel, R. The challenge of treating biofilm-associated bacterial infections. Clin. Pharm. 2007, 82, 204–209. [Google Scholar] [CrossRef]
- Mohammed, Y.H.E.; Manukumar, H.M.; Rakesh, K.P.; Karthik, C.S.; Mallu, P.; Qin, H.L. Vision for medicine: Staphylococcus aureus biofilm war and unlocking key’s for anti-biofilm drug development. Microb. Pathog. 2018, 123, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Toh, Y.S. Anti-biofilm agents: Recent breakthrough against multi-drug resistant Staphylococcus aureus. Pathog. Dis. 2014, 70, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Pozzi, C.; Houston, P.; Humphreys, H.; Robinson, D.A.; Loughman, A.; Foster, T.J.; O’Gara, J.P. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 2008, 190, 3835–3850. [Google Scholar] [CrossRef] [PubMed]
- Boles, B.R.; Horswill, A.R. Staphylococcal biofilm disassembly. Trends Microbiol. 2011, 19, 449–455. [Google Scholar] [CrossRef]
- Yoshii, Y.; Okuda, K.I.; Yamada, S.; Nagakura, M.; Sugimoto, S.; Nagano, T.; Okabe, T.; Kojima, H.; Iwamoto, T.; Kuwano, K.; et al. Norgestimate inhibits staphylococcal biofilm formation and resensitizes methicillin-resistant Staphylococcus aureus to β-lactam antibiotics. NPJ Biofilms Microbiomes 2017, 3, 18. [Google Scholar] [CrossRef]
- Costerton, J.W.; Philip, S.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 5418. [Google Scholar] [CrossRef]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955. [Google Scholar] [CrossRef]
- Mu, H.; Guo, F.; Niu, H.; Liu, Q.; Wang, S.; Duan, J. Chitosan improves anti-biofilm efficacy of gentamicin through facilitating antibiotic penetration. Int. J. Mol. Sci. 2014, 15, 22296–22308. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Subramani, R.; Narayanasamy, M.; Feussner, K. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.; Lema-Alba, R.C.; Dambolena, J.S.; Zygadlo, J.A.; Labaque, M.C.; Marin, R.H. Thymol as natural antioxidant additive for poultry feed: Oxidative stability improvement. Poult. Sci. 2017, 96. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, Z.; Mo, H.; Zhao, Y.; Li, H.; Zhang, H.; Hu, L.; Zhou, X. Thymol mediates bactericidal activity against Staphylococcus aureus by targeting an aldo-keto reductase and consequent depletion of NADPH. J Agric. Food Chem. 2019, 67, 8382–8392. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.W.; Chen, Z.Y.; Gan, Y.Y.; Li, T.; Gu, K.X.; Yin, L.Z. Antibacterial mechanism of thymol on methicillin-resistant Staphylococcus aureus (MRSA). J. South. China Agric. Univ. 2018, 6, 18–23. [Google Scholar] [CrossRef]
- McCarthy, H.; Rudkin, J.K.; Black, N.S.; Gallagher, L.; O’Neill, E.; O’Gara, J.P. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2015, 5, 1. [Google Scholar] [CrossRef]
- O’Gara, J.P.; Humphreys, H. Staphylococcus epidermidis biofilms: Importance and implications. J. Med. Microbiol. 2001, 50, 582–587. [Google Scholar] [CrossRef]
- Nethercott, C.; Mabbett, A.N.; Totsika, M.; Peters, P.; Ortiz, J.C.; Nimmo, G.R.; Coombs, G.W.; Walker, M.J.; Schembri, M.A. Molecular characterization of endocarditis-associated Staphylococcus Aureus. J. Clin. Microbiol. 2013, 51, 2131–2138. [Google Scholar] [CrossRef]
- Xiang, H.; Cao, F.; Ming, D.; Zheng, Y.; Dong, X.; Zhong, X.; Mu, D.; Li, B.; Zhong, L.; Cao, J.; et al. Aloe-emodin inhibits Staphylococcus aureus biofilms and extracellular protein production at the initial adhesion stage of biofilm development. Appl. Microbiol. Biotechnol. 2017, 101, 6671–6681. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS ONE 2016, 11, e0153468. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Figueiredo, A.M.S.; Ferreira, F.A.; Beltrame, C.O.; Côrtes, M.F. The role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit. Rev. Microbiol. 2017, 43, 602–620. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.S.; Otto, M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem. Biol. 2012, 19, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Cue, D.; Lei, M.G.; Lee, C.Y. Genetic regulation of the intercellular adhesion locus in staphylococci. Front. Cell. Infect. Microbiol. 2012, 2, 38. [Google Scholar] [CrossRef]
- Coelho, L.R.; Souza, R.R.; Ferreira, F.A.; Guimarães, M.A.; Ferreira-Carvalho, B.T.; Figueiredo, A.M. Agr RNAIII divergently regulates glucose-induced biofilm formation in clinical isolates of Staphylococcus aureus. Microbiology 2008, 154, 3480–3490. [Google Scholar] [CrossRef]
- Beenken, K.E.; Dunman, P.M.; McAleese, F.; Macapagal, D.; Murphy, E.; Projan, S.J.; Blevins, J.S.; Smeltzer, M.S. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 2004, 186, 4665–4684. [Google Scholar] [CrossRef]
- Tormo, M.A.; Martí, M.; Valle, J.; Manna, A.C.; Cheung, A.L.; Lasa, I.; Penadés, J.R. SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J. Bacteriol. 2005, 187, 2348–2356. [Google Scholar] [CrossRef]
- Valle, J.; Toledo-Arana, A.; Berasain, C.; Ghigo, J.M.; Amorena, B.; Penadés, J.R.; Lasa, I. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 2003, 48, 1075–1087. [Google Scholar] [CrossRef]
- Speziale, P.; Pietrocola, G.; Foster, T.J.; Geoghegan, J.A. Protein-based biofilm matrices in Staphylococci. Front. Cell. Infect. Microbiol. 2014, 4, 171. [Google Scholar] [CrossRef]
- Houston, P.; Rowe, S.E.; Pozzi, C.; Waters, E.M.; O’Gara, J.P. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect. Immun. 2011, 79, 1153. [Google Scholar] [CrossRef]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; LoVetri, K.; Cardona, S.T.; Madhyastha, S.; Sadovskaya, I.; Jabbouri, S.; Izano, E.A. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J. Antibiot. 2012, 65, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, B.; Al Qurashi, Y.M.; Chaieb, K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb. Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-based implant infections in orthopaedics. Adv. Exp. Med. Biol. 2015, 830, 29. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Organ. 2005, 28, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Hou, G.; Wang, L.; Zuo, X.S.; Liu, Z. Protective effects of thymol on LPS-induced acute lung injury in mice. Microb. Pathog. 2018, 116, 8–12. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.; Qu, Z.; Wang, H.; Tian, X.; Johnson, D.W.; Dong, J. Intraperitoneal vancomycin plus either oral moxifloxacin or intraperitoneal ceftazidime for the treatment of peritoneal dialysis−related peritonitis: A randomized controlled pilot study. Am. J. Kidney Dis. 2017, 70, 30–37. [Google Scholar] [CrossRef]
- Eto, D.; Lao, C.; DiToro, D.; Barnett, B.; Escobar, T.C.; Kageyama, R.; Yusuf, I.; Crotty, S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE 2011, 6, e17739. [Google Scholar] [CrossRef]
- Von Rossum, A.; Rey, K.; Enns, W.; Manku, S.; Cheema, R.; MacEwan, G.E.; Choy, J.C. Graft-derived IL-6 amplifies proliferation and survival of effector T cells that drive alloimmune-mediated vascular rejection. Transplantation 2016, 100, 1. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, F.; Xu, Y.; Zhang, J.; Qi, J.; Liu, X.; Wang, R. Thymol alleviates lipopolysaccharide-stimulated inflammatory response via downregulation of RhoA-mediated NF-κB signalling pathway in human peritoneal mesothelial cells. Eur. J. Pharmacol. 2018, 833, 210–220. [Google Scholar] [CrossRef]
- Yuan, Z.; Ouyang, P.; Gu, K.; Rehman, T.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; Shu, G.; et al. The antibacterial mechanism of oridonin against methicillin-resistant Staphylococcus aureus (MRSA). Pharm. Biol. 2019, 57, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, E.; Cedraro, N.; Mangiaterra, G.; Citterio, B.; Mobbili, G.; Minnelli, C.; Bizzaro, D.; Biavasco, F.; Galeazzi, R. Natural alkaloid berberine activity against Pseudomonas aeruginosa MexXY-mediated aminoglycoside resistance: In silico and in vitro studies. J. Nat. Prod. 2019, 7, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.; Pereira, V.; Oliveira, J.; Rodrigues, A.; de Camargo, Z.; Pereira-Neto, W.; Nascimento, N.; Castelo-Branco, D.; Cordeiro, R.; Sidrim, J.; et al. Terpinen-4-ol inhibits the growth of Sporothrix schenckii complex and exhibits synergism with antifungal agents. Future Microbiol. 2019, 14, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Tang, S.; Wu, C.; Wang, Y.; He, T.; Chen, T.; Xiao, X. Synergy between baicalein and penicillins against penicillinase-producing Staphylococcus aureus. Int. J. Med. Microbiol. 2015, 6, 501–504. [Google Scholar] [CrossRef]
- Zhang, B.; Teng, Z.; Li, X.; Lu, G.; Deng, X.; Niu, X.; Wang, J. Chalcone attenuates Staphylococcus aureus virulence by targeting sortase A and alpha-hemolysin. Front. Microbiol. 2017, 8, 1715. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Chi, H.; Sun, L. Pseudomonas fluorescens filamentous hemagglutinin, an iron-regulated protein, is an important virulence factor that modulates bacterial pathogenicity. Front. Microbiol. 2016, 7, 1320. [Google Scholar] [CrossRef]
- Saising, J.; Dube, L.; Ziebandt, A.K.; Voravuthikunchai, S.P.; Nega, M.; Götz, F. Activity of gallidermin on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2012, 56, 5804. [Google Scholar] [CrossRef]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef]
- Freeman, D.J.; Falkiner, F.R.; Keane, C.T. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef]
- Podbielska, A.; Galkowska, H.; Stelmach, E.; Mlynarczyk, G.; Olszewski, W.L. Slime production by Staphylococcus aureus and Staphylococcus epidermidis strains isolated from patients with diabetic foot ulcers. Arch. Immunol. Exp. 2010, 58, 321. [Google Scholar] [CrossRef]
- Rice, K.C.; Mann, E.E.; Endres, J.L.; Weiss, E.C.; Cassat, J.E.; Smeltzer, M.S.; Bayles, K.W. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2007, 104, 8113–8118. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; He, X.; Yuan, Z.W.; Yin, Z.Q.; Fu, H.; Lin, J.; He, C.; Liang, X.; Lv, C.; Shu, G.; et al. Erianin against Staphylococcus aureus Infection via Inhibiting Sortase A. Toxins 2018, 10, 385. [Google Scholar] [CrossRef]
- Thomas, D.S.; Kenneth, J.L. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Christensen, L.D.; van Gennip, M.; Jakobsen, T.H.; Alhede, M.; Hougen, H.P.; Høiby, N.; Bjarnsholt, T.; Givskov, M. Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J. Antimicrob. Chemother. 2012, 67, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Lei, D.; Chen, Y.; Li, Y.; Kong, J.; et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Kabata, T.; Ohtani, K.; Kajino, Y.; Shirai, T.; Tsuchiya, H. Inhibition of biofilm formation on iodine-supported titanium implants. Methods Int. Orthop. 2017, 41, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [CrossRef] [PubMed]

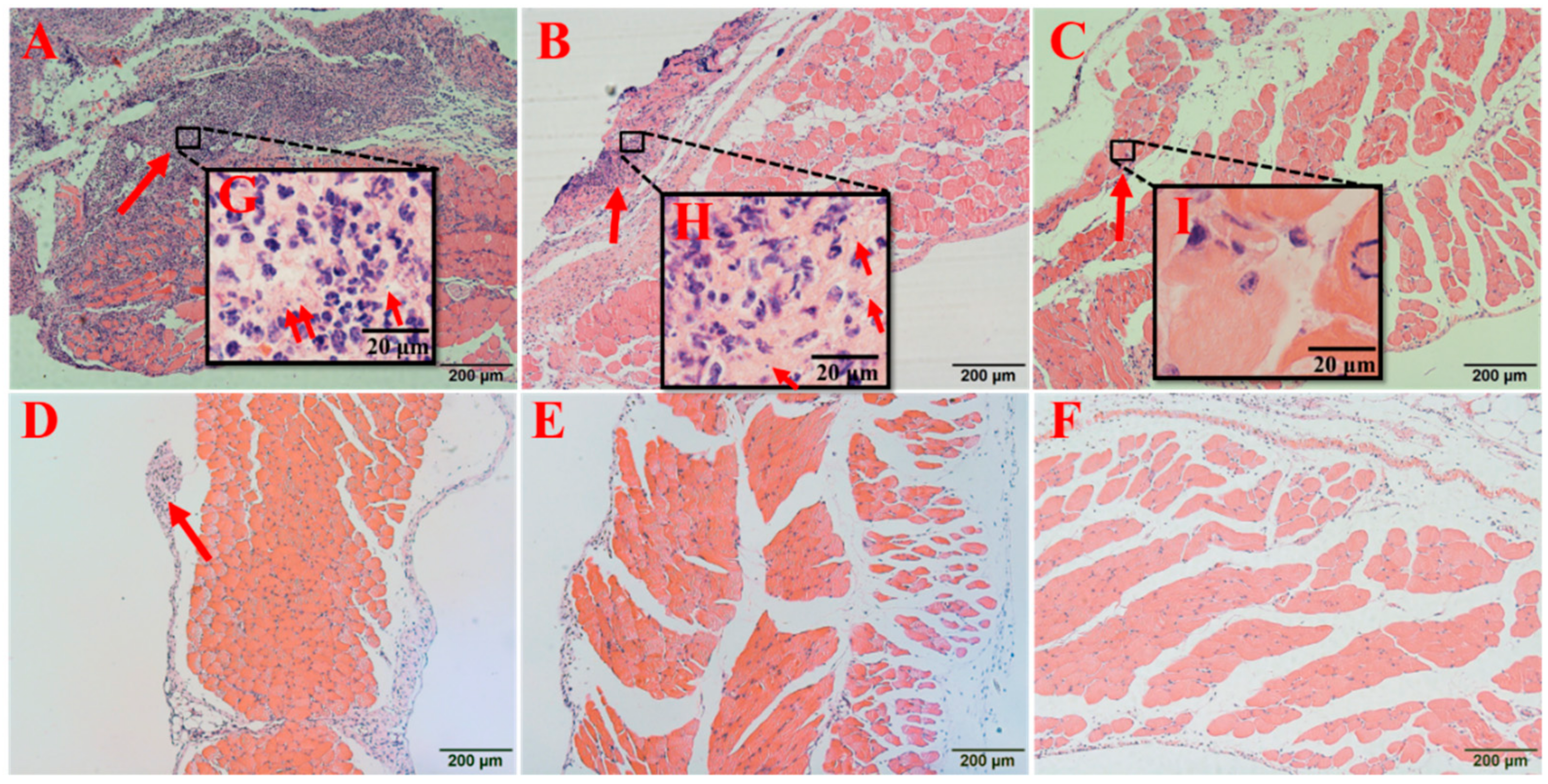
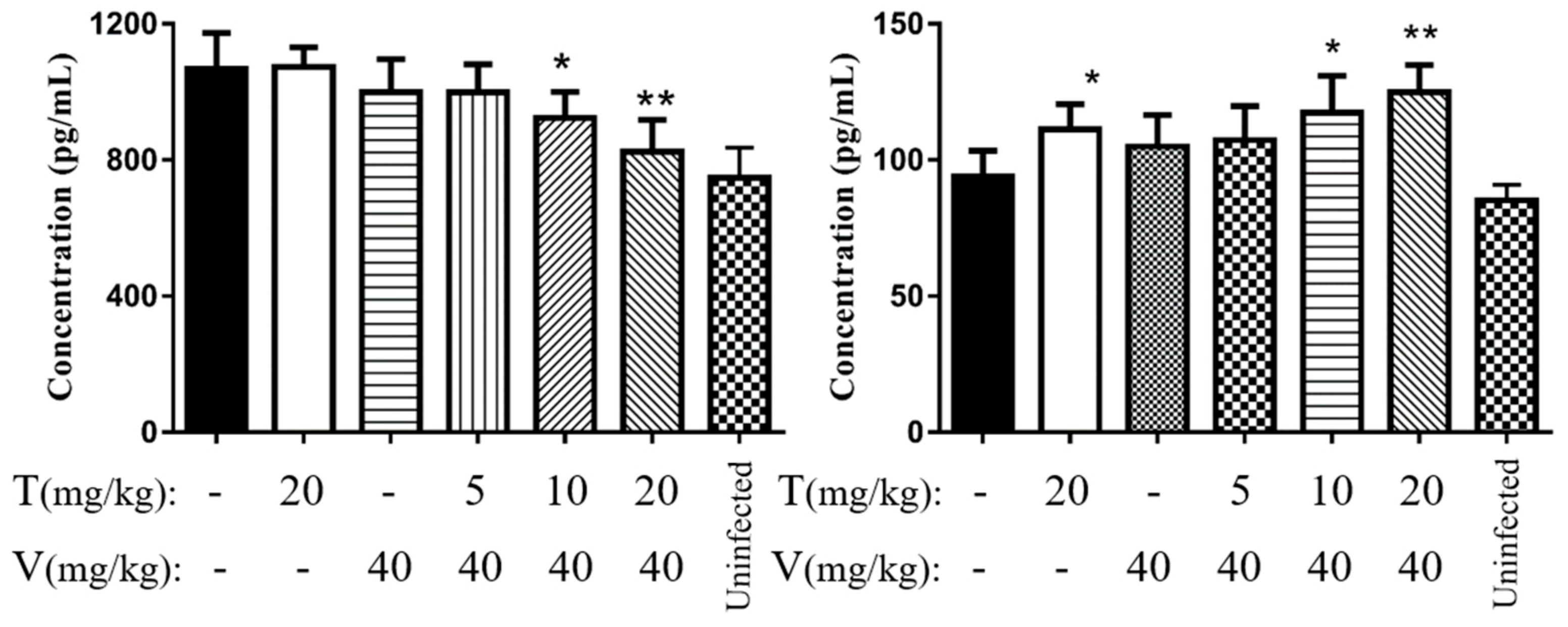
| Group | Treatment | WBC Counts (109/L) |
|---|---|---|
| A | PBS | 7.42 ± 1.94 |
| B | Thymol (20 mg/kg) | 5.10 ± 0.98 * |
| C | Vancomycin hydrochloride (40 mg/kg) | 6.72 ± 1.67 |
| D | Vancomycin hydrochloride (40 mg/kg) + thymol (5 mg/kg) | 4.08 ± 1.89 * |
| E | Vancomycin hydrochloride (40 mg/kg) + thymol (10 mg/kg) | 3.24 ± 1.02 ** |
| F | Vancomycin hydrochloride (40 mg/kg) + thymol (20 mg/kg) | 4.30 ± 2.27 * |
| G | Uninfected | 5.52 ± 1.43 |
| Primer | Sequence (5′→3′) | Product/bp |
|---|---|---|
| icaA-F | TTTCGGGTGTCTTCACTCTAT | 229 |
| icaA-R | CGTAGTAATACTTCGTGTCCC | |
| cidA-F | GATTTTTCATCTTCCCTTAGCCG | 300 |
| cidA-R | GCGTCTACACCTTTACGATGTTTAT | |
| sarA-F | TTGTTTTCGCTGATGTAT | 100 |
| sarA-R | CAATGGTCACTTATGCTG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Dai, Y.; Ouyang, P.; Rehman, T.; Hussain, S.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; et al. Thymol Inhibits Biofilm Formation, Eliminates Pre-Existing Biofilms, and Enhances Clearance of Methicillin-Resistant Staphylococcus aureus (MRSA) in a Mouse Peritoneal Implant Infection Model. Microorganisms 2020, 8, 99. https://doi.org/10.3390/microorganisms8010099
Yuan Z, Dai Y, Ouyang P, Rehman T, Hussain S, Zhang T, Yin Z, Fu H, Lin J, He C, et al. Thymol Inhibits Biofilm Formation, Eliminates Pre-Existing Biofilms, and Enhances Clearance of Methicillin-Resistant Staphylococcus aureus (MRSA) in a Mouse Peritoneal Implant Infection Model. Microorganisms. 2020; 8(1):99. https://doi.org/10.3390/microorganisms8010099
Chicago/Turabian StyleYuan, Zhongwei, Yuyun Dai, Ping Ouyang, Tayyab Rehman, Sajjad Hussain, Tianyi Zhang, Zhongqiong Yin, Hualin Fu, Juchun Lin, Changliang He, and et al. 2020. "Thymol Inhibits Biofilm Formation, Eliminates Pre-Existing Biofilms, and Enhances Clearance of Methicillin-Resistant Staphylococcus aureus (MRSA) in a Mouse Peritoneal Implant Infection Model" Microorganisms 8, no. 1: 99. https://doi.org/10.3390/microorganisms8010099
APA StyleYuan, Z., Dai, Y., Ouyang, P., Rehman, T., Hussain, S., Zhang, T., Yin, Z., Fu, H., Lin, J., He, C., Lv, C., Liang, X., Shu, G., Song, X., Li, L., Zou, Y., & Yin, L. (2020). Thymol Inhibits Biofilm Formation, Eliminates Pre-Existing Biofilms, and Enhances Clearance of Methicillin-Resistant Staphylococcus aureus (MRSA) in a Mouse Peritoneal Implant Infection Model. Microorganisms, 8(1), 99. https://doi.org/10.3390/microorganisms8010099