1. Introduction
Bacterial adhesion is an essential process in inter-species interaction that ultimately enables the development and maturation of multi-species biofilm. Adhesion has been observed among all the genetically distinct bacteria isolated from a number of human sites including the gut, urogenital tract [
1], and the oral cavity [
2]. It is mainly mediated by the interaction of bacterial adhesins to a specific co-receptor in the partner species [
3]. Many adhesins are subject to complex and coordinated regulatory processes in response to quorum sensing, bacterial stress, and host susceptibility, which consequently impacts the development of multi-species biofilms [
4,
5,
6,
7,
8]. Biofilms of the oral cavity are one of the most characterized biofilms as they are easily accessible and the main cause for the progression of diseases like periodontitis and caries [
9].
Fusobacterium nucleatum, an anaerobic, gram-negative commensal and opportunistic pathogen, is one of the most abundant microorganisms of the oral cavity [
10,
11,
12,
13]. It is an important species in dental biofilm ecology as it interacts with both early and late colonizers. The adhesive nature of
Fusobacterium can be attributed to its repertoire of adhesins enabling the binding to various salivary proteins, other microorganisms, and host substrata [
14,
15,
16,
17,
18]. One fusobacterial adhesin in particular, RadD, was identified as the main adhesin to mediate attachment to a number of gram-positive early colonizers [
15] and supports fusobacterial adherence to certain isolates of the periodontal pathogen
Porphyromonas gingivalis [
19]. The RadD adhesin has also been implicated in the induction of cell death in human lymphocytes [
20], indicating that this adhesin may be critical in the establishment of disease. Sequence and transcriptional analyses revealed that RadD is encoded by the last gene of a four-gene operon, which is conserved across all four subspecies of
F. nucleatum [
15]. The gene directly upstream of
radD, previously described as
fad-I, encodes the lipoprotein FAD-I, which is characterized by its ability to induce human β-defensin 2 (hBD-2) in oral epithelial cells in a subspecies-dependent manner [
21,
22]. FAD-I of
F. nucleatum ssp
nucleatum, type strains ATCC 25586 and ATCC 23726, induce expression of hBD-2, while FAD-I of
F. nucleatum ssp
polymorphum, type strain ATCC 10953, fails to do so. This differential antimicrobial peptide induction could have a profound influence on oral community composition. No function has been identified for the two other proteins encoded by the additional genes in the operon, which we denominated as RapA and RapB (RadD-associated proteins). While a recent study highlights the similarity of RapA to the fusobacterial adhesin FadA and suggests it to be FadA2, functional characterization is still missing [
23].
In the present study, we performed a comprehensive investigation of the role of the genes encoded by the radD-containing four-gene operon in interspecies interaction of the F. nucleatum ssp nucleatum type strain ATCC 23726 and F. nucleatum ssp polymorphum type strain ATCC 10953. Our studies revealed that lack of fad-I led to increased binding of fusobacteria to Streptococcus gordonii by triggering significant overexpression of radD, which resulted in an increase in thickness and density of the biofilm formed with this important partner species. Furthermore, we demonstrated that the presence of the RadD adhesin is not needed for FAD-I-mediated regulation of radD expression and that the binding to S. gordonii leads to suppression of radD transcription in a FAD-I-independent manner.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
F. nucleatum ssp
nucleatum strain ATCC 23726,
F. nucleatum ssp
polymorphum strain ATCC 10953, and their respective mutant derivatives generated in this study (
Table 1) were maintained on Columbia agar supplemented with 5% sheep blood or in Columbia broth (CB) (Difco, Detroit, MI) under anaerobic conditions (10% H
2, 10% CO
2, 80% N
2) at 37 °C. Strain
S. gordonii Challis DL1 and its mCherry-expressing derivative [
24] were maintained on Brain Heart Infusion) (BHI) agar or BHI broth (Difco, Detroit, MI) under anaerobic conditions (10% H
2, 10% CO
2, 80% N
2) at 37 °C. Thiamphenicol at 5 μg mL
−1 and clindamycin at 1 μg mL
−1 (MP Biomedicals, Irvine, CA) were used for the selection and maintenance of strains possessing the
catP and
ermB cassette, respectively.
2.2. Mutant Strain and Plasmid Construction
2.2.1. Allelic Exchange Mutagenesis
For the generation of mutants, allelic exchange mutagenesis was used to replace the gene of interest with the
catP gene that confers thiamphenicol resistance in transformable fusobacteria. Briefly, the constructs were generated by fusing upstream (1 KB) and downstream (1 KB) regions of gene of interest to either end of the
catP gene using the primers listed in
Supplementary Table S1. The
catP gene for each construct was amplified from pHS31 [
26]. The primers contained an overlap of 25–30 base pairs to allow fusion in PCR reactions and the fusion PCR was carried out with Phusion HF DNA polymerase (NEB) as described [
28]. The fusion product was cloned into pCR-Blunt II TOPO (Invitrogen) and transformed into One Shot TOP10 competent
E. coli cells according to manufacturer’s protocols to generate the respective plasmids used for gene replacement (
Table 1). The plasmid DNA of the recombinants were isolated with the Qiagen Mini prep kit (Qi-agen, Hilden, Germany) and the presence of the construct was confirmed by restriction digestion and sequencing. Further, purified plasmids were electroporated into the fuso-bacterial strains used in this study to generate the respective derivatives lacking target genes according to previously described protocols (
Supplementary Figure S1 [
29]. The transformed products were plated on selective media containing 5 μg mL
−1 thiamphenicol. The genomic DNA of the colonies obtained after transformation was isolated using GENelute kit (SIGMA) and analyzed by PCR with internal primers for presence of
catP gene and absence of the respective gene.
2.2.2. Complementation
For generation of the strain complementing lack of
fad-I in the Δ
fad-I mutant of
F. nucleatum ssp
nucleatum ATCC 23726, the previously described plasmid pBS5 [
22] was transformed into the Δ
fad-I mutant of ATCC 23726 as described [
29].
2.3. Coaggregation Assay
Coaggregation assays were performed according to previously published protocols [
15,
19] in coaggregation buffer (CAB; 150 mM NaCl, 1 mM Tris, 0.1 mM CaCl
2, 0.1 mM MgCl
2 H
2O [pH 7.5]). Briefly, cells were pelleted and resuspended in CAB to a final concentration of 2 × 10
9 cells (OD
600 of 2). Suspensions of strains to be examined for coaggregation were combined with an equal volume of a test strain adjusted to the same cellular concentration in CAB to a total volume of 400 μL in a reaction tube. The optical densities of reaction mixtures were obtained spectrophotometrically immediately after addition of the second partner strain and vortexing (OD
t = 0 min). After 10 min of incubation, reaction mixtures were centrifuged at low speed (100×
g for 1 min) to pellet coaggregated cells, while leaving non-aggregated bacteria in suspension. Optical densities of the supernatants were measured after the 10 min incubation (OD
t = 10 min) in order to quantify coaggregation. The level of inter-species binding is expressed as % coaggregation, which refers to the proportion of cells that fall out of the original cell suspension due to aggregate formation between partner species. The % coaggregation of the test reactions were calculated as (OD
t = 0 min − OD
t = 10 min)/(OD
t = 0 min). These values were averaged across at least three independent experiments and represented as percentages.
2.4. Co-Incubation of F. Nucleatum with Partner Species
Cells were grown to mid-log phase and 1 mL of 109 cells (OD600 = 1) of F. nucleatum or its mutant derivatives were added to sterile 2 mL microcentrifuge tubes with 1 mL of 109 cells of S. gordonii (OD600 = 1). Control tubes containing 1 mL of 109 cells of F. nucleatum or its mutant derivatives alone were also prepared. Cells were centrifuged for 5 min at 8000× g, the supernatant was discarded, and replaced with 1 mL of CB for F. nucleatum alone tubes, 2 mL of CB for tubes with F. nucleatum ATCC 23726 and S. gordonii. The pellets were incubated anaerobically (10% H2, 10% CO2, 80% N2) at 37 °C for 30 min. Post incubation, the supernatant was removed after a quick centrifugation and the cell pellets were stored in −80 °C.
2.5. Nucleic Acid (DNA and RNA) Isolation and cDNA Generation
Genomic DNA was isolated using the GenElute Bacterial Genomic DNA Kit (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer’s instructions. Total RNA was extracted from cells using the PureLink RNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA), followed by a TURBO DNA-free DNase Treatment (Thermo Fisher Scientific, Waltham, MA, USA), prior to cleaning and concentration with the RNA Clean & Concentrator (Zymo Research, Irvine, CA, USA). One microgram of total RNA was used for cDNA synthesis with the SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol.
2.6. Quantitative (Real-Time) Polymerase Chain Reaction
Gene-specific primers (
Table 2) were used to amplify transcript regions for signal detection by qPCR with the iCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA) in a total reaction volume of 20 μL containing 10 μL of 2X iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA), 0.5 μM each of forward and reverse primers, and 1 μL of 1:10 diluted cDNA.
Amplification and detection were carried out in 96-well optical plates (Thermo Fisher Scientific, Waltham, MA). Each qPCR run was performed with an initial incubation of 10 min at 95 °C followed by 40 cycles of denaturing at 95 °C for 15 s and annealing and elongation at 60 °C for 1 min. After the 40 cycles of amplification, an additional denaturing step was performed at 95 °C for 1 min followed by annealing and elongation at 60 °C for 1 min. A melting curve analysis was completed after each run. Three independent qPCR runs were performed with three technical replicates for each sample to assess reproducibility and inter-run variability. Following amplification, relative expression levels between samples were calculated as fold changes normalized to rpoB reference gene amplification.
2.7. Biofilm Growth
2.7.1. Crystal Violet Assay
Dual-species biofilms of
F. nucleatum ssp
nucleatum (wild-type and its mutant strains) with
S. gordonii were grown in sterile 48-well culture plates (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, fusobacterial and
S. gordonii cells were diluted from overnight cultures to 2 × 10
8 and 1 × 10
5 cells, respectively, into 50% SHI medium [
30] and incubated under anaerobic conditions (10% H
2, 10% CO
2, 90% N
2) for 20 h. The biomass of the biofilms was evaluated by crystal violet (CV) staining according to published procedures [
31]. In brief, supernatants were removed from each well and rinsed once with 500 μL of sterile phosphate-buffered saline (PBS). Plates were inverted and dried. Next, attached bacteria were fixed at room temperature for 15 min by adding 500 μL of methanol into each well. The biofilms were then stained with a 500 μL aqueous solution of 0.5% crystal violet (Thermo Fisher Scientific, Waltham, MA, USA) for 15 min at room temperature, followed by careful rinsing with Millipore water until there was no visible trace of the stain left. Bound stain was extracted by adding 500 μL of 95% ethanol. The optical density of each well was measured at 570 nm and was represented as relative to negative control wells that only contained SHI medium. All wild-type and mutant combinations as well as mono-species control biofilms were grown in at least three biological replicates.
2.7.2. Confocal Microscopy
Dual-species biofilms of
F. nucleatum ATCC 23726 and the mCherry-expressing derivative of
S. gordonii challis DL1 [
24] were grown in 8-well chambers on optical plastic slides (Thermo Fisher Scientific, Waltham, MA), which were coated with microorganism-free pooled saliva and UV-sterilized for 1 h. Biofilms were inoculated and grown as described above for the crystal violet assay and washed with sterile PBS before adding 20 μM SYTO 9 (green) for visualization by confocal microscopy with an LSM-780 confocal microscope (Zeiss, Germany). A Zeiss plan-Apochromat 63× oil immersion objective was used for image acquisition. SYTO 9 fluorescence was imaged using a 488 nm laser with a 500 to 530 nm emission range capture and then pseudo-colored as blue. The mCherry-expressing
S. gordonii in the biofilm was visualized using a 561 nm excitation laser in combination with a 600 to 650 nm emission range capture. Orthogonal sectioning of the z-stacks and height measurements were performed using the Zeiss Zen software. Multiple areas of the biofilm (n = 5) were imaged following a consistent x-y grid. Data from all 3 set of experiments were used to calculate the mean height of the biofilm. The height of the biofilm was determined using the ZEN Blue Image Analysis Module (Zeiss, Germany).
2.8. Statistical Analysis
Student’s t-test was performed to determine statistical significance using Excel (Microsoft, Seattle, WA, USA, Version 2016).
3. Results
In fusobacteria, including the genetically tractable strains of
F. nucleatum ssp
nucleatum ATCC 23726 and
F. nucleatum ssp
polymorphum ATCC 10953, the adhesin RadD is encoded by the last gene of a four-gene operon [
15], comprised of the homologs of FN1529, FN1528, FN1527 (
fad-I) [
21], and FN1526 (
radD). The corresponding homologs of these genes in the
nucleatum subspecies ATCC 23726 are encoded by HMPREF0397_1642, HMPREF0397_1641, HMPREF0397_1640, and HMPREF0397_1639, respectively, and the open reading frames FNP_1046, FNP_1047, FNP_1048, and FNP_1049 in the
polymorphum subspecies ATCC 10953 [
32]. The genes encoded by homologs of FN1529 and FN1528 were previously unnamed and will be referred to in this study as
rapA (radD-
associated
proteins A) and
rapB, respectively. Based on the sequence similarity of RapA to the previously characterized FadA adhesin [
18],
rapA was also suggested to be named
fadA2 by a very recent bioinformatic study [
23]. All the four genes of the operon are predicted to be associated with membrane with Predict Protein (
www.predictprotein.com accessed on 10 October 2019). RapA (127aa) is predicted to be a member of the FadA family [
18,
23], while RapB (123aa) is predicted to be a lipoprotein. We previously identified FAD_I (129aa) as lipoprotein [
21,
22] and RadD (3546aa) as an autotransporter-like adhesin [
15,
20].
In our earlier studies, we inactivated
radD of ATCC 23726 and ATCC 10953 as well as
fad-I of ATCC 10953 [
15,
22,
25] as part of their functional characterization. While we demonstrated a role for RadD as a major adhesin for interaction with streptococcal species, investigation of FAD_I was limited to its role in hBD2 induction in epithelial cells. For a comprehensive investigation of all genes encoded by the
radD-containing four-gene operon in interspecies interaction, we created individual gene inactivation mutants in the
rapA,
rapB, and
fad-I genes in
F. nucleatum ssp
nucleatum ATCC 23726 as well as
rapA and
rapB in
F. nucleatum ssp
polymorphum ATCC 10953 (
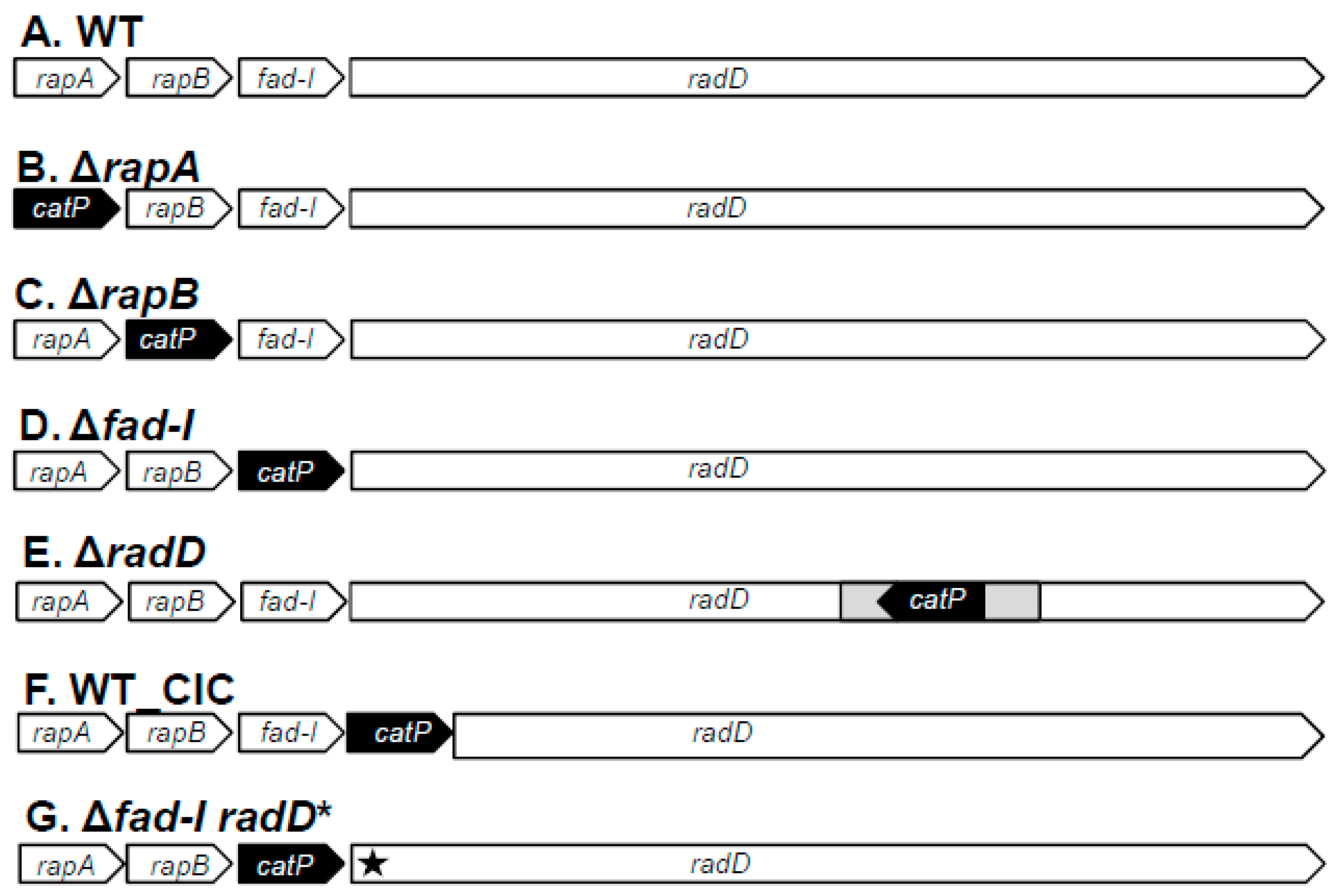
Figure 1) with the approaches described in Material and Methods. As mentioned above, inactivation mutants of ATCC 23726 and ATCC 10953 lacking the remaining genes encoded by the
radD-containing operon were generated in our previous studies [
15,
22,
25]. We also constructed strain WT-CIC (
catP Insertion
Control) in which the
catP resistance cassette was inserted between
fad-I and
radD as a control strain to address possible polar effects of
catP insertion on gene expression (
Figure 1).
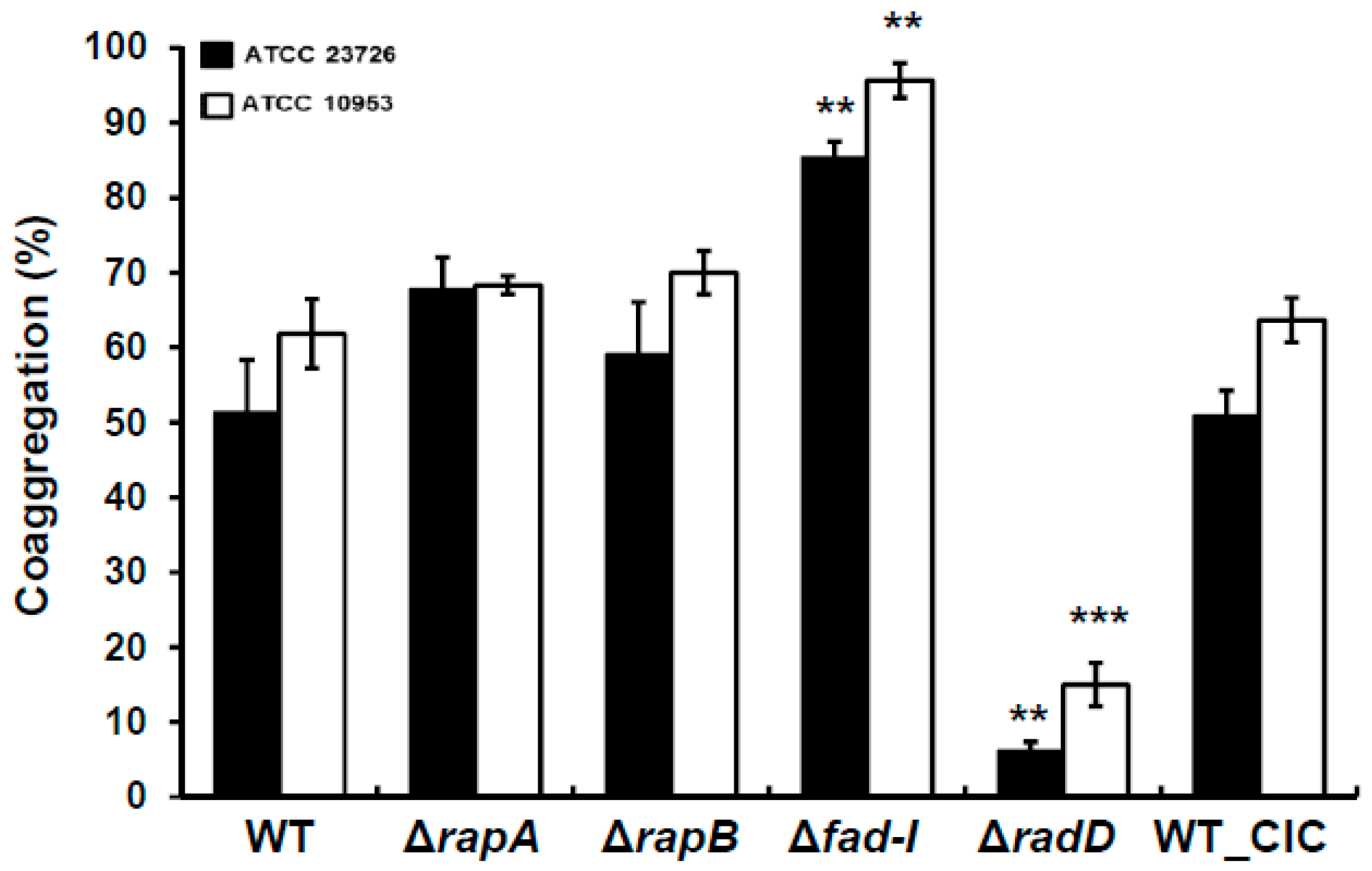
3.1. Inactivation of radD-Operon Genes in F. Nucleatum Alters Coaggregation with S. Gordonii
The Δ
rapA, Δ
rapB, Δ
fad-I, and Δ
radD mutant derivatives of both
F. nucleatum ssp
nucleatum and ssp
polymorphum were subjected to coaggregation assays with
S. gordonii to characterize their binding to this important partner species. Quantitative coaggregation assays revealed similar coaggregation behavior for the corresponding mutants of both fusobacterial subspecies with
S. gordonii (
Figure 2). Individual inactivation of the genes encoding the RapA and RapB proteins did not result in significant changes compared to the wild-type in either one of the two
F. nucleatum subspecies tested. Specifically, coaggregation of
S. gordonii with the Δ
rapA mutants of
F. nucleatum ssp
nucleatum and
F. nucleatum ssp
polymorphum resulted in 67 ± 4% and 68 ± 1% coaggregation, respectively, while coaggregation with the Δ
rapB derivatives was 59 ± 7% for
F. nucleatum ssp
nucleatum and 70 ± 3% for
F. nucleatum ssp
polymorphum compared to 51 ± 7% and 62 ± 4% for the corresponding wild-type strains. In contrast to the Δ
rapA and Δ
rapB mutants, the Δ
fad-I mutants in both subspecies showed significantly increased levels of coaggregation compared to the wild-type (85 ± 2% for
F. nucleatum ssp
nucleatum 23726 and 96 ± 2% for
F. nucleatum ssp
polymorphum 10953) (
Figure 2). Consistent with our previous reports, the
radD mutants for both strains displayed a coaggregation deficient phenotype (
F. nucleatum ssp
nucleatum, 6 ± 1% coaggregation and
F. nucleatum ssp
polymorphum, 15 ± 3% coaggregation), confirming RadD as a major adhesin in the interaction with
S. gordonii. As expected, the WT-CIC strain of both
F. nucleatum ssp
nucleatum and
F. nucleatum ssp
polymorphum exhibited coaggregation levels similar to their wild-type parents with 50 ± 3% and 63 ± 3%, respectively.
3.2. Enhanced Coaggregation in Δfad-I Mutant Correlates with Increased RadD Expression
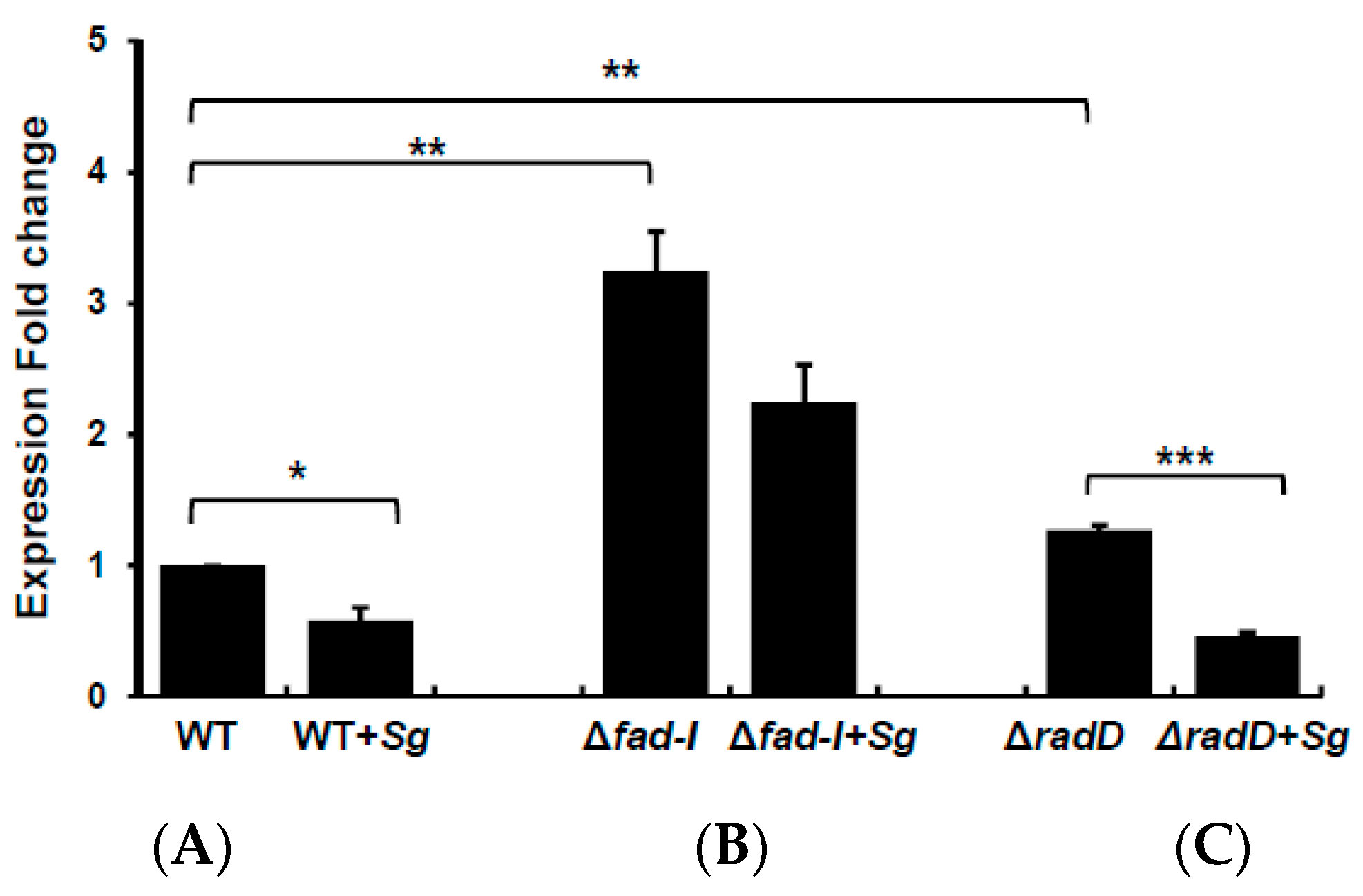
Next, we employed qPCR to determine how the lack of RapA, RapB, FAD-I, and RadD affects
rapA,
rapB,
fad-I, and
radD expression (
Figure 3). As expected, no transcripts were detected in the Δ
rapA, Δ
rapB, and Δ
fad-I mutant derivatives for genes that were replaced by
catP gene (
Figure 1). However,
radD transcript levels similar to wild-type can be detected in the
radD mutants of both
F. nucleatum ssp
nucleatum and
F. nucleatum ssp
polymorphum by using qPCR primer designed upstream of the insertion. Even though some of the differences compared to wild-type gene expression were statistically significant due to the low data variability, they mostly did not exceed or even come close to two-fold changes in expression levels. This included
rapA expression (
Figure 3A) in Fnn_WT_CIC (0.97 ± 0.05), Fnp_Δ
rapB (1.3 ± 0.14), Fnn_Δ
fad-I (1.24 ± 0.29), Fnp_Δ
fad-I (1.30 ± 0.95), Fnn_Δ
radD (0.94 ± 0.06); Fnp_Δ
radD 1.48 ± 0.60),
rapB expression (
Figure 3B) in Fnn_WT_CIC (0.84 ± 0.09), Fnp_WT_CIC (0.52 ± 0.11), Fnn_Δ
rapA (1.92 ± 0.43), Fnp_Δ
rapA (1.29 ± 0.02), Fnn_Δ
fad-I (1.27 ± 0.42), Fnp_Δ
fad-I (1.23 ± 0.13), Fnn_Δ
radD (0.90 ± 0.52), Fnp_Δ
radD (0.95 ± 0.04), and
fad-I expression (
Figure 3C) in Fnn_Δ
rapA (1.72 ± 0.49), Fnp_Δ
rapA (1.38 ± 0.46), Fnn_Δ
rapB (1.42 ± 0.15), Fnp_Δ
rapB (1.69 ± 0.58), Fnn_Δ
radD (0.80 ± 0.07), Fnp_Δ
radD (1.21 ± 0.36), Fnn_WT_CIC (0.67 ± 0.16). Exceptions were somewhat reduced expression of
rapA in Fnn_Δ
rapB (0.41 ± 0.12-fold change) and Fnp_WT_CIC (0.47 ± 0.08 fold change) (
Figure 3A), in addition to lower expression of
fad-I in Fnp_WT_CIC (0.38 ± 0.08 fold change) (
Figure 3C). In contrast to the mostly unaltered expression levels of
rapA,
rapB, and
fad-I,
radD expression levels were significantly (
p ≤ 0.001) elevated by several-fold in some of the mutant derivatives and especially in the Δ
fad-I mutant (
Figure 3D). Interestingly, elevated
radD transcript levels correlated with the observed significant increase in coaggregation with
S. gordonii (
Figure 2). While the enhancing effect on
radD expression was limited in the
F. nucleatum subspecies
nucleatum to the Δ
fad-I mutant (Fnp_Δ
rapA—1.76 ± 0.23; Fnp_Δ
rapB—1.33 ± 0.26; Δ
fad-I—3.49 ± 0.79), in the
polymorphum subspecies, the lack of either one of these genes resulted in increased
radD transcript levels with Fnp_Δ
fad-I exhibiting the largest effect (Fnp_Δ
rapA—2.28 ± 0.27; Fnp_Δ
rapB—3.26 ± 0.23; Fnp_Δ
fad-I—5.95 ± 1.20). Lack of the RadD protein does not seem to affect the expression of the encoding gene as
radD transcript levels were similar to wild-type for Fnn_Δ
radD (0.89 ± 0.13) and Fnp_Δ
radD (0.91 ± 0.09). Furthermore,
radD expression was not elevated in Fnn_WT_CIC (1.06 ± 0.20) and Fnp_WT_CIC (1.22 ± 0.29), which largely rules out polar effects of
catP insertion upstream of
radD as a cause for the observed altered expression.
Since lack of
fad-I produced the strongest phenotype in both subspecies investigated in this study, we constructed an additional mutant strain, Fnp_Δ
fad-
I*, to distinguish if lack of the
fad-I gene encoding sequence in the gene replacement mutants or the lack of the FAD-I protein led to the observed increase in
radD expression. Strain Fnp_Δ
fad-
I* contains the full
fad-I gene sequence but does not produce the FAD-I protein due to a mutated translation start codon (TGA was mutated to AAT). The Fnp_Δ
fad-
I* mutant strain displayed a very similar phenotype to the Fnp_Δ
fad-
I derivative with significantly increased
radD expression and enhanced coaggregation with
S. gordonii (
Supplementary Figure S2). However, the Fnp_Δ
fad-
I* mutant was unstable with a strong tendency to revert to a start codon and was therefore excluded from further investigation.
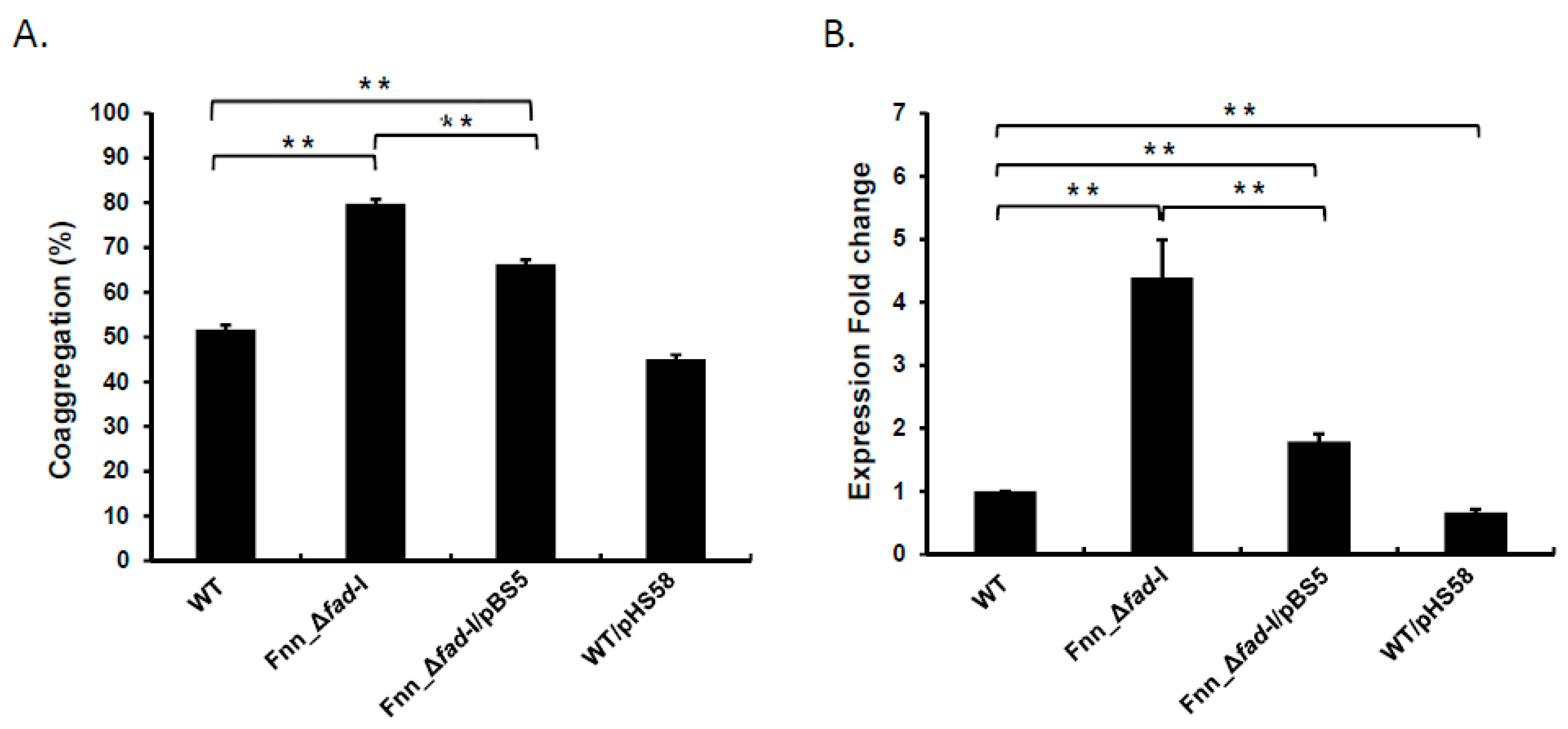
3.3. Plasmid-Based fad-I Complemention Reduced Coaggregation and radD Transcript Levels in Fusobacterial Strains Lacking fad-I
Lack of
fad-I exhibited the most significant increases in
radD transcripts levels as well as coaggregation with
S. gordonii in both fusobacterial subspecies investigated in this study. Strain Fnn_Δ
fad-I carrying the
fad-I-expressing plasmid pBS5 [
22] and pHS58 as empty vector control were assessed for coaggregation with
S. gordonii and
radD expression. We observed a significant reduction in the coaggregation percentage of the Fnn_Δ
fad-I/pBS5 (66.2 ± 2.4) complemented strain compared to the Fnn_Δ
fad-I carrying the empty pHS58 vector control (79.7 ± 1.3), although it was still higher than the wild-type (51.6 ± 2.8) (
Figure 4A). Similarly,
radD transcript levels were significantly reduced from 4.4-fold in the Fnn_Δ
fad-I mutant to 1.8-fold in the Fnn_Δ
fad-I/pBS5 complement strain (
Figure 4B). This reduction, however, was not to the same level as present in the parent strain
F. nucleatum ssp
nucleatum ATCC 23726, indicating that plasmid-based FAD-I production does not allow complementation to wild-type levels.
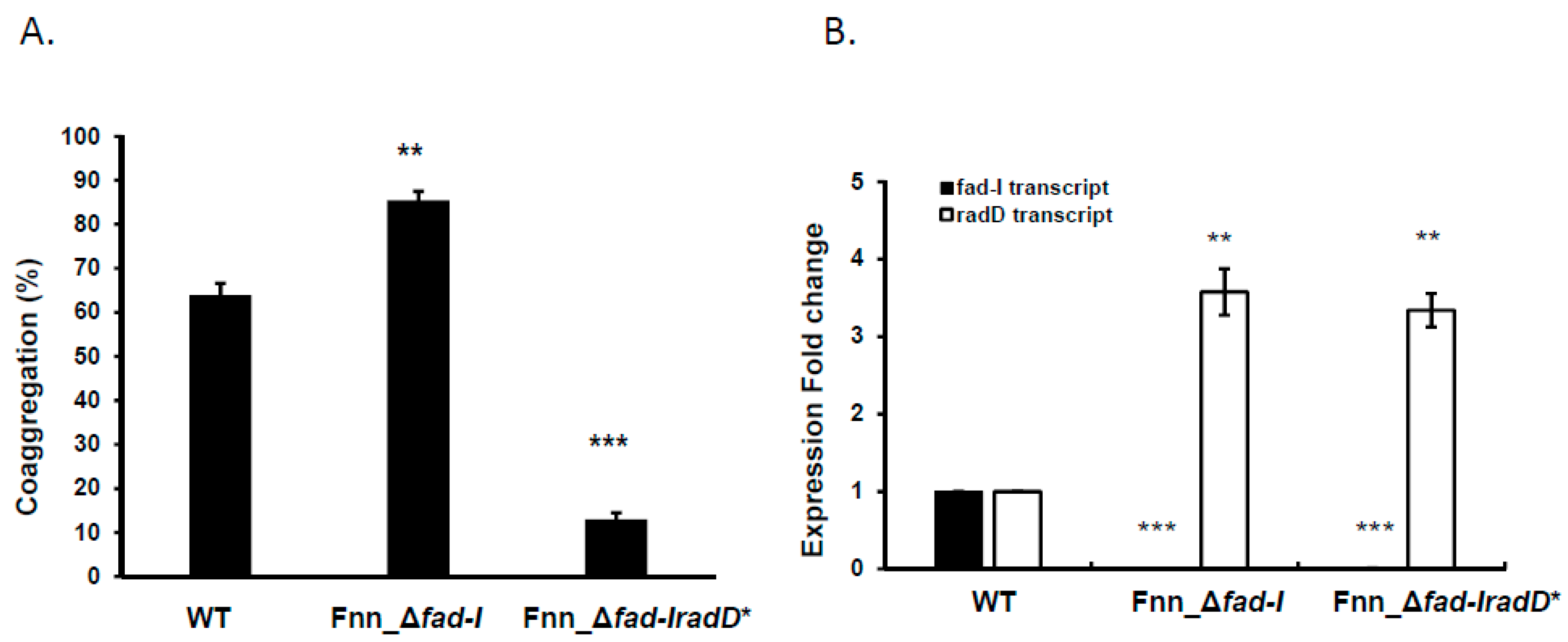
3.4. The Presence of the RadD Adhesin Is Not Required for Increased Expression of radD in Δfad-I Mutant
To investigate if the observed
radD regulation requires the RadD adhesin, we introduced a frameshift mutation in the N-terminal part of the
radD gene in the Fnn_Δ
fad-I mutant background to create a derivative in which
radD is still expressed but not translated into a functional protein (
Figure 1G). The resulting Fnn_Δ
fad-I radD* mutant strain was deficient in coaggregation with
S. gordonii (13.0 ± 2.6% coaggregation compared to 64% for the wild-type parent control) (
Figure 5A), confirming the absence of the RadD adhesin. Transcript levels for
radD in the Fnn_Δ
fad-I radD* derivative remained elevated relative to wild-type (3.3 ± 0.2-fold increase) (
Figure 5B) similar to our findings for the Fnn_Δ
fad-I mutant (
Figure 3D). These results indicate that the RadD adhesin is not needed for the increased
radD transcripts in Δ
fad-I mutant but mediates the enhanced coaggregation phenotype with the fusobacterial partner species
S. gordonii.
3.5. Co-Incubation of F. Nucleatum ssp Nucleatum with S. Gordonii Reduces radD Transcript Levels
F. nucleatum ssp
nucleatum utilizes RadD as a major adhesin for binding to
S. gordonii and other partner species. To examine if the presence of binding partner influences the regulation of this important adhesin on a transcriptional level, we determined
radD expression in the presence and absence of
S. gordonii (
Figure 6). The presence of
S. gordonii reduced
radD transcript levels, when co-incubated with
F. nucleatum ssp
nucleatum ATCC 23726 within the first 30 min. The expression was significantly reduced by almost two-fold upon co-incubation with
S. gordonii. The Δ
radD derivative exhibited a similar significant reduction of
radD expression in the presence of
S. gordonii indicating that this regulatory effect does not require the adhesin. While a relative 1.5-fold reduction of
radD transcript levels was observed in the Fnn_Δ
fad-I mutant during co-incubation with
S. gordonii, the difference was not significant and still resulted in a substantially higher (2.2-fold) expression of
radD compared to the wild-type parent strain.
3.6. RadD Levels Are Important for Dual-Species Biofilm Formation of F. Nucleatum with S. Gordonii
We further investigated if the increase in
radD expression in the Δ
fad-I strain affects biofilm development with
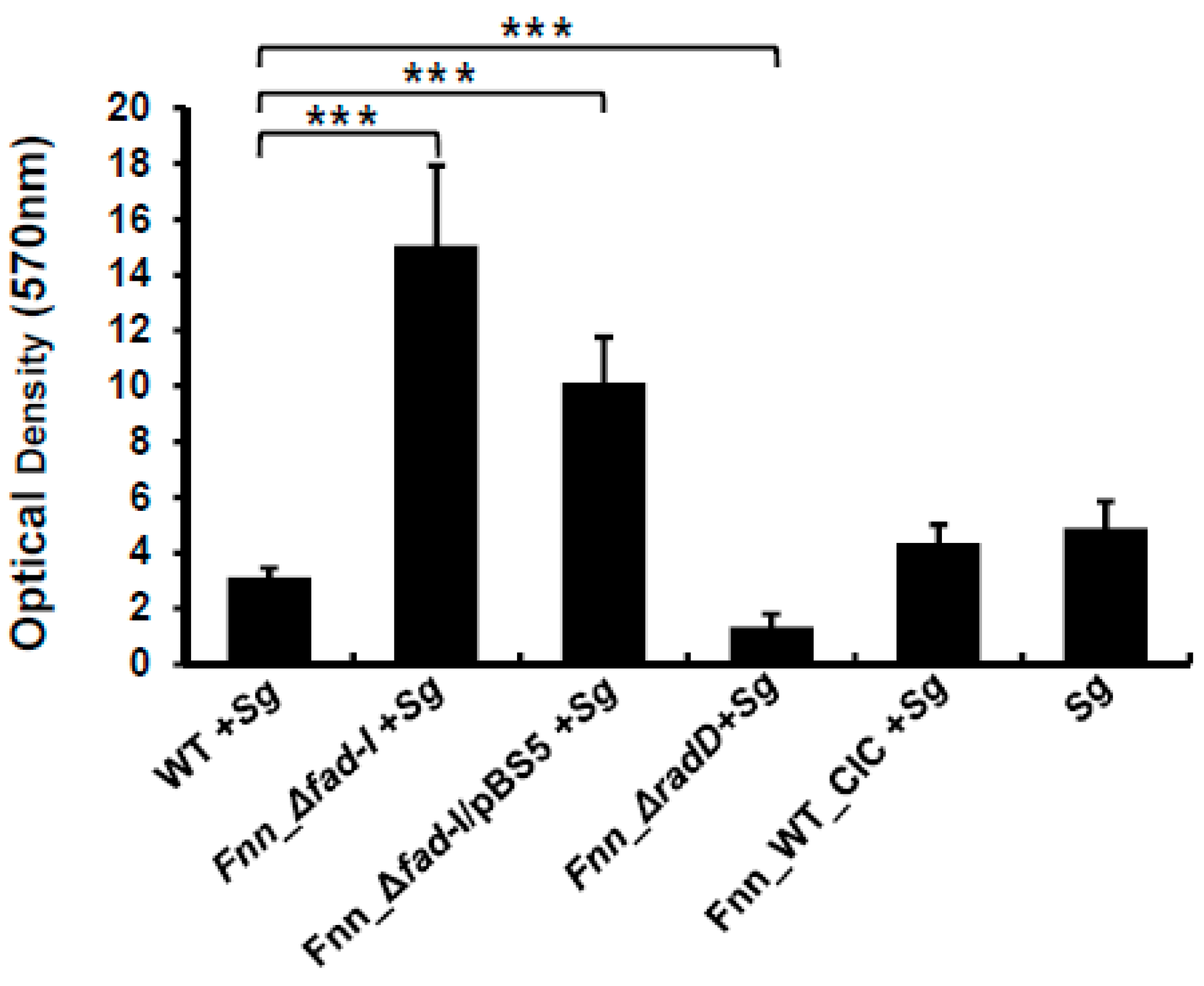
S. gordonii. Staining with crystal violet revealed that the biomass of biofilms developed by
S. gordonii alone (4.8 ± 0.9) was comparable to the biomass of dual-species biofilm formed by
F. nucleatum ssp
nucleatum ATCC 23736 (3.1 ± 0.3) and Fnn_WT_CIC (4.3 ± 0.7) in the presence of
S. gordonii (
Figure 7) under the growth conditions used. Not surprisingly, the corresponding
radD mutant, which is defective in binding to streptococci [
15] produced significantly reduced biomass with
S. gordonii (1.9 ± 0.5). The Fnn_Δ
fad-I mutant generated significantly more biomass (15.0 ± 2.8) during dual-species biofilm formation, consistent with the greatly increased attachment to
S. gordonii (
Figure 2). Similar to our findings above, in which we observed that plasmid-based complementation of lack of
fad-I only partially reduces
radD expression to wild-type levels, the complement strain phenotype exhibits a reduction in biomass production (10.1 ± 1.6) compared to the Fnn_Δ
fad-I mutant strain but remains more than two-fold elevated over wild-type.
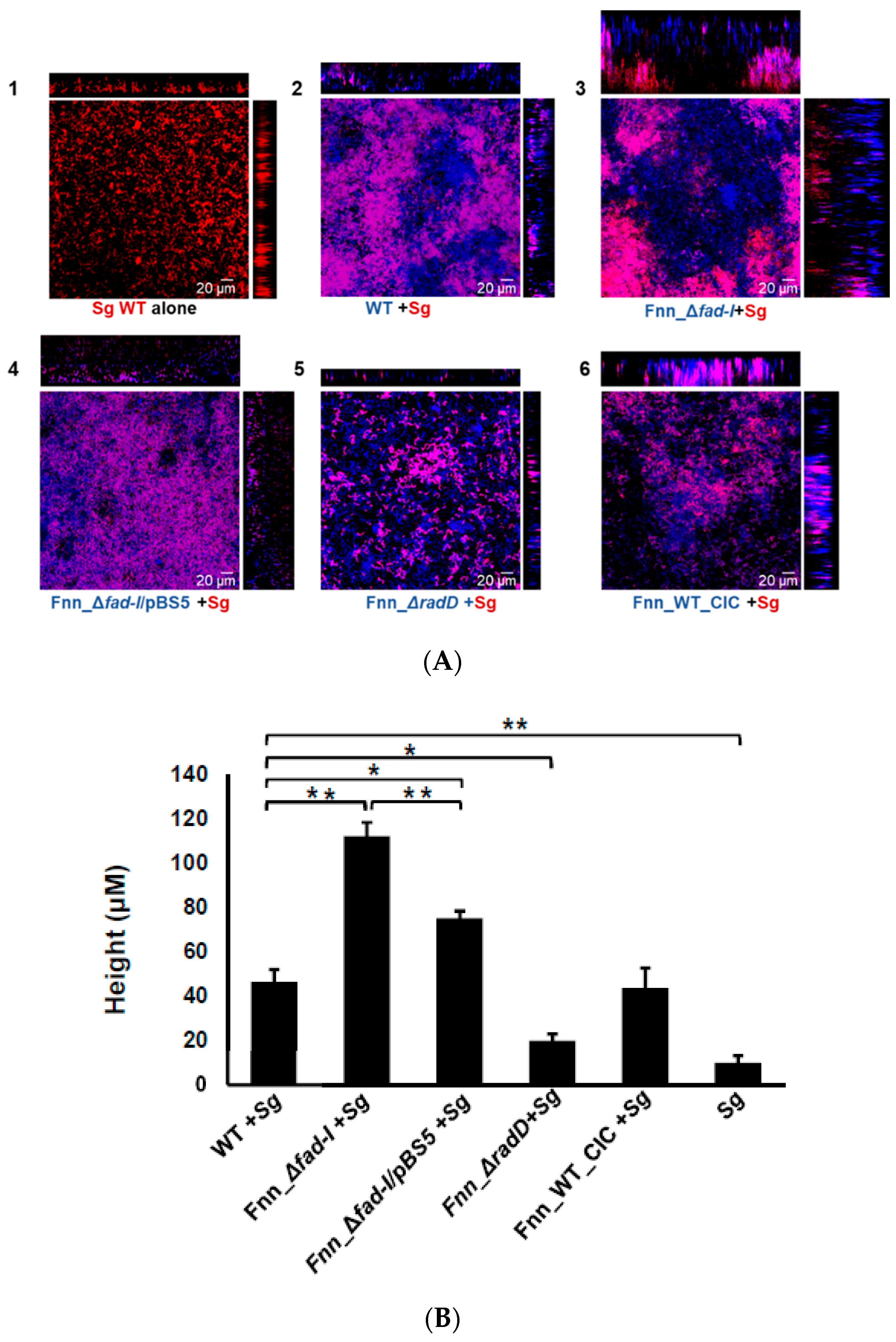
Next, we employed confocal microscopy to examine architectural features of dual-species biofilms by
S. gordonii with
F. nucleatum ssp
nucleatum wild-type and its mutant derivatives investigated in this study (
Figure 8A). Height measurements revealed that dual-species biofilms formed with
F. nucleatum ssp
nucleatum wild-type and the Fnn_WT_CIC control strain were significantly taller (46.30 ± 5.6 μm and 43.5 ± 9.10, respectively) compared to the mono-species biofilms formed by
S. gordonii alone (9.9 ± 3.15 μm) (
Figure 8B). This effect on biofilm height was greatly enhanced when
S. gordonii was combined with the Fnn_Δ
fad-I mutant instead of its wild-type parent. Biofilms with the
F. nucleatum Δ
fad-I (Fnn_Δ
fad-I) mutant were significantly taller (112.16 ± 6.2 μm) confirming the importance of RadD levels in biofilm formation and development. Interestingly, the height of the biofilm formed by the
fad-I complement strain (Fnn_Δ
fad-I/pBS5) (75.1 ± 3.4), was reduced compared to the Fnn_Δ
fad-I mutant, but still higher than the dual-species biofilm formed by the wild-type strain with
S. gordonii. As expected, the Fnn_Δ
radD mutant strain largely failed to integrate into the biofilm, which is reflected by a significantly shallower height (23.8 ± 5.75 μm) compared to those formed with the wild-type.
4. Discussions
In this study, we investigated the roles of the three small proteins (RapA, RapB, FAD-I) encoded upstream of the fusobacterial RadD adhesin. We previously identified RadD as a major adhesin in interspecies interaction with gram-positive species such as oral streptococci [
15] in addition to its function in apoptosis induction in lymphocytes [
20] and strain-specific fusobacterial binding to
P. gingivalis [
19]. The work presented here led to the discovery that the lipoprotein FAD-I, which is part of the
radD-containing operon, controls interspecies interaction via regulation of
radD expression in a RadD-independent manner.
The genes encoding RapA, RapB, and FAD-I were systematically inactivated in the
nucleatum and
polymorphum subspecies of
F. nucleatum and the resulting mutant strains were characterized regarding their effects on interspecies interaction with the important fusobacterial partner strain
S. gordonii. While lack of
rapA and
rapB did not have a consistent significant impact on
F. nucleatum behavior in both subspecies tested, lack of
fad-I produced a striking phenotype characterized by a significant increase of coaggregation as well as biofilm formation with
S. gordonii (
Figure 2,
Figure 7 and
Figure 8). Additional analyses revealed that this phenotype is highly correlated with elevated
radD transcript levels (
Figure 3). We previously identified FAD-I as a small lipoprotein with a subspecies-dependent differential ability to induce human β-defensin 2 (hBD-2) in oral epithelial cells [
21,
22]. In contrast to its function in hBD-2 induction, which is only triggered by FAD-I of
F. nucleatum subspecies
nucleatum but not the subspecies
polymorphum, we found that the FAD-I regulatory effect on
radD expression and role in biofilm formation with its partner species
S. gordonii is similar in both
F. nucleatum subspecies tested in this study (
Figure 2 and
Figure 3). Hence, after establishing this similarity during the initial characterization, we focused on
F. nucleatum ssp
nucleatum ATCC 23726 and its mutant derivatives for more detailed investigation. We revealed that while FAD-I is required for the regulatory effect on
radD expression, a functional RadD adhesin is not (
Figure 4 and
Figure 5,
Supplemental Figure S1), suggesting an independent pathway for FAD-I action. Interestingly, binding to the partner species
S. gordonii also has a regulatory impact and seems to suppress
radD transcription largely independent of FAD-I function (
Figure 6), indicating a multifactorial regulation of
radD expression.
FAD-I is a lipoprotein, and lipoproteins in bacteria are well documented to be involved in a wide variety of functions ranging from adhesion to virulence [
33,
34,
35]. We previously demonstrated that another small fusobacterial lipoprotein, Aid1, is involved in establishing the initial contact with streptococcal binding partners [
27]. As lack of Aid1 results in reduced binding, while overexpression significantly increases attachment to streptococci and alters biofilm architecture, a direct role in fusobacterial interspecies interaction was proposed. In contrast, lack of FAD-I significantly enhances attachment to
S. gordonii, which makes a direct role of FAD-I in the binding process unlikely. Roles in transcriptional regulation similar to what we found in our study for the effect of FAD-I on
radD transcriptional levels have not been previously described for lipoproteins. As FAD-I is a classical small membrane-associated lipoprotein that lacks any possible DNA-binding motif [
22], direct interaction with the promoter driving
radD expression is unlikely. Likewise, RapA, which was recently described as a member of the FadA family [
23] based on its similarity to FadA, a fusobacterial adhesin for binding to eukaryotic cells [
18], as well as the still uncharacterized RapB do not contain any discernible DNA-binding domain. Therefore, the FAD-I-dependent regulation of
radD expression is indirect and the connecting pathways and regulators remain to be discovered.
Regulation of adhesins is important for microorganisms as they have a central role in coordinating the development of multispecies biofilms. This regulation is therefore critical for the versatility of biofilms to respond to both harmful and beneficial factors affecting the polymicrobial community. Previous studies of
F. nucleatum report strain-dependent variation in the adherence properties to host cells and proteins [
36]. This difference in adherence capabilities and the complexity of regulatory mechanisms of adhesins likely reflect the importance of specific expression of adhesins for the survival of bacteria in changing environments. In addition to varying specificities, these differences in attachment are a result of the regulation of adhesins, reflecting the importance of adhesin regulation for the survival of bacteria in changing environments [
37,
38,
39]. To date, there have been few reports of lipoproteins playing roles in adhesion. In addition to our previous characterization of Aid1 [
27], which appears to have a direct role in interspecies binding, the lipoprotein SadB enhances the surface display of the trimeric autotransporter protein SadA in
Salmonella and related enterobacteria [
40]. Contrary to their observation of a direct role in outer membrane protein display for a lipoprotein, we found that the FAD-I lipoprotein regulates RadD on the level of transcription as the absence of FAD-I resulted in increased expression of
radD. Enhanced expression of
radD not only led to increased coaggregation of Δ
fad-I F. nucleatum derivatives with
S. gordonii, but also significantly enhanced dual-species biofilm formation compared to wild-type. The substantial increase in interspecies binding requires the presence of a functional RadD adhesin as a mutant lacking both FAD-I and RadD is unable to bind to streptococcal partner species (
Figure 5). Our findings here support an important biological role for tight multi-level regulation of RadD production, since deregulated
radD expression severely alters interspecies interaction and biofilm architecture. The modulation of RadD mediated interactions by Aid1 [
27] and the results presented in this study demonstrating an effect of FAD-I, as well as binding to
S. gordonii on transcriptional regulation of
radD, suggest that the fine-tuning of interactions involving RadD is essential for maintaining specific interspecies interactions in the oral community.
Our observation that
radD expression is suppressed for wild-type
F. nucleatum spp.
nucleatum as well as its mutant derivatives Δ
fad-I and Δ
radD when it is co-incubated with
S. gordonii (
Figure 6). It is consistent with other studies demonstrating that the expression of adhesin genes is downregulated once attachment is established between bacterial species. Miller et al. [
41] reported similar findings for
P. gingivalis during interaction with
Acinetobacter baumannii where the
fimA and
HagA adhesins were downregulated, presumably to conserve energy. Mostly, adhesins are regulated by a number of environmental factors along with the presence or absence of partner species. This has been well documented for the
fimA adhesin of
P. gingivalis [
42,
43,
44].
In the present study, we provide evidence that lack of fad-I results in increased coaggregation and biofilm formation with S. gordonii. These findings suggest that the FAD-I protein plays an important role in the regulation of the RadD adhesin, however, additional studies are needed to further decode the mechanism of this regulation. Further identification of regulatory mechanisms of fusobacterial adhesins is necessary and will continue to clarify the central role of F. nucleatum interspecies interaction in health and disease.