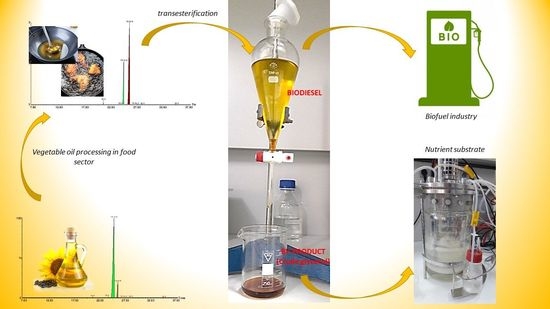
Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Culture Media
2.3. Bioreactor Batch Fermentation
2.4. Assays
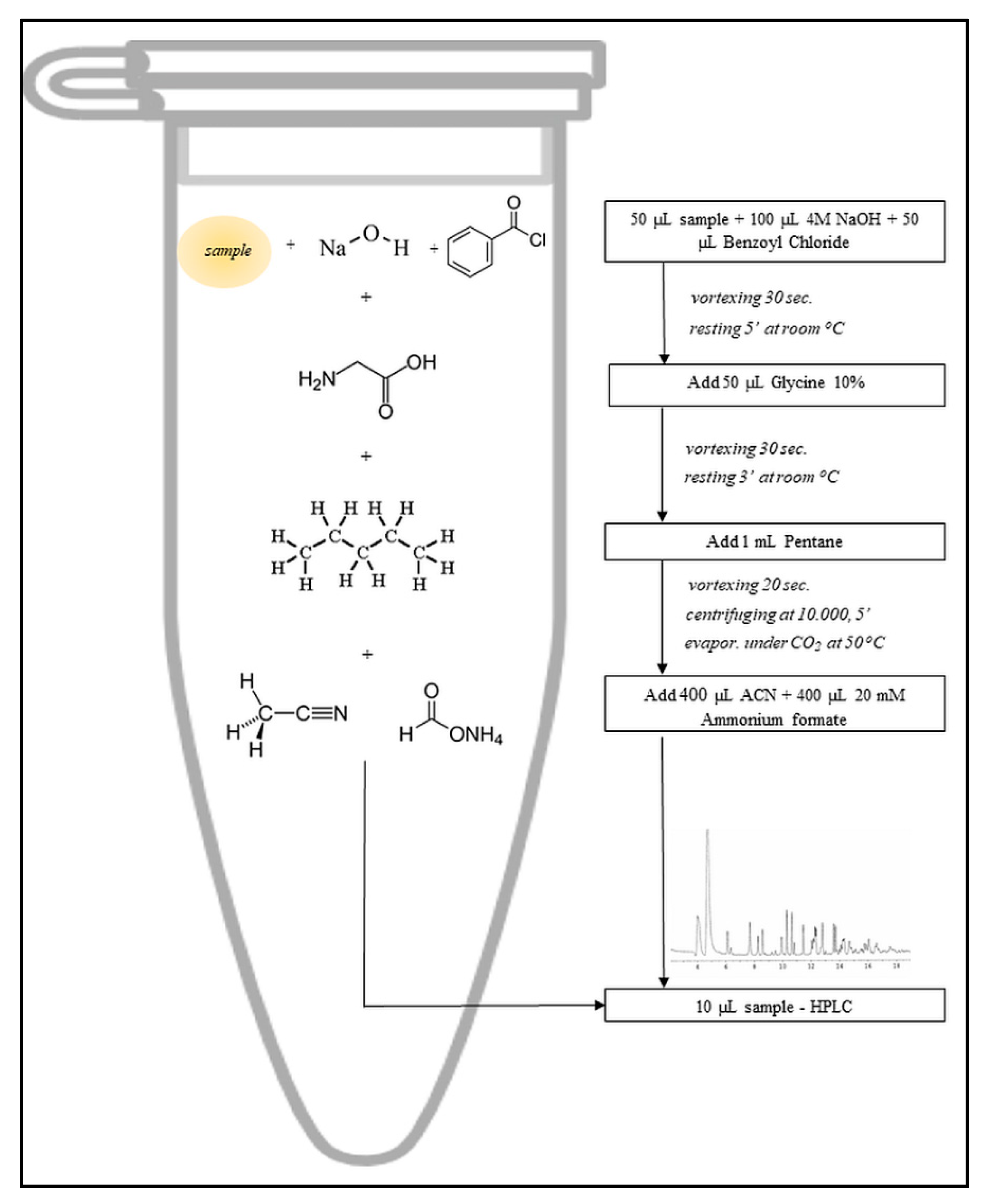
2.4.1. Determination of the Fatty Acids from Processed and Unprocessed Vegetable Oil
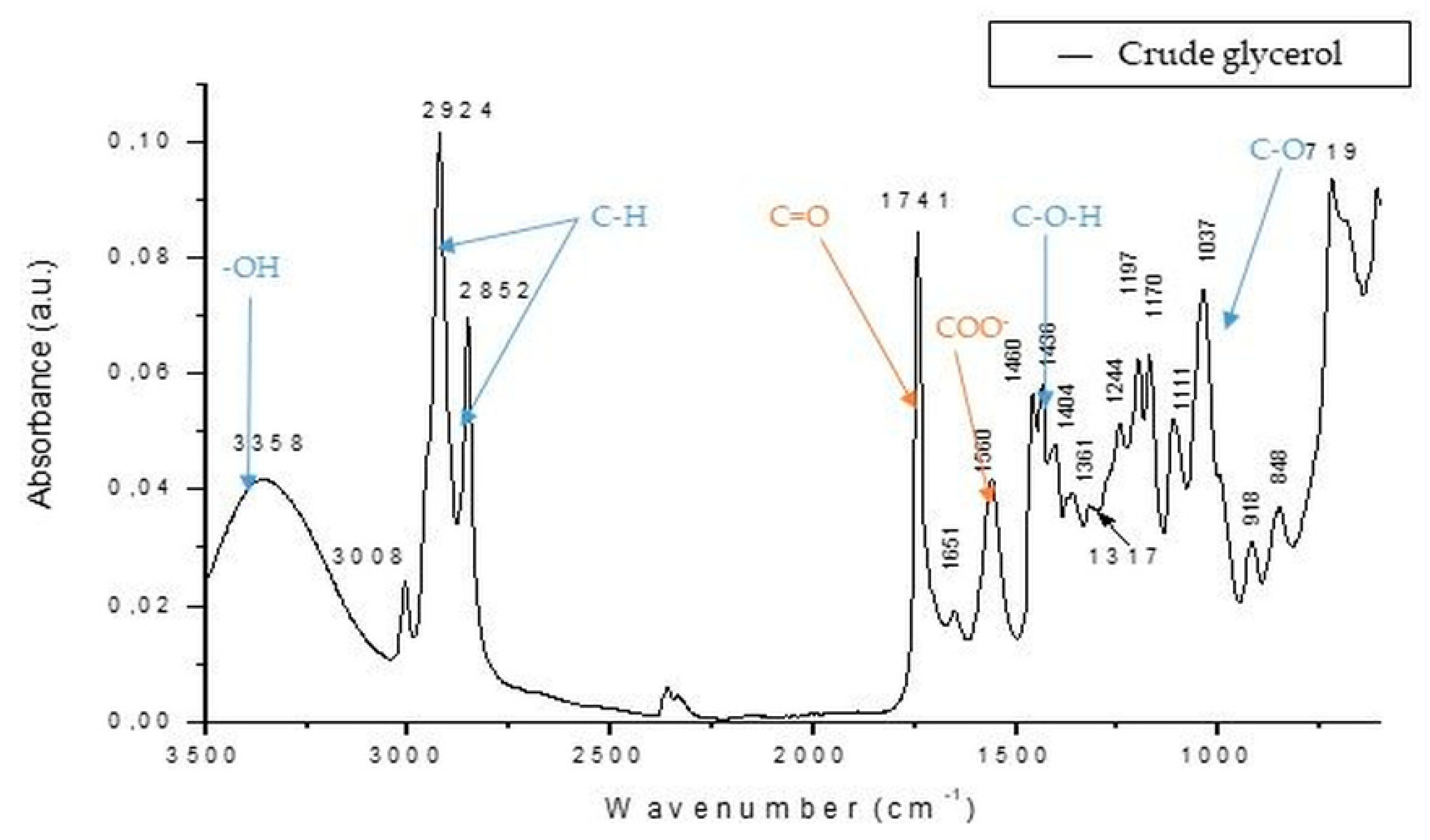
2.4.2. Crude Glycerol Analysis by FTIR
2.4.3. Biomass and Cell Viability
2.4.4. Organic Acids and Substrate Consumption (Glycerol, Glucose) Determination
3. Results and Discussion
3.1. The Fatty Acids Profile from Processed and Unprocessed Vegetable Oil
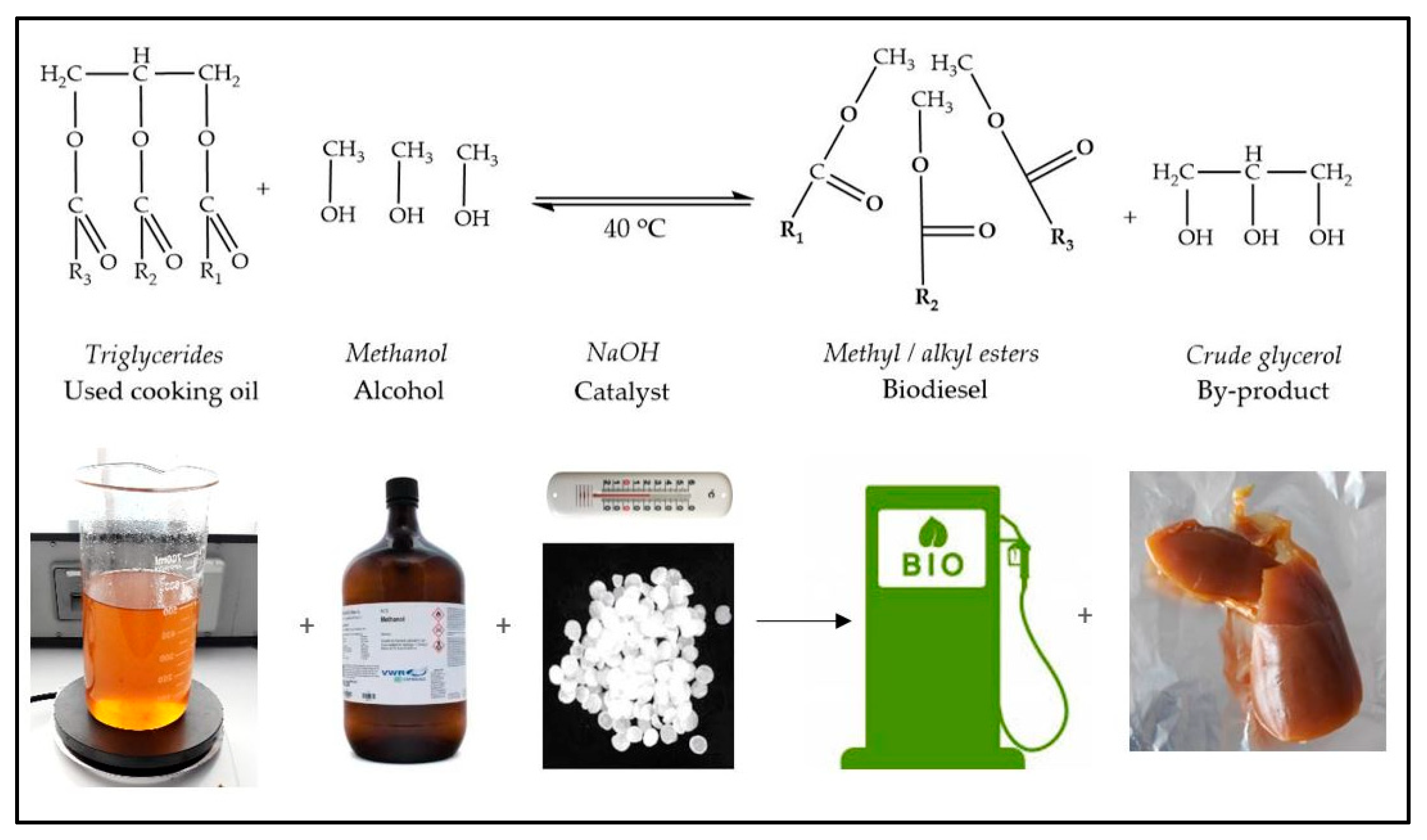
3.2. Crude Glycerol Obtaining Process
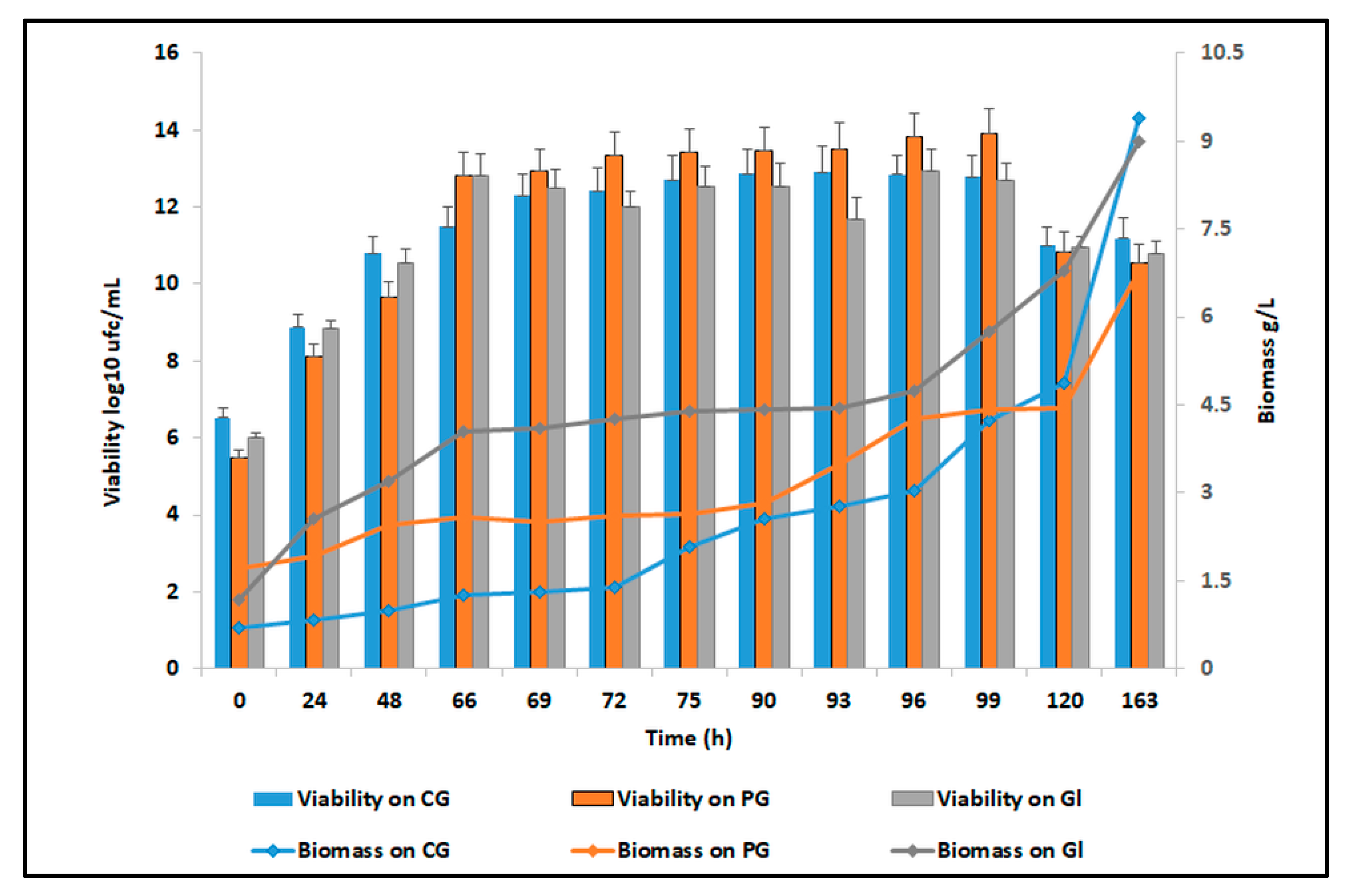
3.3. C. zeylanoides Growth in Different Culture Media
3.4. Succinic and Citric Acids Bio-Synthesis by C. zeylanoides ATCC 20367
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yusuf, N.N.A.N.; Kamarudin, S.K.; Yaakub, Z. Overview on the current trends in biodiesel production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Srivastava, N.; Rathour, R.; Jha, S.; Pandey, K.; Srivastava, M.; Thakur, V.K.; Sengar, S.; Gupta, V.; Mazumder, P.; Khan, A.; et al. Microbial Beta Glucosidase Enzymes: Recent Advances in Biomass Conversation for Biofuels Application. Biomolecules 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of Biodiesel via Acid Catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Dulf, F.V.; Pop, O.L.; Socaciu, C. L (+)-lactic acid production by pellet-form Rhizopus oryzae NRRL 395 on biodiesel crude glycerol. Microb. Cell Factories 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- EU Biofuels Annual. 2018. Available online: https://gain.fas.usda.gov (accessed on 13 June 2019).
- Biofuel Mandates in the EU by Member State in 2018. Available online: https://gain.fas.usda.gov (accessed on 13 June 2019).
- Bezergianni, S.; Dimitriadis, A. Comparison between different types of renewable diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking OilAn Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Lee, E.Y. Nano-Immobilized Biocatalysts for Biodiesel Production from Renewable and Sustainable Resources. Catalysts 2018, 8, 68. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Călinoiu, L.-F.; Mitrea, L.; Precup, G.; Bindea, M.; Rusu, B.; Dulf, F.-V.; Ștefănescu, B.-E.; Vodnar, D.-C. Characterization of Grape and Apple Peel Wastes’ Bioactive Compounds and Their Increased Bioavailability After Exposure to Thermal Process. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2017, 74, 80–89. [Google Scholar] [CrossRef][Green Version]
- Teleky, B.E.; Vodnar, D.C. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers 2019, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ștefănescu, B.E.; Crișan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.N.; Tišma, M.; Budžaki, S.; McMurchie, E.J.; Gonzalez, O.M.M.; Hessel, V.; Ngothai, Y. Scale-up and economic analysis of biodiesel production from recycled grease trap waste. Appl. Energy 2018, 229, 142–150. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste Cooking Oil as an Alternate Feedstock for Biodiesel Production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhou, J.-J.; Sun, Y.-Q.; Xiu, Z.-L. Bioconversion of Raw Glycerol From Waste Cooking-Oil-Based Biodiesel Production to 1,3-Propanediol and Lactate by a Microbial Consortium. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Ataya, F.; Dubé, M.A.; Ternan, M. Acid-Catalyzed Transesterification of Canola Oil to Biodiesel under Single- and Two-Phase Reaction Conditions. Energy Fuels 2007, 21, 2450–2459. [Google Scholar] [CrossRef]
- Patrascoiu, M.; Rathbauer, J.; Negrea, M.; Zeller, R. Perspectives of safflower oil as biodiesel source for South Eastern Europe (comparative study: Safflower, soybean and rapeseed). Fuel 2013, 111, 114–119. [Google Scholar] [CrossRef]
- Jindapon, W.; Ngamcharussrivichai, C. Heterogeneously catalyzed transesterification of palm oil with methanol to produce biodiesel over calcined dolomite: The role of magnesium oxide. Energy Convers. Manag. 2018, 171, 1311–1321. [Google Scholar] [CrossRef]
- Ortega-de la Rosa, N.D.; Vazquez-Vazquez, J.L.; Huerta-Ochoa, S.; Gimeno, M.; Gutierrez-Rojas, M. Stable bioemulsifiers are produced by Acinetobacter bouvetii UAM25 growing in different carbon sources. Bioprocess Biosyst. Eng. 2018, 41, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Ortega, N.; Ramos-Ramírez, E.; Serafín-Muñoz, A.; Zamorategui-Molina, A.; Monjaraz-Vallej, J. Use of Co/Fe-Mixed Oxides as Heterogeneous Catalysts in Obtaining Biodiesel. Catalysts 2019. [Google Scholar] [CrossRef]
- Kong, P.S.; Aroua, M.K.; Wan Daud, W.M.A. Conversion of crude and pure glycerol into derivatives. A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Mitrea, L.; Trif, M.; Cătoi, A.F.; Vodnar, D.C. Utilization of biodiesel derived-glycerol for 1,3-PD and citric acid production. Microb. Cell Fact. 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Ben Hassen Trabelsi, A.; Zaafouri, K.; Baghdadi, W.; Naoui, S.; Ouerghi, A. Second generation biofuels production from waste cooking oil via pyrolysis process. Renew. Energy 2018, 126, 888–896. [Google Scholar] [CrossRef]
- Gerpen, J.V. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Arumugam, A.; Ponnusami, V. Production of biodiesel by enzymatic transesterification of waste sardine oil and evaluation of its engine performance. Heliyon 2017, 3, e00486. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ștefănescu, B.-E.; Pop, I.-D.; Vodnar, D.-C. Isolated Microorganisms for Bioconversion of Biodiesel-Derived Glycerol Into 1,3-Propanediol. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2017, 74, 43–49. [Google Scholar] [CrossRef]
- Busic, A.; Kundas, S.; Morzak, G.; Belskaya, H.; Mardetko, N.; Ivancic Santek, M.; Komes, D.; Novak, S.; Santek, B. Recent Trends in Biodiesel and Biogas Production. Food Technol. Biotechnol. 2018, 56, 152–173. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Shen, J.-T.; Wang, X.-L.; Sun, Y.-Q.; Xiu, Z.-L. Stability and oscillatory behavior of microbial consortium in continuous conversion of crude glycerol to 1,3-propanediol. Appl. Microbiol. Biotechnol. 2018, 102, 8291–8305. [Google Scholar] [CrossRef]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Bindea, M.; Rusu, B.; Rusu, A.; Trif, M.; Leopold, L.F.; Dulf, F.; Vodnar, D.C. Valorification of crude glycerol for pure fractions of docosahexaenoic acid and beta-carotene production by using Schizochytrium limacinum and Blakeslea trispora. Microb. Cell Factories 2018, 17. [Google Scholar] [CrossRef]
- Ma, J.S.; Jiang, H.; Hector, S.B.; Xiao, Z.H.; Li, J.L.; Liu, R.K.; Li, C.Z.; Zeng, B.Q.; Liu, G.Q.; Zhu, Y.H. Adaptability of Klebsiella pneumoniae 2e, a Newly Isolated 1,3-Propanediol-Producing Strain, to Crude Glycerol as Revealed by Genomic Profiling. Appl. Env. Microbiol. 2019, 85, 15. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.-R.; Lee, S.-M.; Heo, S.-Y.; Seo, J.-W.; Kim, C.H. Efficient production of 1,3-propanediol from crude glycerol by repeated fed-batch fermentation strategy of a lactate and 2,3-butanediol deficient mutant of Klebsiella pneumoniae. Microb. Cell Factories 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Samudrala, S.P.; Bhattacharya, S. Toward the Sustainable Synthesis of Propanols from Renewable Glycerol over MoO3-Al2O3 Supported Palladium Catalysts. Catalysts 2018, 8. [Google Scholar] [CrossRef]
- Dolejš, I.; Líšková, M.; Krasňan, V.; Markošová, K.; Rosenberg, M.; Lorenzini, F.; Marr, A.C.; Rebroš, M. Production of 1,3-Propanediol from Pure and Crude Glycerol Using Immobilized Clostridium butyricum. Catalysts 2019, 9, 13. [Google Scholar] [CrossRef]
- Nasirian, N.; Mirzaie, M.; Cicek, N.; Levin, D.B. Lipid and carotenoid synthesis by Rhodosporidium diobovatum, grown on glucose versus glycerol, and its biodiesel properties. Can. J. Microbiol. 2018, 64, 277–289. [Google Scholar] [CrossRef]
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippoussis, A.; Papanikolaou, S. Lipids from yeasts and fungi: Physiology, production and analytical considerations. J. Appl. Microbiol. 2018, 124, 336–367. [Google Scholar] [CrossRef]
- Anastassiadis, S.G.; Rehm, H.-J. Oxygen and temperature effect on continuous citric acid secretion in Candida oleophila. Electron. J. Biotechnol. 2006, 9. [Google Scholar] [CrossRef][Green Version]
- Khan, A.; Bhide, A.; Gadre, R. Mannitol production from glycerol by resting cells of Candida magnoliae. Bioresour. Technol. 2009, 100, 4911–4913. [Google Scholar] [CrossRef]
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Laura Foresti, M. Yarrowia lipolytica: A model yeast for citric acid production. Fems. Yeast Res. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Robak, M.; Lazar, Z.; Tomaszewska, L.; Rymowicz, W. Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenergy 2013, 48, 148–166. [Google Scholar] [CrossRef]
- Xiaoyan, L.; Yu, X.; Lv, J.; Xu, J.; Xia, J.; Wu, Z.; Zhang, T.; Deng, Y. A cost-effective process for the coproduction of erythritol and lipase with Yarrowia lipolytica M53 from waste cooking oil. Food Bioprod. Process. 2017, 103, 86–94. [Google Scholar] [CrossRef]
- Wen, Z.; Bachmann, C.; Grisso, R.; Arogo, J.; Vaughan, D. Making Your Own Biodiesel. 2015. Available online: https://www.researchgate.net/publication/268397302_Making_Your_Own_Biodiesel (accessed on 17 January 2019).
- Takayama, K.; Adachi, T.; Kohata, M.; Hattori, K.; Tomiyama, T. Biotechnical citric acid prepn.-by cultivating yeast mutants having larger iron ion requirements. U.S. Patent US3926724A, 25 March 2019. [Google Scholar]
- Christie, W.W. Preparation of Methyl Ester and Other Derivatives, 1st ed.; Oily Press: Glasgow, UK, 1989. [Google Scholar]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.-H.; Diaconeasa, Z.; Socaciu, C. Liberation and recovery of phenolic antioxidants and lipids in chokeberry (Aronia melanocarpa) pomace by solid-state bioprocessing using Aspergillus niger and Rhizopus oligosporus strains. LWT 2018, 87, 241–249. [Google Scholar] [CrossRef]
- Ziegler, N.R.; Halvorson, H.O. Application of Statistics to Problems in Bacteriology: IV. Experimental Comparison of the Dilution Method, the Plate Count, and the Direct Count for the Determination of Bacterial Populations. J. Bacteriol. 1935, 29, 609–634. [Google Scholar] [PubMed]
- Prakash, M. Methylene Blue Staining. Available online: https://www.protocols.io/view/Methylene-Blue-staining-fd7bi9n (accessed on 13 March 2019).
- Imbert, L.; Saussereau, E.; Lacroix, C. Analysis of Eight Glycols in Serum Using LC-ESI–MS-MS. J. Anal. Toxicol. 2014, 38, 676–680. [Google Scholar] [CrossRef]
- Kongjao, S.; Somsak, D.; Mali, H. Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J. Chem. Eng. 2010, 27, 944–949. [Google Scholar] [CrossRef]
- Kowalski, M.; Kowalska, K.; Wiszniowski, J.; Turek-Szytow, J. Qualitative analysis of activated sludge using FT-IR technique. Chem. Zvesti 2018, 72, 2699–2706. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Hollmann, F.; Grzebyk, P.; Heinrichs, V.; Doderer, K.; Thum, O. On the inactivity of Candida antartica lipase B towards strong acids. J. Mol. Catal. B 2009, 57, 257–261. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Fatykhova, A.R.; Dedyukhina, E.G.; Anastassiadis, S.G.; Golovchenko, N.P.; Morgunov, I.G. Citric Acid Production by Yeast Grown on Glycerol-Containing Waste from Biodiesel Industry. Food Technol. Biotechnol. 2011, 49, 65–74. [Google Scholar]
- Tamakawa, H.; Ikushima, S.; Yoshida, S. Efficient production of l-lactic acid from xylose by a recombinant Candida utilis strain. J. Biosci. Bioeng. 2012, 113, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Thakker, C.; Martínez, I.; Li, W.; San, K.-Y.; Bennett, G.N. Metabolic engineering of carbon and redox flow in the production of small organic acids. J. Ind. Microbiol. Biotechnol. 2015, 42, 403–422. [Google Scholar] [CrossRef] [PubMed]
- Do, D.T.H.; Theron, C.W.; Fickers, P. Organic Wastes as Feedstocks for Non-Conventional Yeast-Based Bioprocesses. Microorganisms 2019, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Galaction, A.I.; Cascaval, D.; Oniscu, C.; Turnea, M. Prediction of oxygen mass transfer coefficients in stirred bioreactors for bacteria, yeasts and fungus broths. Biochem. Eng. J. 2004, 20, 85–94. [Google Scholar] [CrossRef]
- Klein, M.; Islam, Z.-U.; Knudsen, P.B.; Carrillo, M.; Swinnen, S.; Workman, M.; Nevoigt, E. The expression of glycerol facilitators from various yeast species improves growth on glycerol of Saccharomyces cerevisiae. Metab. Eng. Commun. 2016, 3, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Morgunov, I.G. Metabolic peculiarities of the citric acid overproduction from glucose in yeasts Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 433–440. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric Acid Production by Yarrowia lipolytica Yeast on Different Renewable Raw Materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V. Physiologo-biochemical characteristics of citrate-producing yeast Yarrowia lipolytica grown on glycerol-containing waste of biodiesel industry. Appl. Microbiol. Biotechnol. 2015, 99, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mironczuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Morgunov, I.G. Biosynthesis of pyruvic acid from glycerol-containing substrates and its regulation in the yeast Yarrowia lipolytica. Bioresour. Technol. 2018, 266, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk, A.M.; Dobrowolski, A.; Rakicka, M.; Rywińska, A.; Rymowicz, W. Newly isolated mutant of Yarrowia lipolytica MK1 as a proper host for efficient erythritol biosynthesis from glycerol. Process Biochem. 2015, 50, 61–68. [Google Scholar] [CrossRef]
- Visser, W.; Scheffers, W.A.; Batenburg-van der Vegte, W.H.; van Dijken, J.P. Oxygen requirements of yeasts. Appl. Env. Microbiol. 1990, 56, 3785. [Google Scholar]
- Jost, B.; Holz, M.; Aurich, A.; Barth, G.; Bley, T.; Müller, R.A. The influence of oxygen limitation for the production of succinic acid with recombinant strains of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2015, 99, 1675–1686. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hong, S.H.; Lee, S.H.; Park, S.J. Fermentative production of chemicals that can be used for polymer synthesis. Macromol. Biosci. 2004, 4, 157–164. [Google Scholar] [CrossRef]
- Bai, S.; Dai, J.; Xia, M.; Ruan, J.; Wei, H.; Yu, D.; Li, R.; Jing, H.; Tian, C.; Song, L.; et al. Effects of intermediate metabolite carboxylic acids of TCA cycle on Microcystis with overproduction of phycocyanin. Environ. Sci. Pollut. Res. Int. 2015, 22, 5531–5537. [Google Scholar] [CrossRef]
- Yuzbashev, T.V.; Yuzbasheva, E.Y.; Sobolevskaya, T.I.; Laptev, I.A.; Vybornaya, T.V.; Larina, A.S.; Matsui, K.; Fukui, K.; Sineoky, S.P. Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol. Bioeng. 2010, 107, 673–682. [Google Scholar] [CrossRef]

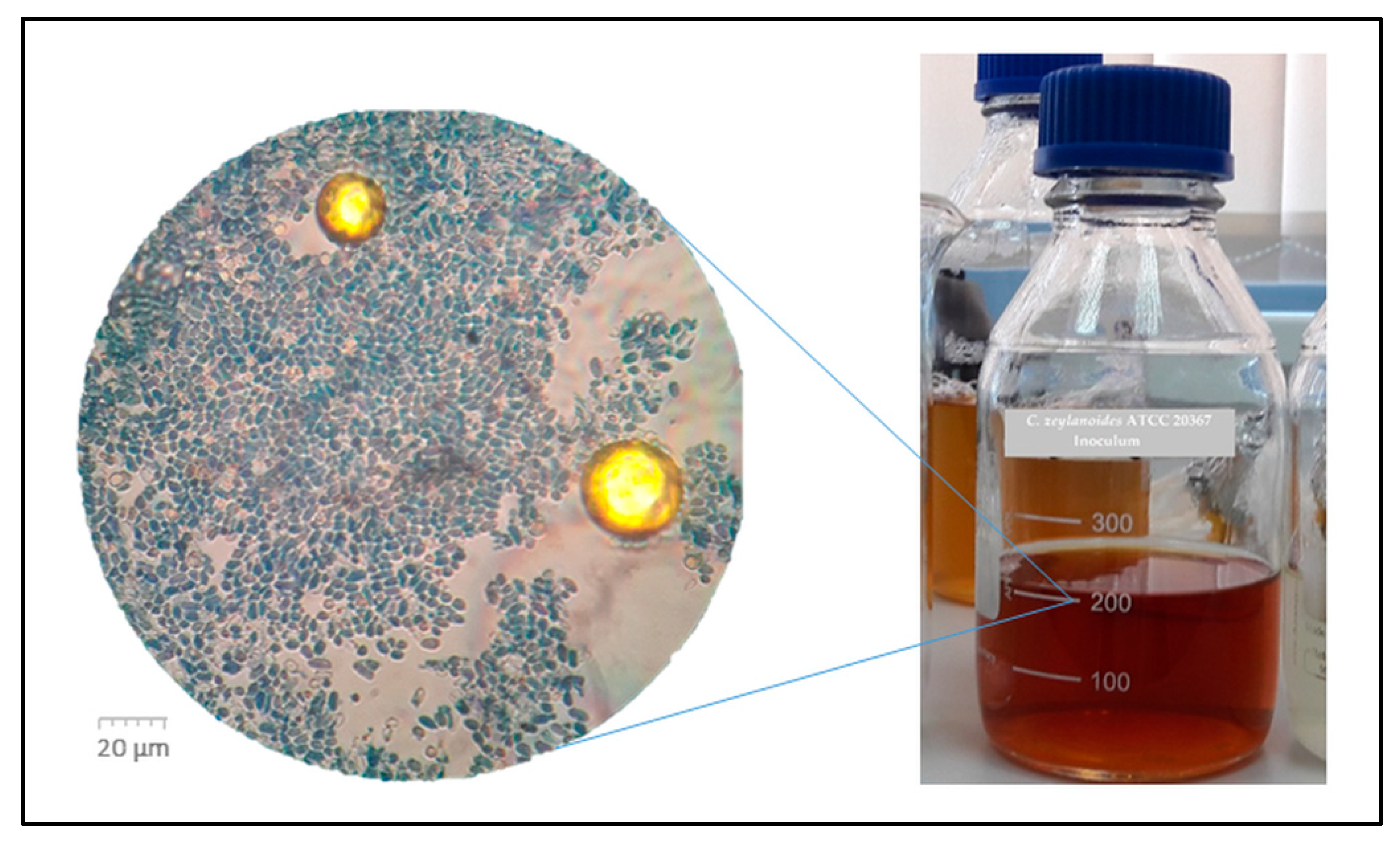

| Nutrients | Batch CG | Batch PG | Batch Gl |
|---|---|---|---|
| n paraffin (mL/L) | 50 | 50 | - |
| Pure glycerol (g/L) | - | 30 | 0.5 |
| Crude glycerol (g/L) | 30 | - | |
| Glucose (g/L) | - | - | 30 |
| NH4Cl (g/L) | 5 | 5 | 5 |
| KH2PO4 (g/L) | 0.5 | 0.5 | 0.5 |
| MgSO4 (g/L) | 0.5 | 0.5 | 0.5 |
| CaCO3 (g/L) * | 80 | 80 | 10 |
| MnSO4 × 4H2O (mg/L) | 2 | 2 | 2 |
| ZnSO4 × 7H2O (mg/L) | 2 | 2 | 2 |
| FeSO4 × 7H2O (mg/L) | 10 | 10 | 10 |
| CuSO4 × 5H2O (µg/L) | 50 | 50 | 50 |
| Thiamine-HCl (µg/L) ** | 100 | 100 | 100 |
| Yield g/g | Batch CG | Batch PG | Batch Gl |
|---|---|---|---|
| YBiomass/Substrate | 0.98 | 0.21 | 0.31 |
| YCitric acid/Substrate | 0.06 | 0.06 | 0.05 |
| YSuccinic acid/Substrate | 0.06 | 0.48 | 1.2 |
| Time (h) | Viability Log10 ufc/mL | Biomass g/L | Citric Acid g/L | Succinic Acid g/L | Residual Glycerol g/L |
|---|---|---|---|---|---|
| 0 | 6.53 ± 0.24 | 0.7 ± 0.02 | 0.35 ± 0.01 | 0.28 ± 0.00 | 9.48 ± 0.45 |
| 24 | 8.88 ± 0.34 | 0.82 ± 0.04 | 0.46 ± 0.02 | 0.43 ± 0.01 | 7.57 ± 0.37 |
| 48 | 10.80 ± 0.42 | 0.99 ± 0.05 | 0.47 ± 0.02 | 0.44 ± 0.02 | 4.31 ± 0.21 |
| 66 | 11.48 ± 0.51 | 1.25 ± 0.03 | 0.47 ± 0.02 | 0.45 ± 0.02 | 3.28 ± 0.16 |
| 69 | 12.30 ± 0.56 | 1.31 ± 0.04 | 0.47 ± 0.00 | 0.45 ± 0.02 | 1.30 ± 0.05 |
| 72 | 12.41 ± 0.62 | 1.40 ± 0.02 | 0.47 ± 0.01 | 0.46 ± 0.01 | 1.00 ± 0.05 |
| 75 | 12.70 ± 0.62 | 2.07 ± 0.01 | 0.49 ± 0.02 | 0.47 ± 0.01 | 0.10 ± 0.00 |
| 90 | 12.86 ± 0.62 | 2.56 ± 0.11 | 0.52 ± 0.02 | 0.48 ± 0.02 | 0.02 ± 0.00 |
| 93 | 12.90 ± 0.66 | 2.76 ± 0.10 | 0.52 ± 0.01 | 0.48 ± 0.02 | 0.00 ± 0.00 |
| 96 | 12.84 ± 0.51 | 3.03 ± 0.15 | 0.53 ± 0.00 | 0.49 ± 0.02 | - |
| 99 | 12.77 ± 0.57 | 4.24 ± 0.13 | 0.55 ± 0.02 | 0.53 ± 0.02 | - |
| 120 | 11.00 ± 0.46 | 4.86 ± 0.15 | 0.63 ± 0.02 | 0.55 ± 0.02 | - |
| 163 | 11.18 ± 0.55 | 9.38 ± 0.26 | 0.66 ± 0.02 | 0.60 ± 0.02 | - |
| Time (h) | Viability Log10 ufc/mL | Biomass g/L | Citric Acid g/L | Succinic Acid g/L | Residual Glycerol g/L |
|---|---|---|---|---|---|
| 0 | 5.48 ± 0.21 | 1.70 ± 0.02 | 0.41 ± 0.02 | 0.33 ± 0.01 | 32.22 ± 1.01 |
| 24 | 8.11 ± 0.31 | 1.93 ± 0.05 | 0.65 ± 0.02 | 1.27 ± 0.02 | 20.22 ± 1.00 |
| 48 | 9.67 ± 0.40 | 2.45 ± 0.10 | 1.02 ± 0.03 | 3.48 ± 0.10 | 7.42 ± 0.35 |
| 66 | 12.83 ± 0.58 | 2.58 ± 0.11 | 1.34 ± 0.05 | 6.31 ± 0.23 | - |
| 69 | 12.92 ± 0.58 | 2.5 ± 0.12 | 1.40 ± 0.06 | 7.23 ± 0.38 | - |
| 72 | 13.32 ± 0.62 | 2.62 ± 0.12 | 1.66 ± 0.03 | 10.20 ± 0.33 | 5.60 ± 0.27 |
| 75 | 13.42 ± 0.62 | 2.65 ± 0.12 | 1.66 ± 0.03 | 9.99 ± 0.32 | - |
| 90 | 13.48 ± 0.61 | 2.83 ± 0.13 | 1.64 ± 0.05 | 9.83 ± 0.44 | - |
| 93 | 13.52 ± 0.66 | 3.48 ± 0.13 | 1.79 ± 0.06 | 9.75 ± 0.45 | - |
| 96 | 13.82 ± 0.62 | 4.25 ± 0.14 | 1.96 ± 0.07 | 12.66 ± 0.56 | 5.01 ± 0.22 |
| 99 | 13.91 ± 0.66 | 4.43 ± 0.11 | 1.93 ± 0.05 | 12.22 ± 0.35 | - |
| 120 | 10.84 ± 0.52 | 4.46 ± 0.15 | 1.95 ± 0.06 | 12.43 ± 0.61 | 3.61 ± 0.17 |
| 163 | 10.54 ± 0.48 | 6.80 ± 0.24 | 2.00 ± 0.07 | 15.66 ± 0.66 | 0.45 ± 0.01 |
| Time (h) | Viability Log10 ufc/mL | Biomass g/L | Citric Acid g/L | Succinic Acid g/L | Residual Glucose g/L |
|---|---|---|---|---|---|
| 0 | 6.00 ± 0.11 | 1.17 ± 0.02 | 0.23 ± 0.01 | 0.28 ± 0.00 | 28.35 ± 1.41 |
| 24 | 8.86 ± 0.17 | 2.57 ± 0.11 | 0.45 ± 0.01 | 0.59 ± 0.02 | 24.84 ± 1.18 |
| 48 | 10.53 ± 0.37 | 3.19 ± 0.12 | 2.41 ± 0.09 | 18.81 ± 0.80 | 19.06 ± 0.92 |
| 66 | 12.80 ± 0.58 | 4.06 ± 0.20 | 2.92 ± 0.10 | 29.76 ± 1.00 | - |
| 69 | 12.49 ± 0.49 | 4.09 ± 0.20 | 2.94 ± 0.12 | 31.02 ± 1.02 | - |
| 72 | 12.00 ± 0.42 | 4.25 ± 0.12 | 2.94 ± 0.14 | 31.03 ± 1.00 | 9.22 ± 0.32 |
| 75 | 12.53 ± 0.53 | 4.39 ± 0.11 | 3.01 ± 0.15 | 34.24 ± 1.52 | - |
| 90 | 12.53 ± 0.61 | 4.43 ± 0.22 | 1.59 ± 0.06 | 16.36 ± 0.80 | - |
| 93 | 11.70 ± 0.55 | 4.44 ± 0.20 | 1.42 ± 0.02 | 8.09 ± 0.20 | - |
| 96 | 12.95 ± 0.56 | 4.74 ± 0.11 | 1.07 ± 0.04 | 3.62 ± 0.10 | 2.66 ± 0.10 |
| 99 | 12.69 ± 0.44 | 5.76 ± 0.19 | 1.57 ± 0.04 | 5.67 ± 0.11 | - |
| 120 | 10.95 ± 0.27 | 6.80 ± 0.20 | 1.37 ± 0.05 | 5.71 ± 0.15 | 1.01 ± 0.02 |
| 163 | 10.77 ± 0.33 | 9.00 ± 0.29 | 1.63 ± 0.07 | 8.71 ± 0.28 | 0.04 ± 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrea, L.; Ranga, F.; Fetea, F.; Dulf, F.V.; Rusu, A.; Trif, M.; Vodnar, D.C. Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367. Microorganisms 2019, 7, 265. https://doi.org/10.3390/microorganisms7080265
Mitrea L, Ranga F, Fetea F, Dulf FV, Rusu A, Trif M, Vodnar DC. Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367. Microorganisms. 2019; 7(8):265. https://doi.org/10.3390/microorganisms7080265
Chicago/Turabian StyleMitrea, Laura, Floricuța Ranga, Florinela Fetea, Francisc Vasile Dulf, Alexandru Rusu, Monica Trif, and Dan Cristian Vodnar. 2019. "Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367" Microorganisms 7, no. 8: 265. https://doi.org/10.3390/microorganisms7080265
APA StyleMitrea, L., Ranga, F., Fetea, F., Dulf, F. V., Rusu, A., Trif, M., & Vodnar, D. C. (2019). Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367. Microorganisms, 7(8), 265. https://doi.org/10.3390/microorganisms7080265