Occupational Exposures to Organic Dust in Irish Bakeries and a Pizzeria Restaurant
Abstract
1. Introduction
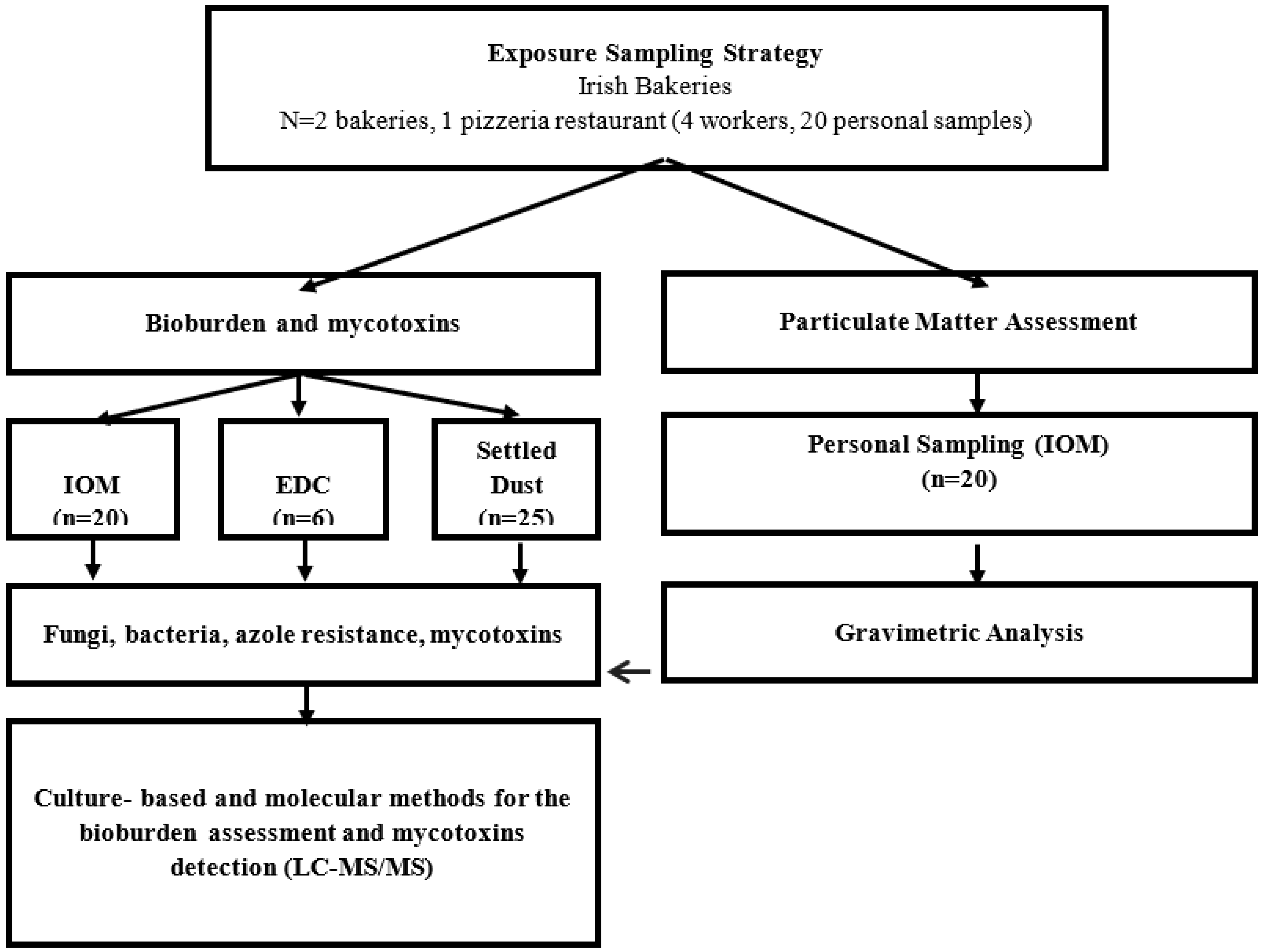
2. Materials and Methods
2.1. Study Participants
2.2. Flour Dust Exposure Assessment
2.3. Environmental Samples
2.3.1. Characteristics of Bacterial Contamination
2.3.2. Fungal Characterization and Azole Resistance Screening Using Culture-Based Methods
2.3.3. Fungal Detection Using Molecular Tools
2.3.4. Mycotoxins Analysis
2.4. Statistical Analysis
3. Results
3.1. Personal Flour Dust Exposure Levels: Total Inhalable Dust
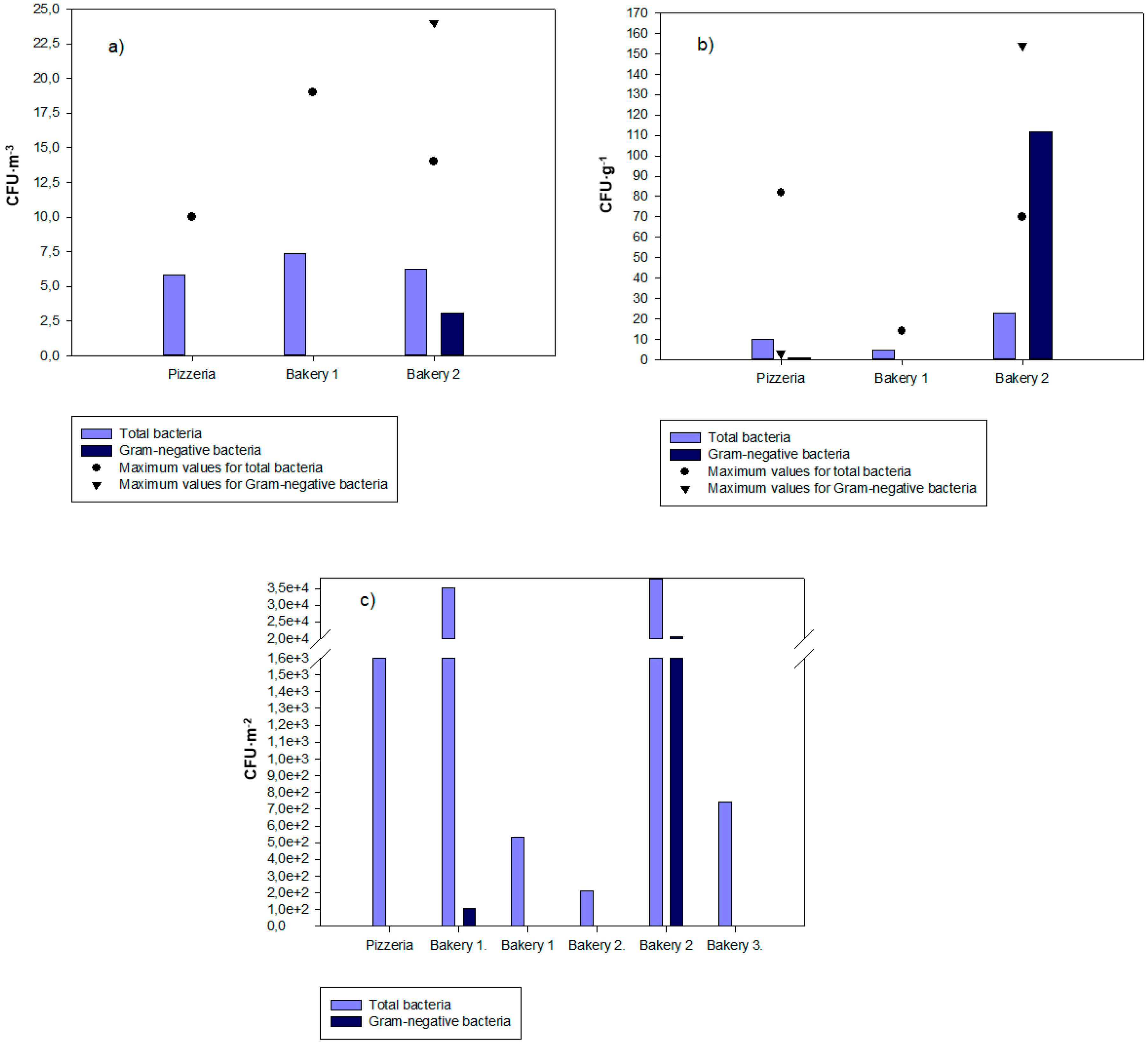
3.2. Bacterial Contamination Distribution
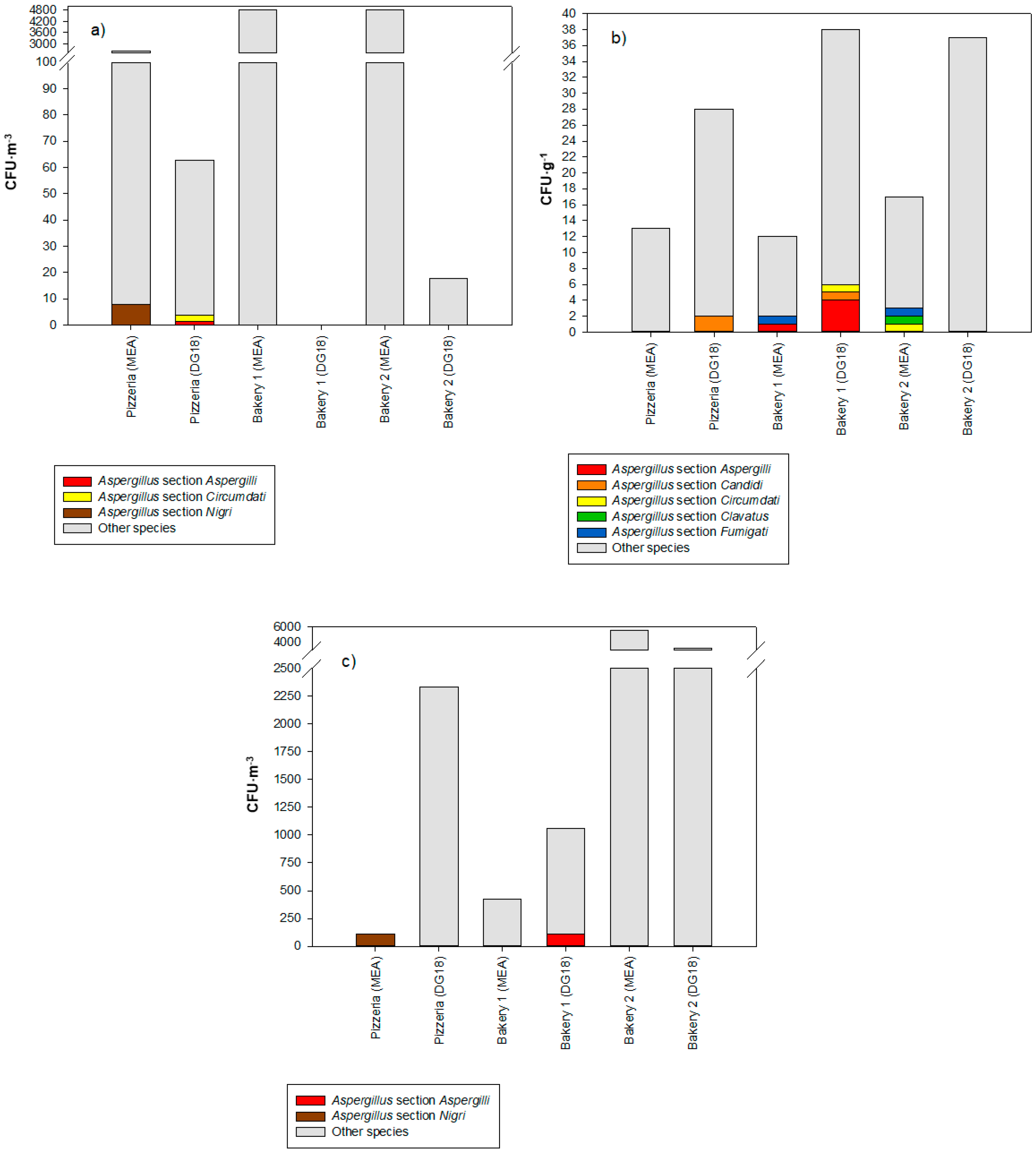
3.3. Fungal Contamination Characterization
3.4. Fungal Load in Azole-Supplemented Media
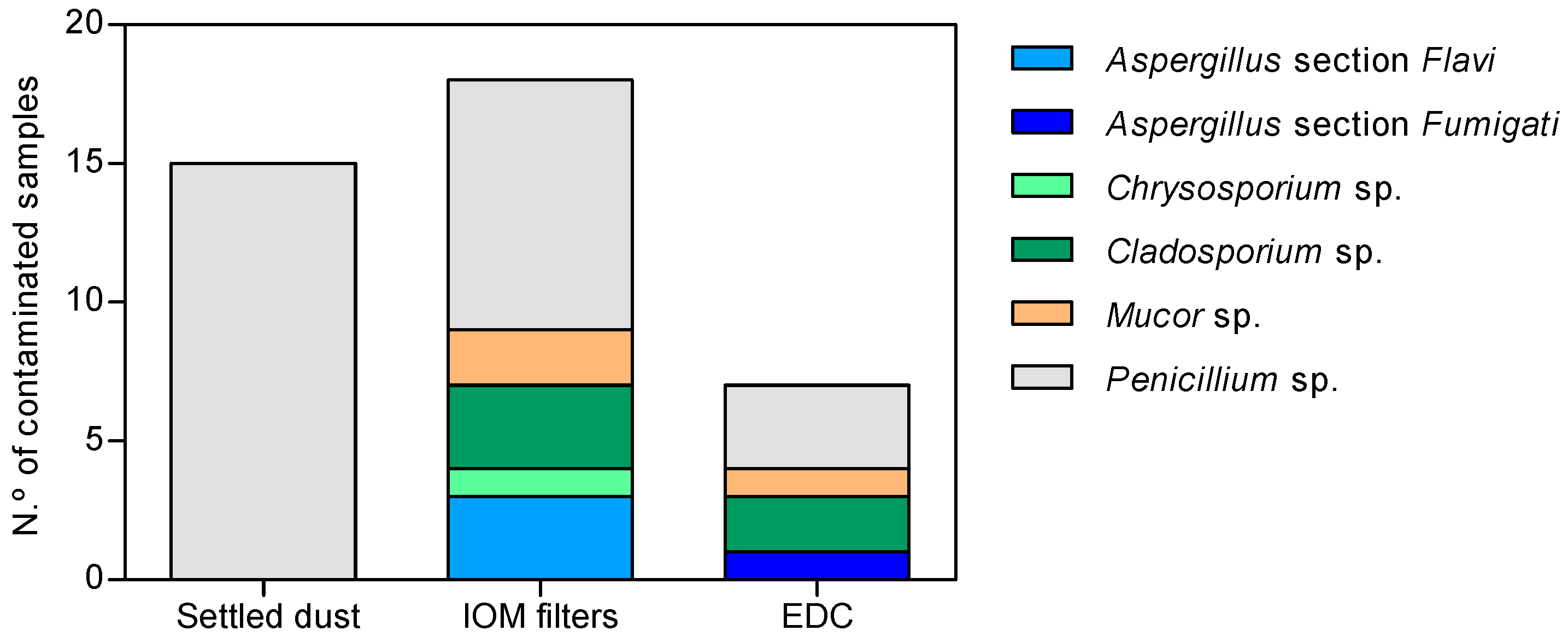
3.5. Fungal Detection
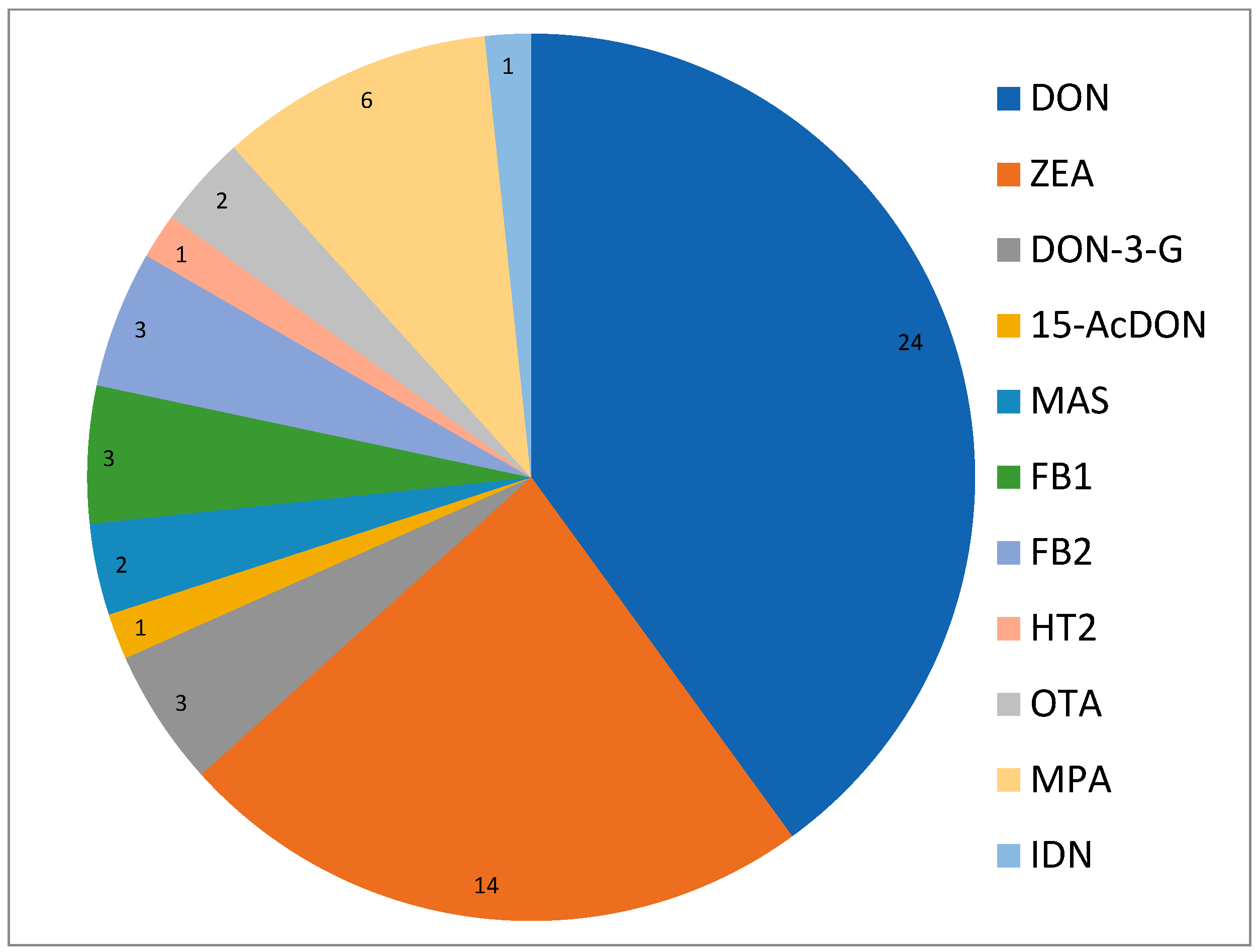
3.6. Mycotoxins Results
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sander, I.; Flagge, A.; Merget, R.; Halder, T.M.; Meyer, H.E.; Baur, X. Identification of wheat flour allergens by means of 2-dimensional immunoblotting. J. Allergy Clin. Immunol. 2001, 107, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Assunção, R.; Nunes, C.; Osteresch, B.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Martins, C.; Alvito, P.; Almeida, A.; et al. Exposure assessment to mycotoxins in a Portuguese fresh bread dough company by using a multi-biomarker approach. Toxins 2018, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Monteiro, A.; Carolino, E.; Viegas, S. Occupational exposure to bioburden in Portuguese bakeries: An approach to sampling viable microbial load. Arch. Ind. Hyg. Toxicol. 2018, 69, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Page, E.H.; Dowell, C.H.; Mueller, C.A.; Biagini, R.E. Evaluation of Sensitization and Exposure to Flour Dust, Spices, and Other Ingredients Among Poultry Breading Workers; National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2013; pp. 1–35.
- Matsuo, H.; Uemura, M.; Yorozuya, M.; Adachi, A.; Morita, E. Identification of IgE-reactive proteins in patients with wheat protein contact dermatitis. Contact Dermat. 2010, 63, 23–30. [Google Scholar] [CrossRef]
- Heederik, D.; Houba, R. An exploratory quantitative risk assessment for high molecular weight sensitizers: Wheat flour. Ann. Occup. Hyg. 2001, 45, 175–185. [Google Scholar] [CrossRef]
- Baatjies, R.; Lopata, A.L.; Sander, I.; Raulf-Heimsoth, M.; Batemane, E.D.; Meijster, T.; Heederik, D.; Robins, T.G.; Jeebhay, M.F. Determinants of asthma phenotypes in supermarket bakery workers. Eur. Respir. J. 2009, 34, 825–833. [Google Scholar] [CrossRef]
- Houba, R.; Van Run, P.; Heederik, D.; Doekes, G. Wheat antigen exposure assessment for epidemiological studies in bakeries using personal dust sampling and inhibition ELISA. Clin. Exp. Allergy 1996, 26, 154–163. [Google Scholar] [CrossRef]
- Tagiyeva, N.; Anua, S.M.; Semple, S.; Dick, F.; Devereux, G. The “take home” burden of workplace sensitizers: Flour contamination in bakers’ families. Environ. Int. 2012, 46, 44–49. [Google Scholar] [CrossRef]
- Salcedo, G.; Quirce, S.; Diaz-Perales, A. Wheat allergens associated with Baker’s asthma. J. Investig. Allergol. Clin. Immunol. 2011, 21, 81–92. [Google Scholar]
- Blanco Carmona, J.G.; Juste Picon, S.; Garces Sotillos, M. Occupational asthma in bakeries caused by sensitivity to alpha-amylase. Allergy 1991, 46, 274–276. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Twarużekf, M.; Kosicki, R.; Marchand, G.; Viegas, S. Bioburden in healthcare centers: Is the compliance with Portuguese legislation enough to prevent and control infection? Build. Environ. 2019, 160, 106226. [Google Scholar] [CrossRef]
- Kirkeleit, J.; Hollund, B.E.; Riise, T.; Eduard, W.; Bråtveit, M.; Storaas, T. Bakers’ exposure to flour dust. J. Occup. Environ. Hyg. 2016, 14, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Van Tongeren, M.; Galea, K.S.; Ticker, J.; While, D.; Kromhout, H.; Cherrie, J.W. Temporal trends of flour dust exposure in the United Kingdom, 1985-2003. J. Environ. Monit. 2009, 11, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Health Safety Executive (HSE). Annual Statitistics: Work Related Asthma in Great Britain. 2018. Available online: http://www.hse.gov.uk/statistics/causdis/ (accessed on 15 January 2020).
- Ameille, J.; Pauli, G.; Calastreng-Crinquand, A.; Vervloet, D.; Iwatsubo, Y.; Popin, E.; Bayeux-Dunglas, M.C.; Kopferschmitt-Kubler, M.C. Reported incidence of occupational asthma in France, 1996–1999: The ONAP programme. Occup. Environ. Med. 2003, 60, 136–141. [Google Scholar] [CrossRef]
- Leira, H.L.; Bratt, U.; Slastad, S. Notified cases of occupational asthma in Norway: Exposure and consequences for health and income. Am. J. Ind. Med. 2005, 48, 359–364. [Google Scholar] [CrossRef]
- Karjalainen, A.; Leppänen, M.; Leskinen, J.; Torvela, T.; Pasanen, P.; Tissari, J.; Miettinen, M. Concentrations and number size distribution of fine and nanoparticles in a traditional Finnish bakery. J. Occup. Environ. Hyg. 2018, 15, 194–203. [Google Scholar] [CrossRef]
- Wiszniewska, M.; Walusiak-Skorupa, J. Diagnosis and frequency of work-exacerbated asthma among bakers. Ann. Allergy Asthma Immunol. 2013, 111, 370–375. [Google Scholar] [CrossRef]
- Money, A.; Carder, M.; Agius, R. The Incidence of Work-Related Ill-Health as Reported to the Health and Occupation Research (THOR) Network by Physicians in the Republic of Ireland between 2005 and 2016. 2017. Available online: https://www.hsa.ie/eng/Workplace_Health/Illness_Reports/ROI_THOR_2017_annual_report.pdf (accessed on 15 January 2020).
- Buczaj, A.; Pawlak, H.; Tarasinska, J.; Maksym, P.; Brzana, W. Evaluation of work conditions in a pasta manufacturing plant with particular consideration of dustiness. Ann. Agric. Environ. Med. 2012, 19, 810–816. [Google Scholar]
- Meijster, T.; Tielemans, E.; Pater, N.D.; Heederik, D. Modelling exposure in flour processing sectors in The Netherlands: A baseline measurement in the context of an intervention program. Ann. Occup. Hyg. 2007, 51, 293–304. [Google Scholar]
- Elms, J.; Robinson, E.; Rahman, S.; Garrod, A. Exposure to flour dust in UK bakeries: Current use of control measures. Ann. Occup. Hyg. 2005, 49, 85–91. [Google Scholar]
- Baatjies, R.; Meijster, T.; Lopata, A.; Sander, I.; Raulf-Heimsoth, M.; Heederik, D.; Jeebhay, M. Exposure to flour dust in South African supermarket bakeries: Modeling of baseline measurements of an intervention study. Ann. Occup. Hyg. 2010, 54, 309–318. [Google Scholar] [PubMed]
- Nieuwenhuijsen, M.J.; Sandiford, C.P.; Lowson, D.; Tee, R.D.; Venables, K.M.; Newman Taylor, A.J. Peak exposure concentrations of dust and flour aeroallergen in flour mills and bakeries. Ann. Occup. Hyg. 1995, 39, 193–201. [Google Scholar] [CrossRef]
- Bord Bia Vantage. Cakes and Pastries Research. 2019. Available online: https://www.bordbia.ie/industry/ (accessed on 15 January 2020).
- Irish Central Statistics Office (CSO). Irish Business Register, Nace 5610 Irish Restaurants and Mobile Food Services (Personal Communication 2019). 2019. Available online: https://www.cso.ie/en/releasesandpublications/er/bd/businessdemography2016/ (accessed on 15 January 2020).
- HSE. General Methods for Sampling and Gravimetric Analysis of Respirable, Thoracic and Inhalable Aerosols. MDHS14/4; Health and Safety Executive: London, UK, 2014. Available online: http://www.hse.gov.uk/pubns/mdhs/pdfs/mdhs101.pdf (accessed on 15 January 2020).
- Madsen, A.M.; Matthiesen, C.B.; Frederiksen, M.W.; Frederiksen, M.; Frankel, M.; Spilak, M.; Gunnarsen, L.; Timm, M. Sampling, extraction and measurement of bacteria, endotoxin, fungi and inflammatory potential of settling indoor dust. J. Environ. Monit. 2012, 14, 3230–3239. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Pacífico, C.; Faria, T.; de Oliveira, A.C.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Fungal contamination in green coffee beans samples: A public health concern. J. Toxicol. Environ. Health Part A Curr. Issues 2017, 80, 719–728. [Google Scholar] [CrossRef] [PubMed]
- ISO. Microbiology of Food and Animal Feeding Stuffs: Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95; ISO Pressed: Geneva, Switzerland, 2008. [Google Scholar]
- ISO. Microbiology of Food and Animal Feeding Stuffs: Horizontal Method for the Enumeration of Yeasts and Moulds. Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95; ISO Pressed: Geneva, Switzerland, 2008. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Antifungal Agents Breakpoint Tables for Interpretation of MICs; Version 8.1; 2017. Available online: http://www.eucast.org (accessed on 25 September 2019).
- Arendrup, M.C.; Verweij, P.E.; Mouton, J.W.; Lagrou, K.; Meletiadis, J. Multicentre validation of 4-well azole agar plates as a screening method for detection of clinically relevant azole-resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 2017, 72, 3325–3333. [Google Scholar] [CrossRef]
- De Hoog, D.; Guarro, J.; Gene, G.; Figueras, M. Atlas of Clinical Fungi—The Ultimate Benchtool for Diagnosis; Version 4.1.4; Utr Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2016. [Google Scholar]
- Viegas, C.; Monteiro, A.; dos Santos, M.; Faria, T.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Marchand, G.; Lacombe, N.; Viegas, S. Filters from taxis air conditioning system: A tool to characterize driver’s occupational exposure to bioburden? Environ. Res. 2018, 164, 522–529. [Google Scholar] [CrossRef]
- Brisman, J.; Järvholm, B.; Lillienberg, L. Exposure-response relations for self reported asthma and rhinitis in bakers. Occup. Environ. Med. 2000, 57, 335–340. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; Monteiro, A.; Aranha Caetano, L.; Carolino, E.; Quintal Gomes, A.; Viegas, S. A Novel Multi-Approach Protocol for the Characterization of Occupational Exposure to Organic Dust—Swine Production Case Study. Toxics 2018, 6, 5. [Google Scholar] [CrossRef]
- Adhikari, A.; Kettleson, E.M.; Vesper, S.; Kumar, S.; Popham, D.L.; Schaffer, C.; Indugula, R.; Chatterjee, K.; Allam, K.K.; Grinshpun, S.A.; et al. Dustborne and Airborne Gram-Positive and Gram-Negative Bacteria in High versus Low ERMI Homes. Sci. Total Environ. 2014, 482, 92–99. [Google Scholar]
- Viegas, S.; Assunção, R.; Martins, C.; Nunes, C.; Osteresch, B.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Ribeiro, E.; Viegas, C. Occupational Exposure to Mycotoxins in Swine Production: Environmental and Biological Monitoring Approaches. Toxins 2019, 11, 78. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Twaruzek, M.; Kosicki, R.; Viegas, S. Characterization of Occupational Exposure To Fungal Burden in Portuguese Bakeries. Microorganisms 2019, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Viegas, C.; Oppliger, A. Occupational Exposure to Mycotoxins: Current Knowledge and Prospects. Ann. Work Expo. Health 2018, 62, 923–941. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, E.A. Exposure to inhalable flour dust in Canadian flour mills. Appl. Occup. Environ. Hyg. 2003, 18, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Caetano, L.A.; Faria, T.; Crespo Batista, A.; Viegas, S.; Viegas, C. Assessment of occupational exposure to azole resistant fungi in 10 Portuguese bakeries. AIMS Microbiol. 2017, 3, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Degois, J.; Clerc, F.; Simon, X.; Bontemps, C.; Leblond, P.; Duquenne, P. First metagenomic survey of the microbial diversity in bioaerosols emitted in waste sorting plants. Ann. Work Expo. Health 2017, 61, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.G.; Meis, J.F. Azole resistance in aspergillus fumigatus: Can we retain the clinical use of mold-active antifungal azoles? Clin. Infect. Dis. 2016, 62, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Perlin, D.S. Azole resistance in Aspergillus: A growing public health menace. Future Microbiol. 2011, 6, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Pasqualotto, A.C. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med. Mycol. 2009, 47, 261–270. [Google Scholar] [CrossRef]
- Mccormick, A.; Loeffler, J.; Ebel, F. Aspergillus fumigatus: Contours of an opportunistic human pathogen. Cell. Microbiol. 2010, 12, 1535–1543. [Google Scholar] [CrossRef]
- Goncalves, S.S.; Souza, A.C.; Chowdhary, A.; Meis, J.F.; Colombo, A.L. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses 2016, 59, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012, 54, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Meletiadis, J.; Mouton, J.W.; Meis, J.F.; Verweij, P.E. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 2003, 51, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Chakrabarti, A.; Chowdhary, A.; Cordoba, S.; Dannaoui, E.; Dufresne, P.; Fothergill, A.; Ghannoum, M.; Gonzalez, G.M.; Guarro, J.; et al. Multicenter evaluation of MIC distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of Mucorales. Antimicrob. Agents Chemother. 2015, 59, 1745–1750. [Google Scholar] [CrossRef]
- Caetano, L.A.; Faria, T.; Springer, J.; Loeffler, J.; Viegas, C. Antifungal-resistant Mucorales in different indoor environments. Mycology 2019, 10, 75–83. [Google Scholar] [CrossRef]
- Mortensen, K.L.; Mellado, E.; Lass-Florl, C.; Rodriguez-Tudela, J.L.; Johansen, H.K.; Arendrup, M.C. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 2010, 54, 4545–4549. [Google Scholar] [CrossRef]
- Snelders, E.; Huis In ’t Veld, R.A.; Rijs, A.J.; Kema, G.H.; Melchers, W.J.; Verweij, P.E. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 2009, 75, 4053–4057. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Parada, H.; Brandão, J.; Viegas, C.; Carolino, E.; Clemons, K.V.; Stevens, D.A. Molecular screening of 246 Portuguese Aspergillus isolates among different clinical and environmental sources. Med. Mycol. 2014, 52, 519–529. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Aspergillus spp. prevalence in different Portuguese occupational environments: What is the real scenario in high load settings? J. Occup. Environ. Hyg. 2017, 14, 771–785. [Google Scholar] [CrossRef]
- Resendiz Sharpe, A.; Lagrou, K.; Meis, J.F.; Chowdhary, A.; Lockhart, S.R.; Verweij, P.E.; ISHAM ECMM Aspergillus Resistance Surveillance Working Group. Triazole resistance surveillance in Aspergillus fumigatus. Med. Mycol. 2018, 56, 83–92. [Google Scholar] [CrossRef]
- Brera, C.; de Santis, B.; Debegnach, F.; Miano, B.; Moretti, G.; Lanzone, A.; Del Sordo, G.; Buonsenso, D.; Chiaretti, A.; Hardie, L.; et al. Experimental Study of Deoxynivalenol Biomarkers in Urine; EFSA supporting publication 2015, EN-818; European Food Safety Authority: Parma, Italy, 2015; p. 136.
- Assunção, R.; Martins, C.; Viegas, S.; Viegas, C.; Jakobsen, L.S.; Pires, S.; Alvito, P. Climate change and the health impact of aflatoxins exposure in Portugal: An overview. Food Addit. Contam. Part A 2018, 35, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
| Sample ID | Business Type | Flour Used | Amount of Flour Used (kg/day) | Dough Produced (pcs or kg/day) | Number of Pizza/Bakeries Produced (per day) | Work Activities or Area | Number of Samples Collected | Ventilation | Work Area (m2) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Restaurant Pizzeria | WF; CF | 18 (WF) and 0.5–1 (CF) | 42 pcs | 20–65 pcs | Mixing | 5 | Natural | 6 |
| Preparing pizza | 20 | ||||||||
| 2. | Commercial Bakeries | SRF, WF, and PF | 7–11 (SF), 3 (WF), and 1–2 (PF) | 0 | >50 pcs | General duties of bakery tasks (mixing, molding, baking, etc.) | 5 | Room Ventilation (Fan) | 28 |
| 3. | Commercial Bakeries | T.65 (TF), (SF), and (RF) | >100 (TF), 3–9 (SP), and 3–5 (RF) | >100 kg | >800 pcs | General duties of bakery tasks (mixing, molding, baking, etc.) | 5 | Room ventilation (fan) | 120 |
| 5 | 120 |
| Mycotoxins | Limit of Detection (LOD; ng/g) | Limit of Quantification (LOQ; ng/g) |
|---|---|---|
| Patulin | 1.1 | 3.6 |
| Nivalenol | 4.5 | 14.9 |
| Deoxynivalenol-3-glucoside | 5.4 | 17.8 |
| Deoxynivalenol | 2.7 | 8.9 |
| Fusarenon-X | 4.8 | 15.8 |
| Deepoxy-deoxynivalenol | 4.2 | 13.9 |
| α-Zearalanol | 2.0 | 6.6 |
| β-Zearalanol | 0.9 | 3.0 |
| β-Zearalenol | 1.4 | 4.6 |
| α-Zearalenol | 1.0 | 3.3 |
| Zearalanone | 0.5 | 1.7 |
| Zearalenone | 0.2 | 0.7 |
| T2 Tetraol | 5.4 | 17.8 |
| Deepoxydeoxynivalenol | 0.4 | 1.3 |
| Neosolaniol | 0.1 | 0.3 |
| 15-Acetyldeoxynivalenol | 0.8 | 2.6 |
| 3-Acetyldeoxynivalenol | 0.8 | 2.6 |
| Monoacetoxyscirpenol | 0.1 | 0.3 |
| Diacetoxyscirpenol | 0.3 | 1.0 |
| Aflatoxin M1 | 0.1 | 0.3 |
| Aflatoxin B1 | 0.1 | 0.3 |
| Aflatoxin B2 | 0.1 | 0.3 |
| Aflatoxin G1 | 0.1 | 0.3 |
| Aflatoxin G2 | 0.1 | 0.3 |
| Fumonisin B1 | 0.5 | 1.7 |
| Fumonisin B2 | 0.4 | 1.3 |
| Fumonisin B3 | 0.5 | 1.7 |
| T2 Triol | 0.3 | 1.0 |
| Roquefortine C | 0.2 | 0.7 |
| Griseofulvin | 0.1 | 0.3 |
| T2 | 0.1 | 0.3 |
| HT2 | 0.3 | 1.0 |
| Ochratoxin A | 0.1 | 0.3 |
| Ochratoxin B | 0.1 | 0.3 |
| Mycophenolic acid | 0.2 | 0.7 |
| Sampling Location | n | Sample Duration (m) | Inhalable Dust (mg m−3) | |||
|---|---|---|---|---|---|---|
| AM | GM | GSD | Range | |||
| Pizzeria | 5 | 300–385 | 1.08 | 0.87 | 1.97 | 0.46–2.61 |
| Bakery 1 | 5 | 185–300 | 3.58 | 2.66 | 2.19 | 1.29–9.91 |
| Bakery 2 | 10 | 185–250 | 11.1 | 10.14 | 1.57 | 5.82–18.29 |
| Bakery total | 15 | 185–300 | 8.59 | 6.49 | 2.35 | 1.29–18.29 |
| Location/Sample Number | Total Inhalable Dust (8 h TWA) (mg·m−3) | Fungal Isolates MEA (CFU/m3) | Fungal Isolates MEA (CFU/m3) | Fungal Isolates DG18 (CFU/m3) | Total Bacteria Isolates (CFU/m3) | Gram-Negative Bacteria (CFU/m3) |
|---|---|---|---|---|---|---|
| P01 | 1.05 | 646 | 0 | 0 | 0 | 0 |
| P02 | 2.61 | 661 | 9 | 27 | 8 | 0 |
| P03 | 0.46 | 656 | 23 | 34 | 4 | 0 |
| P04 | 0.59 | 660 | 0 | 1 | 7 | 0 |
| P05 | 0.70 | 2 | 2 | 0 | 10 | 0 |
| B101 | 9.91 | 1201 | 0 | 0 | 2 | 0 |
| B102 | 2.49 | 1120 | 0 | 0 | 11 | 0 |
| B103 | 2.56 | 1179 | 0 | 0 | 19 | 0 |
| B104 | 1.66 | 2 | 2 | 0 | 0 | 0 |
| B105 | 1.29 | 1331 | 0 | 0 | 5 | 0 |
| B201 | 13.39 | 0 | 0 | 0 | 12 | 0 |
| B202 | 5.79 | 1100 | 0 | 0 | 2 | 0 |
| B203 | 13.73 | 2 | 2 | 2 | 14 | 2 |
| B204 | 7.38 | 0 | 0 | 0 | 2 | 0 |
| B205 | 16.45 | 0 | 0 | 4 | 8 | 24 |
| B206 | 15.46 | 2 | 2 | 0 | 4 | 0 |
| B207 | 5.82 | 2 | 2 | 0 | 9 | 2 |
| B208 | 8.09 | 1167 | 0 | 0 | 0 | 0 |
| B209 | 18.29 | 1325 | 0 | 0 | 8 | 0 |
| B210 | 6.68 | 1195 | 17 | 12 | 9 | 0 |
| Sampling Location | MEA | DG18 | ||||
|---|---|---|---|---|---|---|
| ID | CFU·m−3 | % | ID | CFU·m−3 | % | |
| Pizzeria Restaurant | Chrysonilia sitophila | 2591.43 | 98.78 | Penicillium sp. | 57.71 | 91.77 |
| Penicillium sp. | 24.22 | 0.92 | Aspergillus sp. | 3.87 | 6.16 | |
| Aspergillus sp. | 7.71 | 0.29 | Cladosporium sp. | 1.30 | 2.07 | |
| Total | 2623.36 | 100.00 | Total | 62.89 | 100.00 | |
| Bakery 1 | C. sitophila | 4831.13 | 99.95 | |||
| Penicillium sp. | 2.33 | 0.05 | ||||
| Total | 4833.46 | 100.00 | ||||
| Bakery 2 | C. sitophila | 4770.79 | 99.52 | Penicillium sp. | 15.76 | 88.64 |
| Penicillium sp. | 18.16 | 0.38 | Cladosporium sp. | 2.02 | 11.36 | |
| Chrysosporium sp. | 4.71 | 0.10 | Total | 17.78 | 100.00 | |
| Total | 4793.66 | 100.00 | ||||
| Sampling Location | MEA | DG18 | ||||
|---|---|---|---|---|---|---|
| ID | CFU·g−1 | % | ID | CFU·g−1 | % | |
| Pizzeria Restaurant | Penicillium sp. | 13 | 100.00 | Penicillium sp. | 24 | 85.71 |
| Aspergillus sp. | 2 | 7.14 | ||||
| Chrysosporium sp. | 1 | 3.57 | ||||
| Cladosporium sp. | 1 | 3.57 | ||||
| Total | 28 | 100.00 | ||||
| Bakery 1 | Penicillium sp. | 9 | 75.00 | Penicillium sp. | 30 | 78.95 |
| Aspergillus sp. | 2 | 16.67 | Aspergillus sp. | 6 | 15.79 | |
| Mucor sp. | 1 | 8.33 | Chrysosporium sp. | 2 | 5.26 | |
| Total | 12 | 100.00 | Total | 38 | 100.00 | |
| Bakery 2 | Penicillium sp. | 4 | 57.14 | Penicillium sp. | 37 | 100.00 |
| Aspergillus sp. | 3 | 42.86 | ||||
| Total | 7 | 100.00 | ||||
| Sampling Location | MEA | DG18 | ||||
|---|---|---|---|---|---|---|
| ID | CFU·m−2 | % | ID | CFU·m−2 | % | |
| Pizzeria Restaurant | Aspergillus sp. | 106.16 | 100.00 | Penicillium sp. | 2229.30 | 95.45 |
| Mucor sp. | 106.16 | 4.55 | ||||
| Total | 2335.46 | 100.00 | ||||
| Bakery 1 | Penicillium sp. | 318.47 | 75.00 | Penicillium sp. | 849.26 | 80 |
| Mucor sp. | 106.16 | 25.00 | Aspergillus sp. | 106.16 | 10 | |
| Total | 424.63 | 100.00 | Monascus ruber | 106.16 | 10 | |
| Total | 1061.57 | 100 | ||||
| Bakery 2 | Penicillium sp. | 4989.38 | 88.68 | Penicillium sp. | 2335.46 | 70.97 |
| Chrysosporium sp. | 530.79 | 9.43 | Cladosporium sp. | 955.41 | 29.03 | |
| Cladosporium sp. | 106.16 | 1.89 | Total | 3290.87 | 100 | |
| Total | 5626.327 | 100.00 | ||||
| Sampling Location | ID | SDA | ITRA | VORI | POSA | ||||
|---|---|---|---|---|---|---|---|---|---|
| CFU·m−3 | % | CFU·m−3 | % | CFU·m−3 | % | CFU·m−3 | % | ||
| Pizzeria Restaurant | Penicillium sp. | 16 | 94.12 | 4 | 66.67 | 17 | 100.00 | ||
| Aspergillus section Flavi | 1 | 16.67 | |||||||
| Cladosporium sp. | 1 | 16.67 | |||||||
| Chrysosporium sp. | 1 | 5.88 | |||||||
| Total | 17 | 100.00 | 6 | 100.00 | 17 | 100.00 | |||
| Bakery 1 | Penicillium sp. | 2 | 66.67 | ||||||
| Aspergillus section Flavi | 1 | 100.00 | |||||||
| Cladosporium sp. | 1 | 33.33 | |||||||
| Total | 3 | 100.00 | 1 | 100.00 | |||||
| Bakery 2 | Penicillium sp. | 4 | 80.00 | 1 | 50.00 | ||||
| Mucor sp. | 1 | 20.00 | 1 | 50.00 | |||||
| Total | 5 | 100.00 | 2 | 100.00 | |||||
| Sampling Location | ID | SDA | ITRA | VORI | POSA | ||||
|---|---|---|---|---|---|---|---|---|---|
| CFU·g−1 | % | CFU·g−1 | % | CFU·g−1 | % | CFU·g−1 | % | ||
| Pizzeria Restaurant | Penicillium sp. | 3 | 100.00 | ||||||
| Bakery 1 | Penicillium sp. | 1 | 100.00 | ||||||
| Bakery 2 | Penicillium sp. | 2 | 100.00 | ||||||
| Sampling Location | SDA | ITRA | VORI | POSA | |||||
|---|---|---|---|---|---|---|---|---|---|
| ID | CFU·m−2 | % | CFU·m−2 | % | CFU·m−2 | % | CFU·m−2 | % | |
| Pizzeria Restaurant | n.d. | ||||||||
| Total | |||||||||
| Bakery 1 | Penicillium sp. | 106.16 | 100.00 | 106.16 | 50.00 | ||||
| Cladosporium sp. | 106.16 | 50.00 | |||||||
| Total | 106.16 | 100.00 | 212.31 | 100.00 | |||||
| Bakery 2 | Penicillium sp. | 3609.34 | 94.44 | 1804.67 | 100.00 | ||||
| Mucor sp. | 106.16 | 2.78 | 106.157 | 50.00 | 106.16 | 100.00 | |||
| A. section Fumigati | 106.16 | 2.78 | |||||||
| Cladosporium sp. | 106.157 | 50.00 | |||||||
| Total | 3821.66 | 100.00 | 212.31 | 100.00 | 106.16 | 100.00 | |||
| Sampling Location | Environmental Matrix | CT |
|---|---|---|
| Pizzeria | Settled dust | 34.44 |
| IOM Filters | 37.50 | |
| 37.22 * | ||
| 37.42 * | ||
| 39.50 | ||
| Bakery 1 | IOM Filters | 36.10 |
| 39.52 | ||
| 36.34 | ||
| 36.97 | ||
| 38.19 | ||
| Bakery 2 | IOM Filters | 37.95 |
| 37.78 | ||
| 33.50 |
| Samples Type | Culture Media | Settled Dust | Personal Samplers | EDC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DG18 | TSA | RB | MEA | DG18 | TSA | RB | MEA | DG18 | TSA | RB | ||
| Settled dust | MEA | 0.420 * | 0.116 | −0.345 | 0.021 | −0.015 | −0.026 | −0.066 | −0.642 | −0.971 ** | −0.588 | −0.418 |
| DG18 | 0.035 | −0.273 | −0.029 | 0.271 | 0.075 | 0.187 | −0.221 | −0.116 | 0.116 | 0.412 | ||
| TSA | −0.125 | −0.015 | 0.277 | 0.021 | −0.004 | −0.493 | −0.152 | −0.395 | −0.108 | |||
| RB | −0.371 | 0.091 | 0.009 | 0.413 | 0.814 * | 0.463 | 0.494 | 0.237 | ||||
| Personal samplers | MEA | −0.067 | −0.056 | −0.437 | −0.145 | −0.200 | −0.886 * | −0.676 | ||||
| DG18 | 0.195 | 0.284 | −0.531 | 0.393 | 0.393 | 0.465 | ||||||
| TSA | 0.329 | −0.118 | 0.000 | 0.232 | 0.686 | |||||||
| RB | - | - | - | - | ||||||||
| EDC | MEA | 0.551 | 0.203 | 0.017 | ||||||||
| DG18 | 0.486 | 0.372 | ||||||||||
| TSA | 0.845* | |||||||||||
| Culture Media | Sampling Location | n | Ranks | Test Statistics | Kruskal–Wallis Multiple Comparisons | |||
|---|---|---|---|---|---|---|---|---|
| Mean Rank | Chi-Square | df | p | |||||
| Settled dust | MEA | Pizzeria | 10 | 12.70 | 9.778 | 2 | 0.008 * | Bakery 1 ≠ Bakery 2 (p = 0.005) |
| Bakery 1 | 5 | 21.30 | ||||||
| Bakery 2 | 10 | 9.15 | ||||||
| Total | 25 | |||||||
| DG18 | Pizzeria | 10 | 11.50 | 4.118 | 2 | 0.128 | ||
| Bakery 1 | 5 | 18.70 | ||||||
| Bakery 2 | 10 | 11.65 | ||||||
| Total | 25 | |||||||
| TSA | Pizzeria | 10 | 11.70 | 0.629 | 2 | 0.730 | ||
| Bakery 1 | 5 | 13.50 | ||||||
| Bakery 2 | 10 | 14.05 | ||||||
| Total | 25 | |||||||
| RB | Pizzeria | 10 | 10.30 | 15.436 | 2 | 0.000 * | Bakery 1 ≠ Bakery 2 (p = 0.001) | |
| Bakery 1 | 5 | 5.50 | Pizzeria ≠ Bakery 2 (p = 0.011) | |||||
| Bakery 2 | 10 | 19.45 | ||||||
| Total | 25 | |||||||
| Personal samplers | MEA | Pizzeria | 5 | 9.60 | 3.649 | 2 | 0.161 | |
| Bakery 1 | 5 | 14.80 | ||||||
| Bakery 2 | 10 | 8.80 | ||||||
| Total | 20 | |||||||
| DG18 | Pizzeria | 5 | 13.80 | 4.329 | 2 | 0.115 | ||
| Bakery 1 | 5 | 7.50 | ||||||
| Bakery 2 | 10 | 10.35 | ||||||
| Total | 20 | |||||||
| TSA | Pizzeria | 5 | 9.50 | 0.204 | 2 | 0.903 | ||
| Bakery 1 | 5 | 10.60 | ||||||
| Bakery 2 | 10 | 10.95 | ||||||
| Total | 20 | |||||||
| RB | Pizzeria | 5 | 9.00 | 3.333 | 2 | 0.189 | ||
| Bakery 1 | 5 | 9.00 | ||||||
| Bakery 2 | 10 | 12.00 | ||||||
| Total | 20 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, C.; Fleming, G.T.A.; Kadir, A.; Almeida, B.; Caetano, L.A.; Quintal Gomes, A.; Twarużek, M.; Kosicki, R.; Viegas, S.; Coggins, A.M. Occupational Exposures to Organic Dust in Irish Bakeries and a Pizzeria Restaurant. Microorganisms 2020, 8, 118. https://doi.org/10.3390/microorganisms8010118
Viegas C, Fleming GTA, Kadir A, Almeida B, Caetano LA, Quintal Gomes A, Twarużek M, Kosicki R, Viegas S, Coggins AM. Occupational Exposures to Organic Dust in Irish Bakeries and a Pizzeria Restaurant. Microorganisms. 2020; 8(1):118. https://doi.org/10.3390/microorganisms8010118
Chicago/Turabian StyleViegas, Carla, Gerard T. A. Fleming, Abdul Kadir, Beatriz Almeida, Liliana Aranha Caetano, Anita Quintal Gomes, Magdalena Twarużek, Robert Kosicki, Susana Viegas, and Ann Marie Coggins. 2020. "Occupational Exposures to Organic Dust in Irish Bakeries and a Pizzeria Restaurant" Microorganisms 8, no. 1: 118. https://doi.org/10.3390/microorganisms8010118
APA StyleViegas, C., Fleming, G. T. A., Kadir, A., Almeida, B., Caetano, L. A., Quintal Gomes, A., Twarużek, M., Kosicki, R., Viegas, S., & Coggins, A. M. (2020). Occupational Exposures to Organic Dust in Irish Bakeries and a Pizzeria Restaurant. Microorganisms, 8(1), 118. https://doi.org/10.3390/microorganisms8010118










