A Transcriptional Activator of Ascorbic Acid Transport in Streptococcus pneumoniae Is Required for Optimal Growth in Endophthalmitis in a Strain-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Preparation and Sequence Analysis of Transposon Libraries
2.3. Construction of ulaR2 Deletion Mutants
2.4. S. pneumoniae Pangenome Construction
2.5. Growth Experiments in Vitro
2.6. Growth and Virulence in Vivo
3. Results
3.1. Transposon Mutant Library Analysis
3.2. Creation of ulaR2 Deletion in S. pneumoniae
3.3. Pangenome Analysis
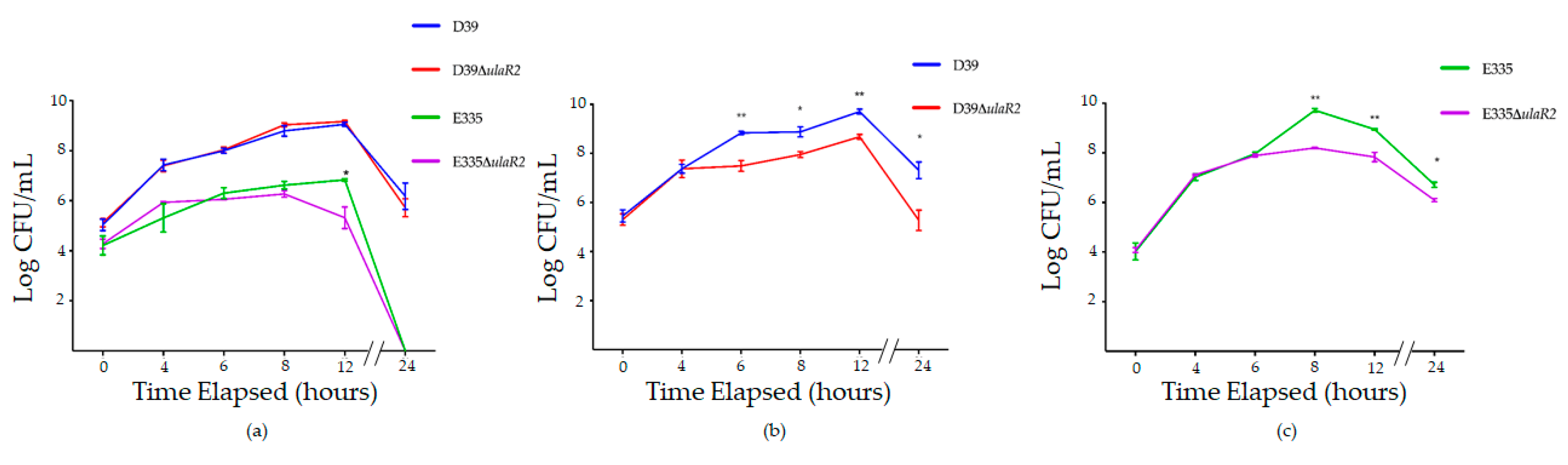
3.4. Growth of D39, D39ΔulaR2, E335, and E335ΔulaR2 in Vitro
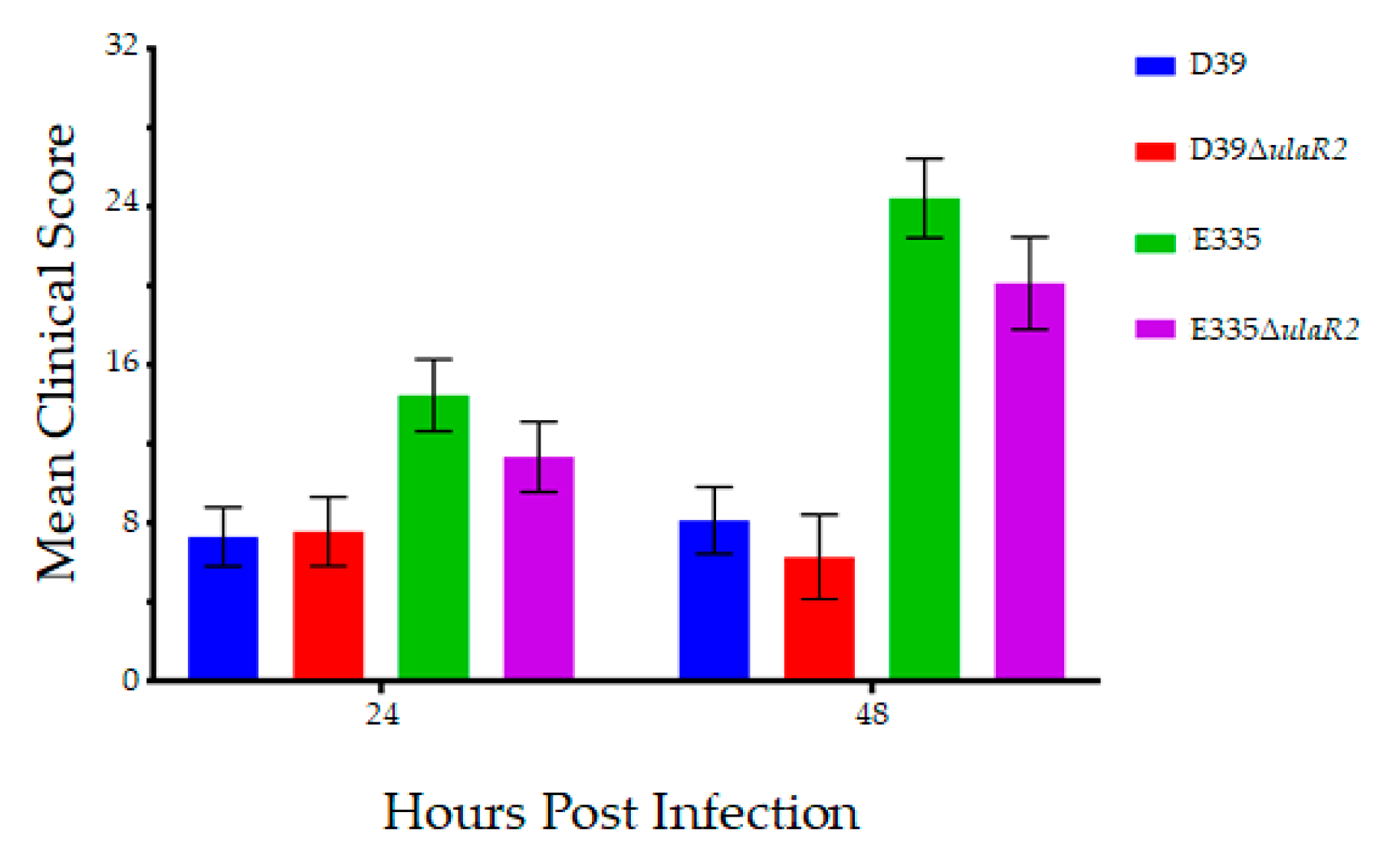
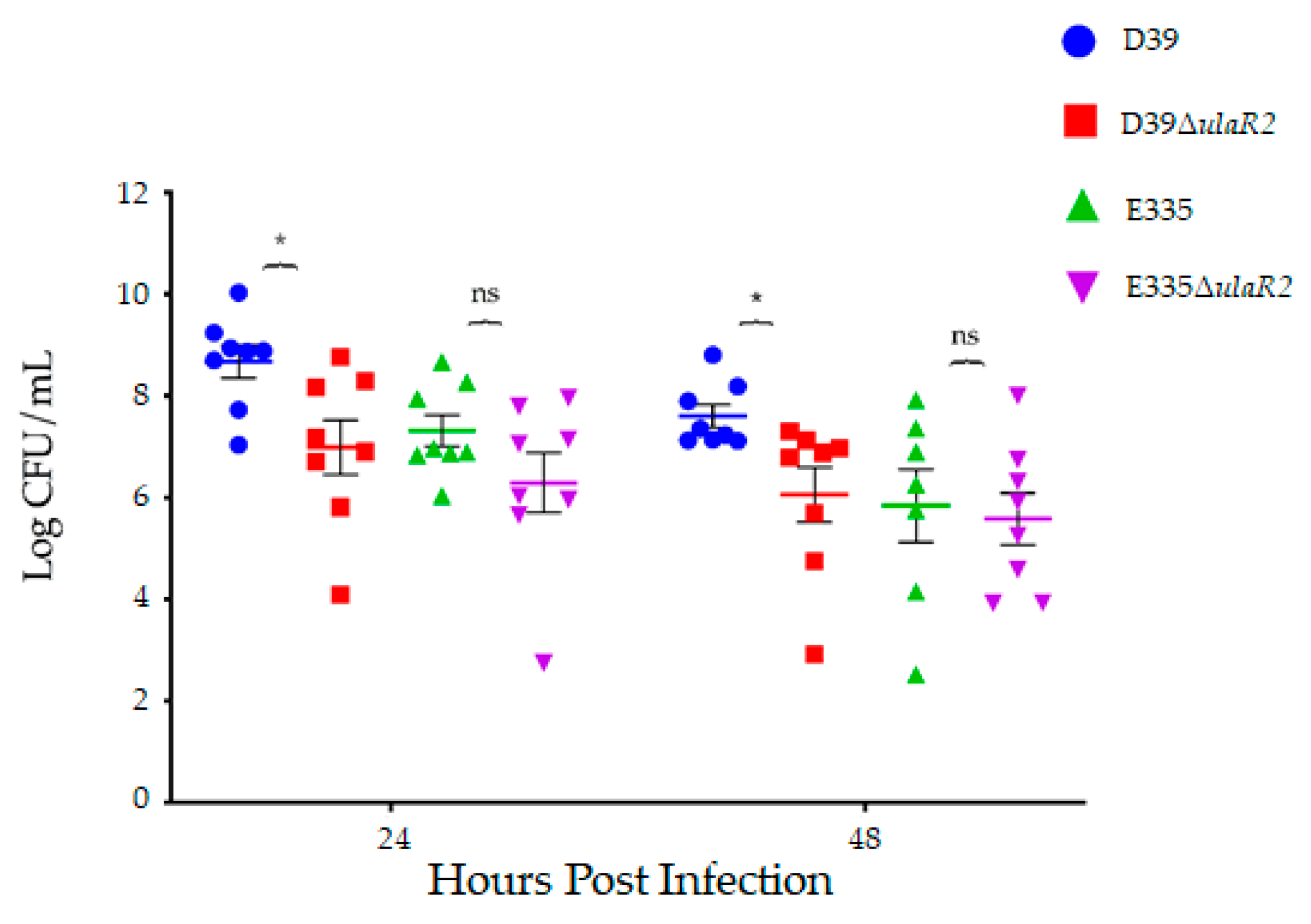
3.5. Growth and Virulence of D39, D39ΔulaR2, E335, and E335ΔulaR2 in Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dave, T.V.; Dave, V.P.; Sharma, S.; Karolia, R.; Joseph, J.; Pathengay, A.; Pappuru, R.R.; Das, T. Infectious endophthalmitis leading to evisceration: Spectrum of bacterial and fungal pathogens and antibacterial susceptibility profile. J. Ophthalmic. Inflamm. Infect. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.P.; Angbue Te, N.; Zagora, S.L.; Symes, R.J.; Yates, W.; Chang, A.A.; McCluskey, P.J.; Simunovic, M.P. Post-surgical versus post-intravitreal injection endophthalmitis: Changing patterns in causative flora. Clin. Exp. Ophthalmol. 2019, 47, 57–62. [Google Scholar] [CrossRef]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial profile of ocular infections: A systematic review. BMC Ophthalmol. 2017, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.L. Bacterial and fungal endophthalmitis. Clin Microbiol Rev 2017, 30, 597–613. [Google Scholar] [CrossRef] [PubMed]
- McCannel, C.A. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: Causative organisms and possible prevention strategies. Retina 2011, 31, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, E.D.; Rocke, J.R.; Sandhu, S.S.; Allen, P.J. Predictors of visual outcome and the role of early vitrectomy in streptococcal endophthalmitis. Clin. Exp. Ophthalmol. 2018, 46, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Scott, I.U.; Flynn, H.W., Jr.; Smiddy, W.E.; Corey, R.P.; Miller, D. Endophthalmitis caused by Streptococcus pneumoniae. Am. J. Ophthalmol. 2004, 138, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.W.; Costa, J.R.; Samiy, N.; Ruoff, K.L.; Connolly, E.; Cousins, F.V.; D’Amico, D.J. Contribution of pneumolysin and autolysin to the pathogenesis of experimental pneumococcal endophthalmitis. Retina 2002, 22, 622–632. [Google Scholar] [CrossRef]
- Ng, E.W.; Samiy, N.; Rubins, J.B.; Cousins, F.V.; Ruoff, K.L.; Baker, A.S.; D’Amico, D.J. Implication of pneumolysin as a virulence factor in Streptococcus pneumoniae endophthalmitis. Retina 1997, 17, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Norcross, E.W.; Moore, Q.C., III; Fratkin, J.; Thompson, H.; Marquart, M.E. Immunization with pneumolysin protects against both retinal and global damage caused by Streptococcus pneumoniae endophthalmitis. J. Ocul. Pharmacol. Ther. 2010, 26, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Norcross, E.W.; Robertson, Z.M.; Moore, Q.C., III; Fratkin, J.; Thompson, H.; Marquart, M.E. The Streptococcus pneumoniae capsule is required for full virulence in pneumococcal endophthalmitis. Invest Ophthalmol. Vis. Sci. 2011, 52, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Norcross, E.W.; Moore, Q.C.; Onwubiko, C.; King, L.B.; Fratkin, J.; Marquart, M.E. A comparison of pneumolysin activity and concentration in vitro and in vivo in a rabbit endophthalmitis model. Clin. Ophthalmol. 2008, 2, 793–800. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lundquist, O.; Osterlin, S. Glucose concentration in the vitreous of nondiabetic and diabetic human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 1994, 232, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Benton, A.H.; Fulton, L.K.; Marquart, M.E. Exogenous Streptococcus pneumoniae endophthalmitis in diabetic rabbits. Sci. Rep. 2017, 7, 46196. [Google Scholar] [CrossRef] [PubMed]
- McGahan, M.C. Ascorbic acid levels in aqueous and vitreous humors of the rabbit: Effects of inflammation and ceruloplasmin. Exp. Eye Res. 1985, 41, 291–298. [Google Scholar] [CrossRef]
- Reiss, G.R.; Werness, P.G.; Zollman, P.E.; Brubaker, R.F. Ascorbic acid levels in the aqueous humor of nocturnal and diurnal mammals. Arch. Ophthalmol. 1986, 104, 753–755. [Google Scholar] [CrossRef]
- Rose, R.C.; Richer, S.P.; Bode, A.M. Ocular oxidants and antioxidant protection. Proc. Soc. Exp. Biol. Med. 1998, 217, 397–407. [Google Scholar] [CrossRef]
- Bidossi, A.; Mulas, L.; Decorosi, F.; Colomba, L.; Ricci, S.; Pozzi, G.; Deutscher, J.; Viti, C.; Oggioni, M.R. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoS ONE 2012, 7, e33320. [Google Scholar] [CrossRef]
- Yamane, K.; Minamoto, A.; Yamashita, H.; Takamura, H.; Miyamoto-Myoken, Y.; Yoshizato, K.; Nabetani, T.; Tsugita, A.; Mishima, H.K. Proteome analysis of human vitreous proteins. Mol. Cell Proteomics 2003, 2, 1177–1187. [Google Scholar] [CrossRef]
- Aretz, S.; Krohne, T.U.; Kammerer, K.; Warnken, U.; Hotz-Wagenblatt, A.; Bergmann, M.; Stanzel, B.V.; Kempf, T.; Holz, F.G.; Schnölzer, M.; et al. In-depth mass spectrometric mapping of the human vitreous proteome. Proteome Sci. 2013, 11, 22. [Google Scholar] [CrossRef]
- Kokavec, J.; Min, S.H.; Tan, M.H.; Gilhotra, J.S.; Newland, H.S.; Durkin, S.R.; Grigg, J.; Casson, R.J. Biochemical analysis of the living human vitreous. Clin. Exp. Ophthalmol. 2016, 44, 597–609. [Google Scholar] [CrossRef] [PubMed]
- van Opijnen, T.; Lazinski, D.W.; Camilli, A. Genome-wide fitness and genetic interactions determined by Tn-seq, a high-throughput massively parallel sequencing method for microorganisms. Curr. Protoc. Microbiol. 2015, 36. [Google Scholar] [CrossRef]
- Iyer, R.; Baliga, N.S.; Camilli, A. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 2005, 187, 8340–8349. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.M.; Hunt, H.D.; Ho, S.N.; Pullen, J.K.; Pease, L.R. Engineering hybrid genes without the use of restriction enzymes: Gene splicing by overlap extension. Gene 1989, 77, 61–68. [Google Scholar] [CrossRef]
- Yother, J.; McDaniel, L.S.; Briles, D.E. Transformation of encapsulated Streptococcus pneumoniae. J. Bacteriol. 1986, 168, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Callegan, M.C.; Jett, B.D.; Hancock, L.E.; Gilmore, M.S. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect Immun. 1999, 67, 3357–3366. [Google Scholar] [PubMed]
- Williams, R.N.; Paterson, C.A.; Eakins, K.E.; Bhattacherjee, P. Quantification of ocular inflammation: Evaluation of polymorphonuclear leucocyte infiltration by measuring myeloperoxidase activity. Curr. Eye Res. 1982, 2, 465–470. [Google Scholar] [CrossRef]
- Afzal, M.; Shafeeq, S.; Henriques-Normark, B.; Kuipers, O.P. UlaR activates expression of the ula operon in Streptococcus pneumoniae in the presence of ascorbic acid. Microbiology 2015, 161, 41–49. [Google Scholar] [CrossRef][Green Version]
- Afzal, M.; Shafeeq, S.; Kuipers, O.P. Ascorbic acid-dependent gene expression in Streptococcus pneumoniae and the activator function of the transcriptional regulator UlaR2. Front. Microbiol. 2015, 6, 72. [Google Scholar] [CrossRef]
- De Ste Croix, M.; Chen, K.Y.; Vacca, I.; Manso, A.S.; Johnston, C.; Polard, P.; Kwun, M.J.; Bentley, S.D.; Croucher, N.J.; Bayliss, C.D.; et al. Recombination of the phase-variable spnIII locus is independent of all known pneumococcal site-specific recombinases. J. Bacteriol. 2019, 201, e00233-19. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.J.; Cesbron, Y.; Shaw, S.L.; Bazan Villicana, J.; Tsui, H.T.; Boersma, M.J.; Ye, Z.A.; Tovpeko, Y.; Dekker, C.; Holden, S.; et al. Movement dynamics of divisome proteins and PBP2x:FtsW in cells of Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 2019, 116, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; Hakala, P.; Singh, A.K.; Lapatto, H.A.K.; King, S.J.; Meri, S.; Jokiranta, T.S.; Haapasalo, K. Role of pneumococcal NanA neuraminidase activity in peripheral blood. Front. Cell Infect. Microbiol. 2019, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, C.M.; King, S.J. Pneumococcal carbohydrate transport: Food for thought. Trends Microbiol. 2012, 20, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Van Opijnen, T.; Camilli, A. A fine scale phenotype-genotype virulence map of a bacterial pathogen. Genome Res. 2012, 22, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Valentino, M.D.; Foulston, L.; Sadaka, A.; Kos, V.N.; Villet, R.A.; Santa Maria, J., Jr.; Lazinski, D.W.; Camilli, A.; Walker, S.; Hooper, D.C.; et al. Genes contributing to Staphylococcus aureus fitness in abscess- and infection-related ecologies. MBio 2014, 5, e01729-14. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Sequence (5′ to 3′) |
|---|---|
| spd1961_upF | ACACATCGTAAGGATAGATGCGGA |
| spd1961_upR | CATCAAGCTTATCGATACCGTTCTCTTCCTTTCTAACTAC |
| spd1961_downF | GAAGGTTTTTATATTACAGCTCCAATGTTAAAAATTGGTACAGC |
| spd1961_downR | TATTTTTGCACAAATGCTGGGGA |
| Gene | Description | Reduction in Fitness | p-value 1 |
|---|---|---|---|
| spd_0006 | Transcription repair coupling factor | 86.06% | 0.026 |
| spd_0095 | Conserved hypothetical protein | 7.42% | 0.022 |
| spd_1004 | Glyceraldehyde-3-phosphate dehydrogenase, NADP-dependent | 88.53% | 0.016 |
| spd_1225 | Conserved hypothetical protein | 38.43& | 0.014 |
| spd_1489 | N-acetylneuraminate lyase, putative | 11.92% | 0.028 |
| spd_1846 | Phosphotransferase system IIB component | 65.13% | 0.020 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benton, A.H.; Jackson, M.D.; Wong, S.M.; Dees, J.L.; Akerley, B.J.; Marquart, M.E. A Transcriptional Activator of Ascorbic Acid Transport in Streptococcus pneumoniae Is Required for Optimal Growth in Endophthalmitis in a Strain-Dependent Manner. Microorganisms 2019, 7, 290. https://doi.org/10.3390/microorganisms7090290
Benton AH, Jackson MD, Wong SM, Dees JL, Akerley BJ, Marquart ME. A Transcriptional Activator of Ascorbic Acid Transport in Streptococcus pneumoniae Is Required for Optimal Growth in Endophthalmitis in a Strain-Dependent Manner. Microorganisms. 2019; 7(9):290. https://doi.org/10.3390/microorganisms7090290
Chicago/Turabian StyleBenton, Angela H., Mary Darby Jackson, Sandy M. Wong, Justine L. Dees, Brian J. Akerley, and Mary E. Marquart. 2019. "A Transcriptional Activator of Ascorbic Acid Transport in Streptococcus pneumoniae Is Required for Optimal Growth in Endophthalmitis in a Strain-Dependent Manner" Microorganisms 7, no. 9: 290. https://doi.org/10.3390/microorganisms7090290
APA StyleBenton, A. H., Jackson, M. D., Wong, S. M., Dees, J. L., Akerley, B. J., & Marquart, M. E. (2019). A Transcriptional Activator of Ascorbic Acid Transport in Streptococcus pneumoniae Is Required for Optimal Growth in Endophthalmitis in a Strain-Dependent Manner. Microorganisms, 7(9), 290. https://doi.org/10.3390/microorganisms7090290




