Taxonomic Distribution of Cytochrome P450 Monooxygenases (CYPs) among the Budding Yeasts (Sub-Phylum Saccharomycotina)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sequence Retrieval
2.2. Phylogenetic Analysis
3. Results
3.1. Sequence Collection and Initial Sorting
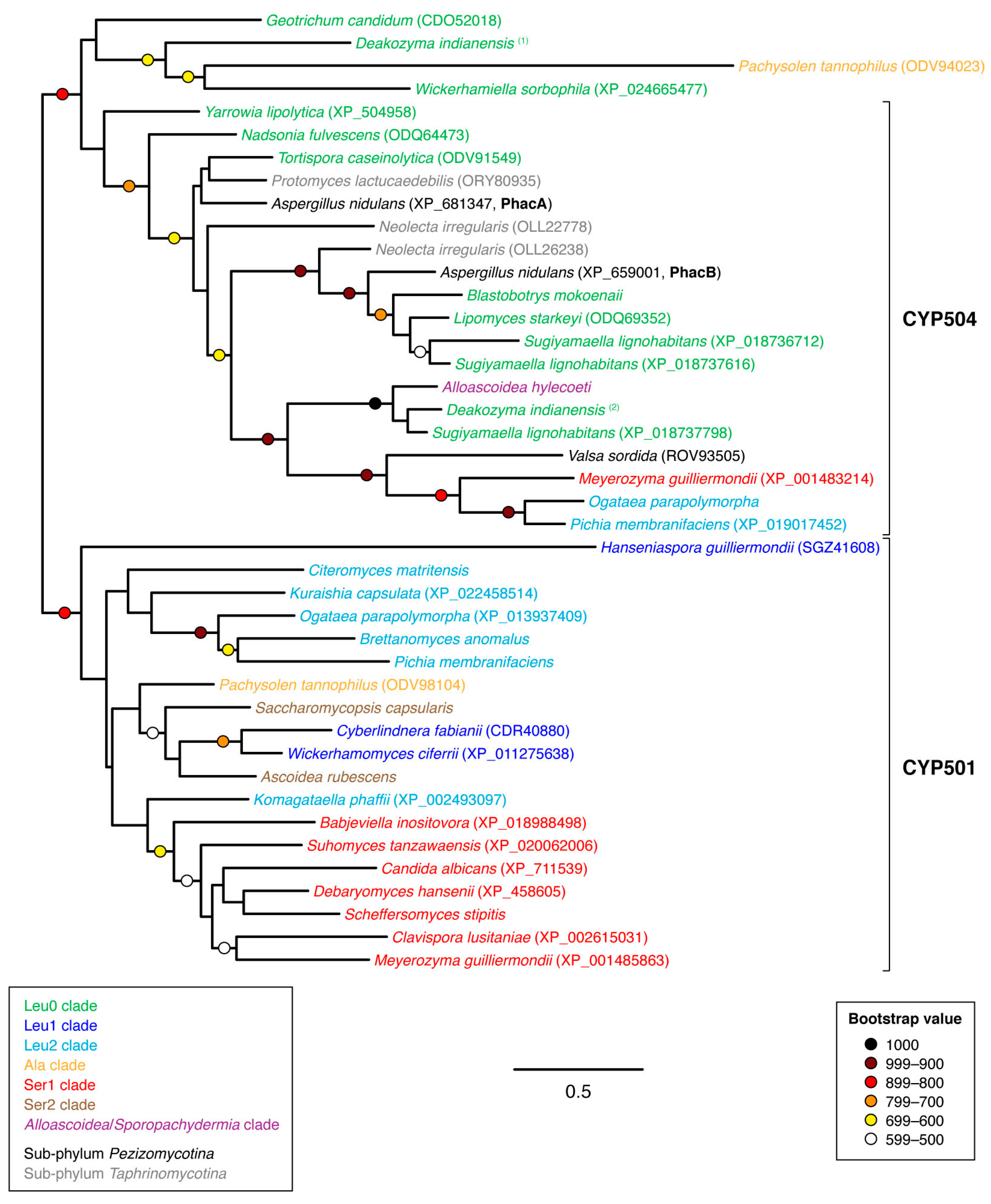
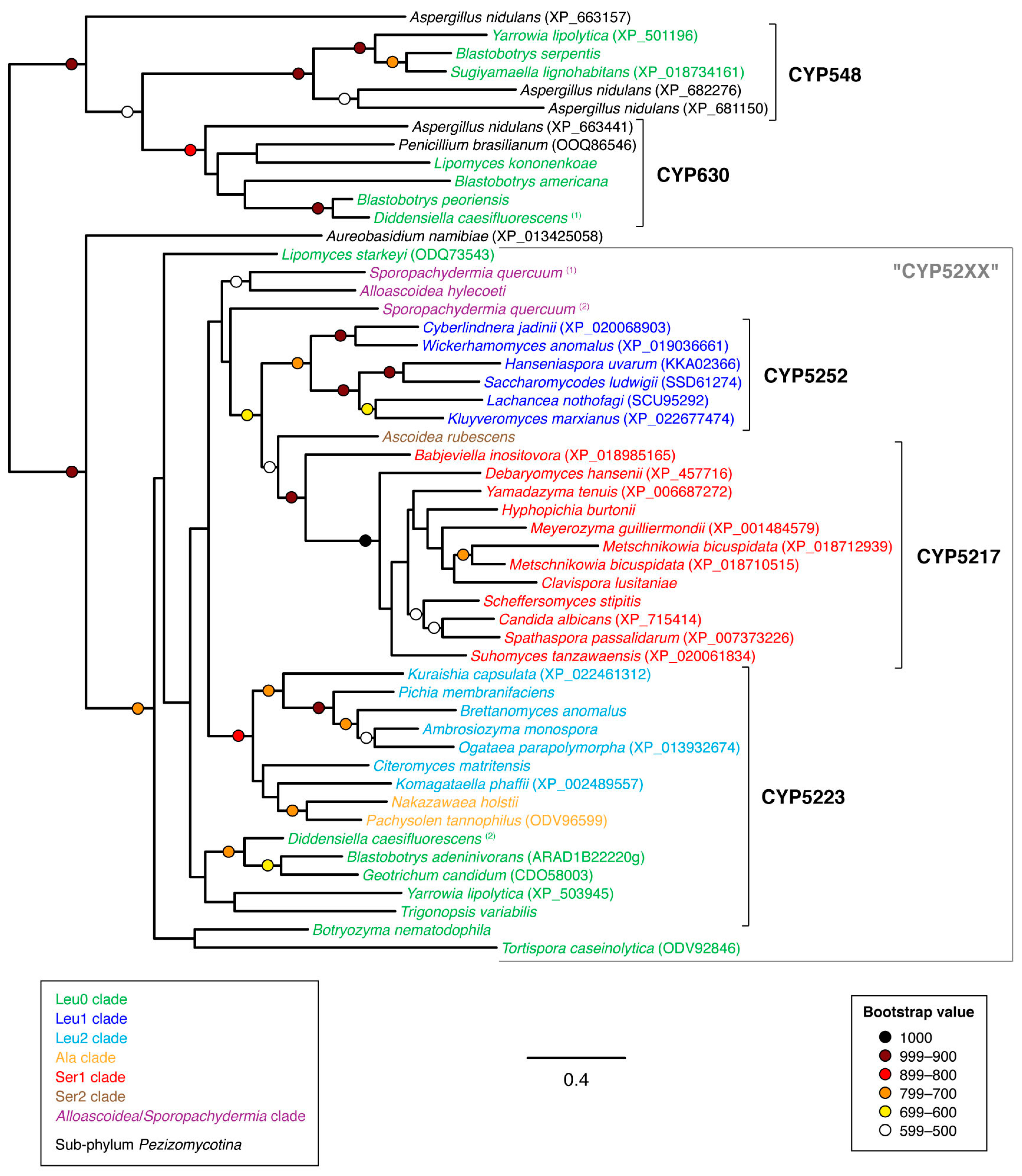
3.2. Phylogenetic Analyses
4. Discussion
Funding
Conflicts of Interest
Appendix A
| Species | Genomic Accession 1 | Strand | Coordinates |
|---|---|---|---|
| CYP56 | |||
| Aciculoconidium aculeatum | PPJB01000050 2 | + | 74966–76441 |
| Alloascoidea hylecoeti | BCKZ01000006 | − | 381233–382128, 382188–382782 |
| Barnettozyma populi | PPLZ02000015 | + | 151344–152804 |
| Dipodascus albidus | PPJE01000011 | + | 4500–5072, 5133–5489, 5549–6016, 6083–6148 |
| Diutina catenulata | PJEZ01000006 2 | + | 67720–69054 |
| Kodamaea ohmeri | PPNN01000011 2 | − | 275828–277339 |
| Magnusiomyces ingens | UIDE01000004 | − | 435148–435696, 435779–436135, 436192–436770 |
| Priceomyces haplophilus | BCIF01000002 2 | + | 1110008–1111489 |
| Phaffomyces opuntiae | PPNH01000004 | − | 4783–6303 |
| Starmera amethionina | PPNC01000057 | + | 57884–59377 |
| Teunomyces kruisii | PPLR01000028 2 | + | 76692–78167 |
| Wickerhamia fluorescens | BCGE01000005 2 | − | 792413–793876 |
| Yueomyces sinensis | PPMT01000005 | − | 193708–195165 |
| Zygotorulaspora mrakii | PPHZ01000005 | + | 324089–325603 |
| CYP52 | |||
| Alloascoidea hylecoeti | BCKZ01000001 | − | 888959–890518 |
| Diddensiella caesifluorescens (1) | PPJD01000017 | + | 195224–196783 |
| Diddensiella caesifluorescens (2) | PPJD01000017 | + | 197357–198934 |
| Nadsonia starkeyi-henricii (1) | QBLK01000108 | − | 23137–24687 |
| Nadsonia starkeyi-henricii (2) | QBLK01000108 | − | 21081–22637 |
| Saccharomycopsis fodiens (1) | JNFV01000009 2 | − | 683357–684952 |
| Saccharomycopsis fodiens (2) | JNFV01000003 2 | + | 6643–8226 |
| Saccharomycopsis fodiens (3) | JNFV01000009 2 | + | 7380–8951 |
| Saccharomycopsis fodiens (4) | JNFV01000001 2 | + | 2891–4453 |
| Starmerella bombicola (1) | NRDR01000004 | − | 591579–593135 |
| Starmerella bombicola (2) | NRDR01000004 | − | 318040–319596 |
| Starmerella bombicola (3) | NRDR01000025 | + | 62522–64117 |
| Starmerella bombicola (4) | NRDR01000003 | − | 308477–310045 |
| Starmerella bombicola (5) | NRDR01000005 | − | 9970–11538 |
| Starmerella bombicola (6) | NRDR01000005 | − | 6608–8176 |
| Trigonopsis variabilis | PPXM02000002 | − | 1137995–1139512 |
| Wickerhamiella versatilis (1) | NRED01000001 | − | 1258441–1259970 |
| Wickerhamiella versatilis (2) | NRED01000011 | − | 25516–27039 |
| Wickerhamiella versatilis (3) | NRED01000004 | + | 480766–482298 |
| Zygoascus ofunaensis | PPMC02000012 | − | 276041–277603 |
| CYP501, CYP504 | |||
| Alloascoidea hylecoeti | BCKZ01000004 | − | 123913–125610 |
| Ascoidea rubescens | NW_017962913 2 | + | 1016180–1017844 |
| Blastobotrys mokoenaii | PPJM02000008 | − | 17335–18903 |
| Brettanomyces anomalus | LCTY01000003 | − | 955844–957598 |
| Citeromyces matritensis | PPHV01003366 | + | 46403–47908 |
| Deakozyma indianensis (1) | PPLG02000002 | + | 204898–206562 |
| Deakozyma indianensis (2) | PPLG02000014 | − | 73651–75282 |
| Ogataea parapolymorpha | NC_027864 | − | 1260033–1261667 |
| Pichia membranifaciens | NW_017566986 | − | 510782–512500 |
| Saccharomycopsis capsularis | PPIG01000060 2 | + | 55413–56858 |
| Scheffersomyces stipitis | NC_009047 2 | − | 132488–134149 |
| CYP548, CYP630, CYP5217, CYP5223, CYP5252 | |||
| Alloascoidea hylecoeti | BCKZ01000023 | − | 227089–228648 |
| Ambrosiozyma monospora | BCIP01000010 | − | 36354–38219 |
| Ascoidea rubescens | NW_017962915 2 | − | 986327–988084 |
| Blastobotrys americana | PPJN02000012 | + | 34235–35698 |
| Blastobotrys peoriensis | PPJJ02000009 | − | 104592–106094 |
| Blastobotrys serpentis | PPJG01000004 | − | 306605–308191 |
| Botryozyma nematodophila | PPJC01001041 | − | 443–1888 |
| Brettanomyces anomalus | LCTY01000007 | + | 286500–288059 |
| Citeromyces matritensis | PPHV01003001 | − | 5839–7305 |
| Clavispora lusitaniae | NW_003101576 2 | − | 792130–793887 |
| Diddensiella caesifluorescens (1) | PPJD01000014 | + | 6451–7920 |
| Diddensiella caesifluorescens (2) | PPJD01000011 | − | 56654–58084 |
| Hyphopichia burtonii | NW_017963729 2 | + | 1954183–1955925 |
| Lipomyces kononenkoae | PPJW01000001 | + | 112103–112111, 112147–112381, 112443–113550, 113613–113838 |
| Nakazawaea holstii | PPKU01000003 3 | + | 174994–176508 |
| Pichia membranifaciens | NW_017566985 | + | 474706–476289 |
| Scheffersomyces stipitis | NC_009047 2 | + | 1074603–1076360 |
| Sporopachydermia quercuum (1) | BCGN01000006 | − | 1022779–1024251 |
| Sporopachydermia quercuum (2) | BCGN01000011 | + | 318748–320439 |
| Trigonopsis variabilis | PPXM02000001 | − | 657214–658683 |
| CYP5078 | |||
| Ambrosiozyma kashinagacola | BCGA01000001 | + | 1475803–1477413 |
| Ambrosiozyma maleeae | PPLV01000007 | + | 15809–17437 |
| Ambrosiozyma oregonensis | PPKY02000013 | + | 3245–4852 |
| Ambrosiozyma philentoma | PPKZ02000016 | − | 79288–80895 |
| Ambrosiozyma pseudovanderkliftii | PPLW02000009 | − | 377701–379308 |
| Ambrosiozyma vanderkliftii | PPHW01000114 | − | 32141–33748 |
| Lipomyces suomiensis (1) | PPJQ02000001 | + | 628395–629990 |
| Lipomyces suomiensis (2) | PPJQ02000011 | − | 223225–224796 |
| Lipomyces suomiensis (3) | PPJQ02000011 | + | 248970–249275, 249341–249604, 249662–250591 |
| Lipomyces suomiensis (4) | PPJQ02000063 | + | 4147–4461, 4542–4781, 4846–5757 |
| Ogataea glucozyma | PPKO01000004 | + | 345713–347290 |
| Ogataea henricii | PPHT01000003 | + | 42457–44139 |
| Ogataea methylivora | PPKQ01000003 | + | 112748–114325 |
| Ogataea populi-albae | PPIX02000004 | + | 115779–117362 |
| Ogataea trehaloabstinens | PPKJ01000015 | + | 25059–26642 |
| Ogataea zsoltii | PPKH02000011 | − | 45513–47093 |
| Peterozyma toletana | PPKG01000007 3 | − | 78966–80543 |
| Peterozyma xylosa | PPKF01000009 3 | − | 77489–79066 |
| Sporopachydermia lactativora (1) | PPID01000125 | − | 23315–24868 |
| Sporopachydermia lactativora (2) | PPID01000036 | + | 26664–28286 |
| Sporopachydermia quercuum | BCGN01000002 | + | 250317–251900 |
References
- Hittinger, C.T.; Rokas, A.; Bai, F.Y.; Boekhout, T.; Gonçalves, P.; Jeffries, T.W.; Kominek, J.; Lachance, M.A.; Libkind, D.; Rosa, C.A.; et al. Genomics and the making of yeast biodiversity. Curr. Opin. Genet. Dev. 2015, 35, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.; Haridas, S.; Wolfe, K.H.; Lopes, M.R.; Hittinger, C.T.; Göker, M.; Salamov, A.A.; Wisecaver, J.H.; Long, T.M.; Calvey, C.H.; et al. Comparative genomics of biotechnologically important yeasts. Proc. Natl. Acad. Sci. USA 2016, 113, 9882–9887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.X.; Opulente, D.A.; Kominek, J.; Zhou, X.; Steenwyk, J.L.; Buh, K.V.; Haase, M.A.B.; Wisecaver, J.H.; Wang, M.; Doering, D.T.; et al. Tempo and mode of genome evolution in the budding yeast subphylum. Cell 2018, 175, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Pribat, A.; Waller, J.C.; de Crécy-Lagard, V. ‘Unknown’ proteins and ‘orphan’ enzymes: The missing half of the engineering parts list--and how to find it. Biochem. J. 2009, 425, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R. Cytochrome P450 diversity in the tree of life. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Crešnar, B.; Petrič, S. Cytochrome P450 enzymes in the fungal kingdom. Biochim. Biophys. Acta 2011, 1814, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, F.; Bichet, A.; Ewen, K.M.; Bernhardt, R. Cytochrome P450 systems—Biological variations of electron transport chains. Biochim. Biophys. Acta 2007, 1770, 330–344. [Google Scholar] [CrossRef]
- Nebert, D.W.; Nelson, D.R.; Coon, M.J.; Estabrook, R.W.; Feyereisen, R.; Fujii-Kuriyama, Y.; Gonzalez, F.J.; Guengerich, F.P.; Gunsalus, I.C.; Johnson, E.F.; et al. The P450 superfamily: Update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991, 10, 1–14. [Google Scholar] [CrossRef]
- Kelly, S.L.; Lamb, D.C.; Corran, A.J.; Baldwin, B.C.; Parks, L.W.; Kelly, D.E. Purification and reconstitution of activity of Saccharomyces cerevisiae P450 61, a sterol delta 22-desaturase. FEBS Lett. 1995, 377, 217–220. [Google Scholar] [CrossRef]
- Turi, T.G.; Kalb, V.F.; Loper, J.C. Cytochrome P450 lanosterol 14 alpha-demethylase (ERG11) and manganese superoxide dismutase (SOD1) are adjacent genes in Saccharomyces cerevisiae. Yeast 1991, 7, 627–630. [Google Scholar] [CrossRef]
- Briza, P.; Eckerstorfer, M.; Breitenbach, M. The sporulation-specific enzymes encoded by the DIT1 and DIT2 genes catalyze a two-step reaction leading to a soluble ll-dityrosine-containing precursor of the yeast spore wall. Proc. Natl. Acad. Sci. USA 1994, 91, 4524–4528. [Google Scholar] [CrossRef] [PubMed]
- Briza, P.; Winkler, G.; Kalchhauser, H.; Breitenbach, M. Dityrosine is a prominent component of the yeast ascospore wall. A proof of its structure. J. Biol. Chem. 1986, 261, 4288–4294. [Google Scholar] [PubMed]
- Sanglard, D.; Loper, J.C. Characterization of the alkane-inducible cytochrome P450 (P450alk) gene from the yeast Candida tropicalis: Identification of a new P450 gene family. Gene 1989, 76, 121–136. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.; De Mey, M.; Develter, D.; Soetaert, W.; Vandamme, E.J. Importance of the cytochrome P450 monooxygenase CYP52 family for the sophorolipid-producing yeast Candida bombicola. FEMS Yeast Res. 2009, 9, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.J.; Kominek, J.; Opulente, D.A.; Shen, X.X.; Zhou, X.; Langdon, Q.K.; DeVirgilio, J.; Hulfachor, A.B.; Kurtzman, C.P.; Rokas, A.; et al. Functional and evolutionary characterization of a secondary metabolite gene cluster in budding yeasts. Proc. Natl. Acad. Sci. USA 2018, 115, 11030–11035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore-Lloyd, D.; Sumann, I.; Brachmann, A.O.; Schneeberger, K.; Ortiz-Merino, R.A.; Moreno-Beltrán, M.; Schläfli, M.; Kirner, P.; Santos Kron, A.; Rueda-Mejia, M.P.; et al. Snf2 controls pulcherriminic acid biosynthesis and antifungal activity of the biocontrol yeast Metschnikowia pulcherrima. Mol. Microbiol. 2019, 112, 317–332. [Google Scholar] [CrossRef]
- Nelson, D.R. The cytochrome p450 homepage. Hum. Genom. 2009, 4, 59–65. [Google Scholar]
- Moktali, V.; Park, J.; Fedorova-Abrams, N.D.; Park, B.; Choi, J.; Lee, Y.H.; Kang, S. Systematic and searchable classification of cytochrome P450 proteins encoded by fungal and oomycete genomes. BMC Genom. 2012, 13, 525. [Google Scholar] [CrossRef]
- Chen, W.; Lee, M.K.; Jefcoate, C.; Kim, S.C.; Chen, F.; Yu, J.H. Fungal cytochrome P450 monooxygenases: Their distribution, structure, functions, family expansion, and evolutionary origin. Genome Biol. Evol. 2014, 6, 1620–1634. [Google Scholar] [CrossRef]
- Katoh, K.; Kuma, K.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef] [Green Version]
- Le, S.; Gascuel, O. An improved general amino-acid replacement matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.E.; Krasevec, N.; Mullins, J.; Nelson, D.R. The CYPome (Cytochrome P450 complement) of Aspergillus nidulans. Fungal Genet. Biol. 2009, 46, S53–S61. [Google Scholar] [CrossRef]
- Krassowski, T.; Coughlan, A.Y.; Shen, X.X.; Zhou, X.; Kominek, J.; Opulente, D.A.; Riley, R.; Grigoriev, I.V.; Maheshwari, N.; Shields, D.C.; et al. Evolutionary instability of CUG-Leu in the genetic code of budding yeasts. Nat. Commun. 2018, 9, 1887. [Google Scholar] [CrossRef] [Green Version]
- Kitazume, T.; Takaya, N.; Nakayama, N.; Shoun, H. Fusarium oxysporum fatty-acid subterminal hydroxylase (CYP505) is a membrane-bound eukaryotic counterpart of Bacillus megaterium cytochrome P450BM3. J. Biol. Chem. 2000, 275, 39734–39740. [Google Scholar] [CrossRef]
- Nakayama, N.; Takemae, A.; Shoun, H. Cytochrome P450foxy, a catalytically self-sufficient fatty acid hydroxylase of the fungus Fusarium oxysporum. J. Biochem. 1996, 119, 435–440. [Google Scholar] [CrossRef]
- Venter, P.; Kock, J.L.; Kumar, G.S.; Botha, A.; Coetzee, D.J.; Botes, P.J.; Bhatt, R.K.; Falck, J.R.; Schewe, T.; Nigam, S. Production of 3R-hydroxy-polyenoic fatty acids by the yeast Dipodascopsis uninucleata. Lipids 1997, 32, 1277–1283. [Google Scholar] [CrossRef]
- Smith, D.P.; Kock, J.L.F.; van Wyk, P.W.J.; Venter, P.; Coetzee, D.J.; van Heerden, E.; Linke, D.; Nigam, S. The occurrence of 3-hydroxy oxylipins in the ascomycetous yeast family Lipomycetaceae. S. Afr. J. Sci. 2000, 96, 247–249. [Google Scholar]
- Melo, N.R.; Moran, G.P.; Warrilow, A.G.; Dudley, E.; Smith, S.N.; Sullivan, D.J.; Lamb, D.C.; Kelly, D.E.; Coleman, D.C.; Kelly, S.L. CYP56 (Dit2p) in Candida albicans: Characterization and investigation of its role in growth and antifungal drug susceptibility. Antimicrob. Agents Chemother. 2008, 52, 3718–3724. [Google Scholar] [CrossRef] [PubMed]
- Van Bogaert, I.N.; Holvoet, K.; Roelants, S.L.; Li, B.; Lin, Y.C.; Van de Peer, Y.; Soetaert, W. The biosynthetic gene cluster for sophorolipids: A biotechnological interesting biosurfactant produced by Starmerella bombicola. Mol. Microbiol. 2013, 88, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Saerens, K.M.; Saey, L.; Soetaert, W. One-step production of unacetylated sophorolipids by an acetyltransferase negative Candida bombicola. Biotechnol. Bioeng. 2011, 108, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Saerens, K.M.; Roelants, S.L.; Van Bogaert, I.N.; Soetaert, W. Identification of the UDP-glucosyltransferase gene UGTA1, responsible for the first glucosylation step in the sophorolipid biosynthetic pathway of Candida bombicola ATCC 22214. FEMS Yeast Res. 2011, 11, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Saerens, K.M.; Zhang, J.; Saey, L.; Van Bogaert, I.N.; Soetaert, W. Cloning and functional characterization of the UDP-glucosyltransferase UgtB1 involved in sophorolipid production by Candida bombicola and creation of a glucolipid-producing yeast strain. Yeast 2011, 28, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Gojković, Z.; Sandrini, M.P.; Piskur, J. Eukaryotic beta-alanine synthases are functionally related but have a high degree of structural diversity. Genetics 2001, 158, 999–1011. [Google Scholar] [PubMed]
- Kurtzman, C.P.; Price, N.P.; Ray, K.J.; Kuo, T.M. Production of sophorolipid biosurfactants by multiple species of the Starmerella(Candida) bombicola yeast clade. FEMS Microbiol. Lett. 2010, 311, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, X.; Zhang, H.; Qu, Y.B.; Miao, J.Y. Production, structure elucidation and anticancer properties of sophorolipid from Wickerhamiella domercqiae. Enzyme Microb. Technol. 2006, 39, 501–506. [Google Scholar] [CrossRef]
- Mingot, J.M.; Peñalva, M.A.; Fernández-Cañón, J.M. Disruption of phacA, an Aspergillus nidulans gene encoding a novel cytochrome P450 mono-oxygenase catalyzing phenylacetate 2-hydroxylation, resulting in penicillin overproduction. J. Biol. Chem. 1999, 274, 14545–14550. [Google Scholar] [CrossRef]
- Ferrer-Sevillano, F.; Fernández-Cañón, J.M. Novel phacB-encoded cytochrome P450 monooxygenase from Aspergillus nidulans with 3-hydroxyphenylacetate 6-hydroxylase and 3, 4-dihydroxyphenylacetate 6-hydroxylase activities. Eukaryot. Cell 2007, 6, 514–520. [Google Scholar] [CrossRef]
- Middelhoven, W.J. Catabolism of benzene compounds by ascomycetous and basidiomycetous yeasts and yeastlike fungi. A literature review and an experimental approach. Antonie Van Leeuwenhoek 1993, 63, 125–144. [Google Scholar] [CrossRef]
- Nelson, D.R. Metazoan cytochrome P450 evolution. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 121, 15–22. [Google Scholar] [CrossRef]
- Steenwyk, J.L.; Opulente, D.A.; Kominek, J.; Shen, X.X.; Zhou, X.; Labella, A.L.; Bradley, N.P.; Eichman, B.F.; Čadež, N.; Libkind, D.; et al. Extensive loss of cell-cycle and DNA repair genes in an ancient lineage of bipolar budding yeasts. PLoS Biol. 2019, 17, e3000255. [Google Scholar] [CrossRef]
- Hausjell, J.; Halbwirth, H.; Spadiut, O. Recombinant production of eukaryotic cytochrome P450s in microbial cell factories. Biosci. Rep. 2018, 38, BSR20171290. [Google Scholar] [CrossRef]
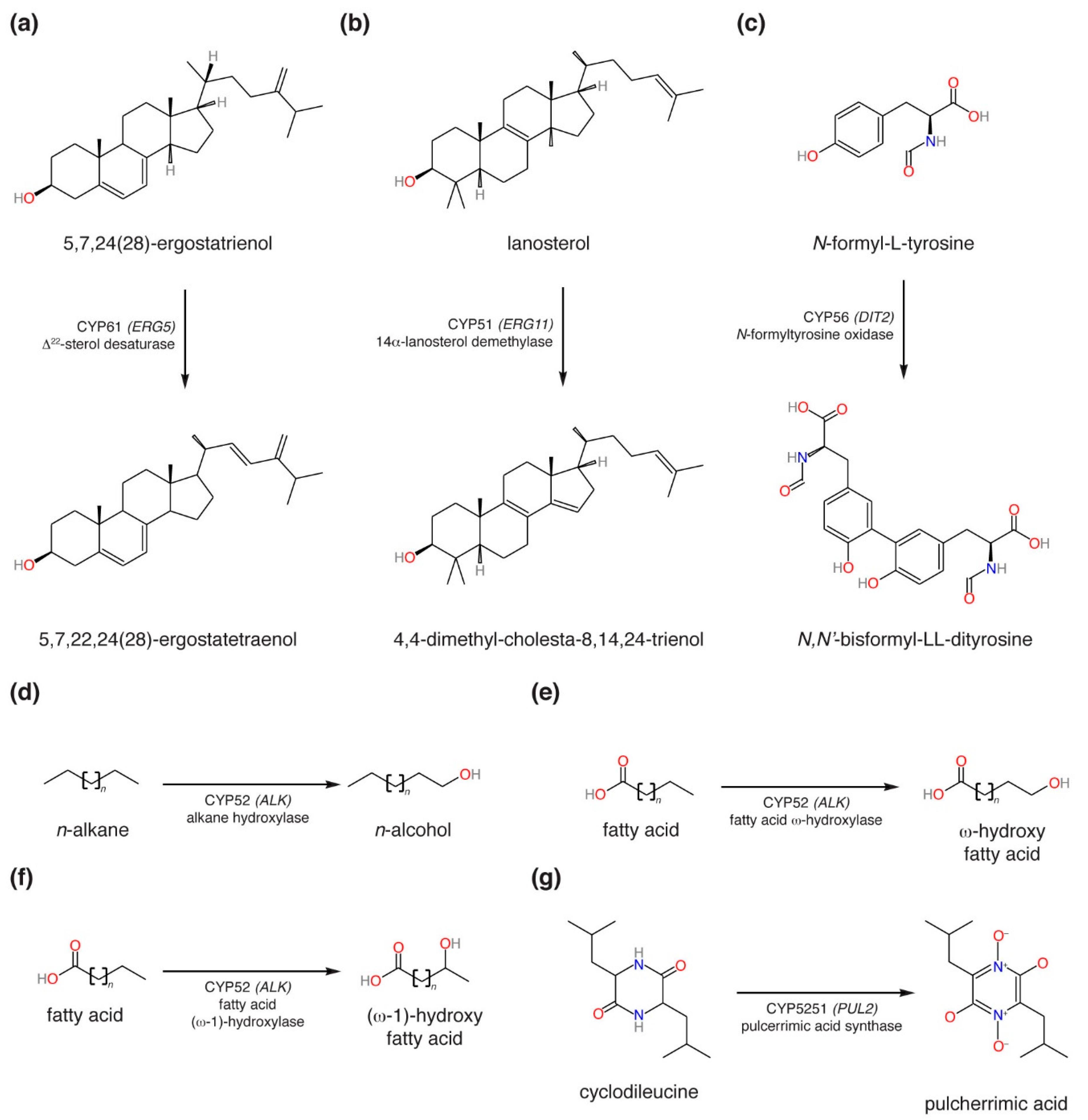

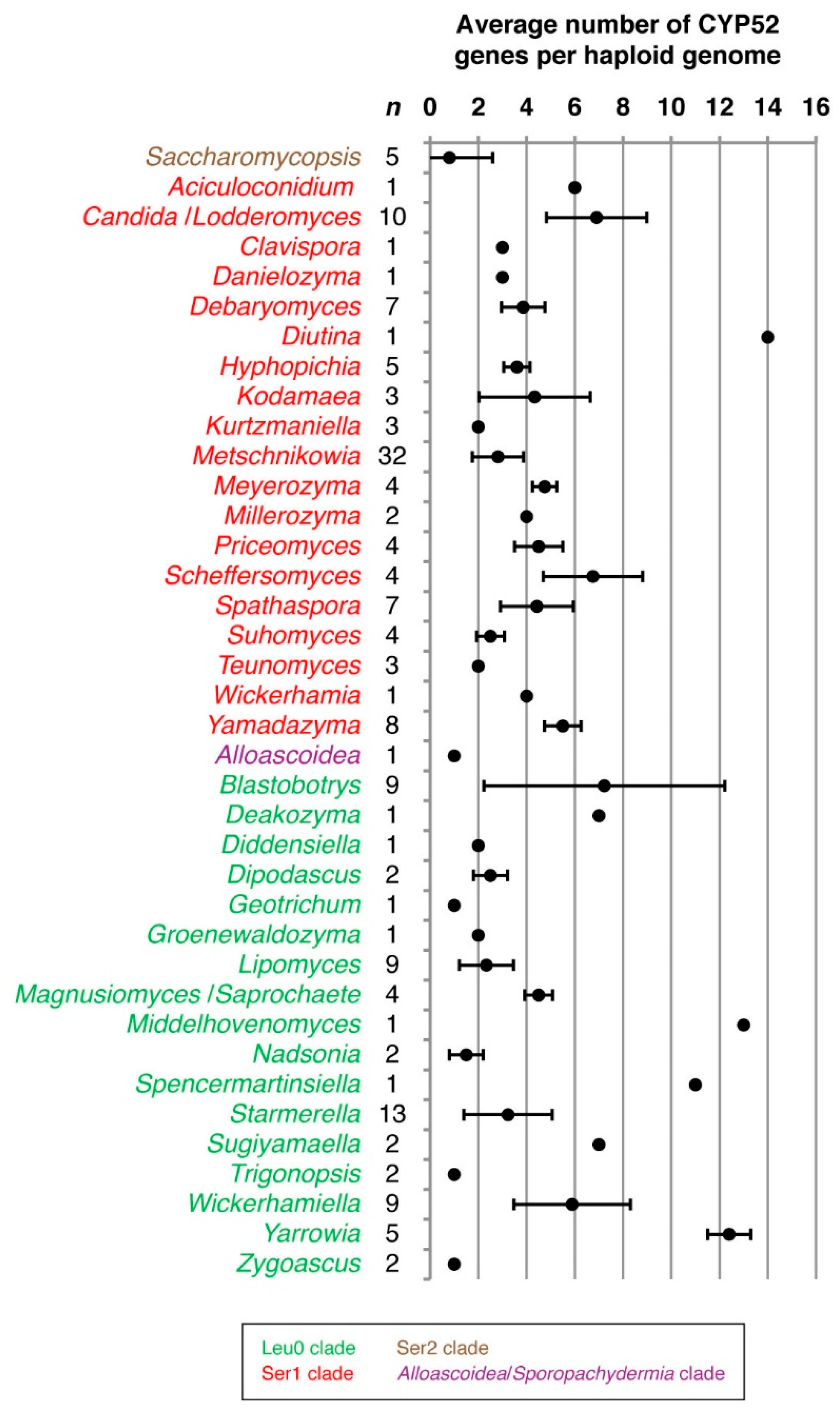

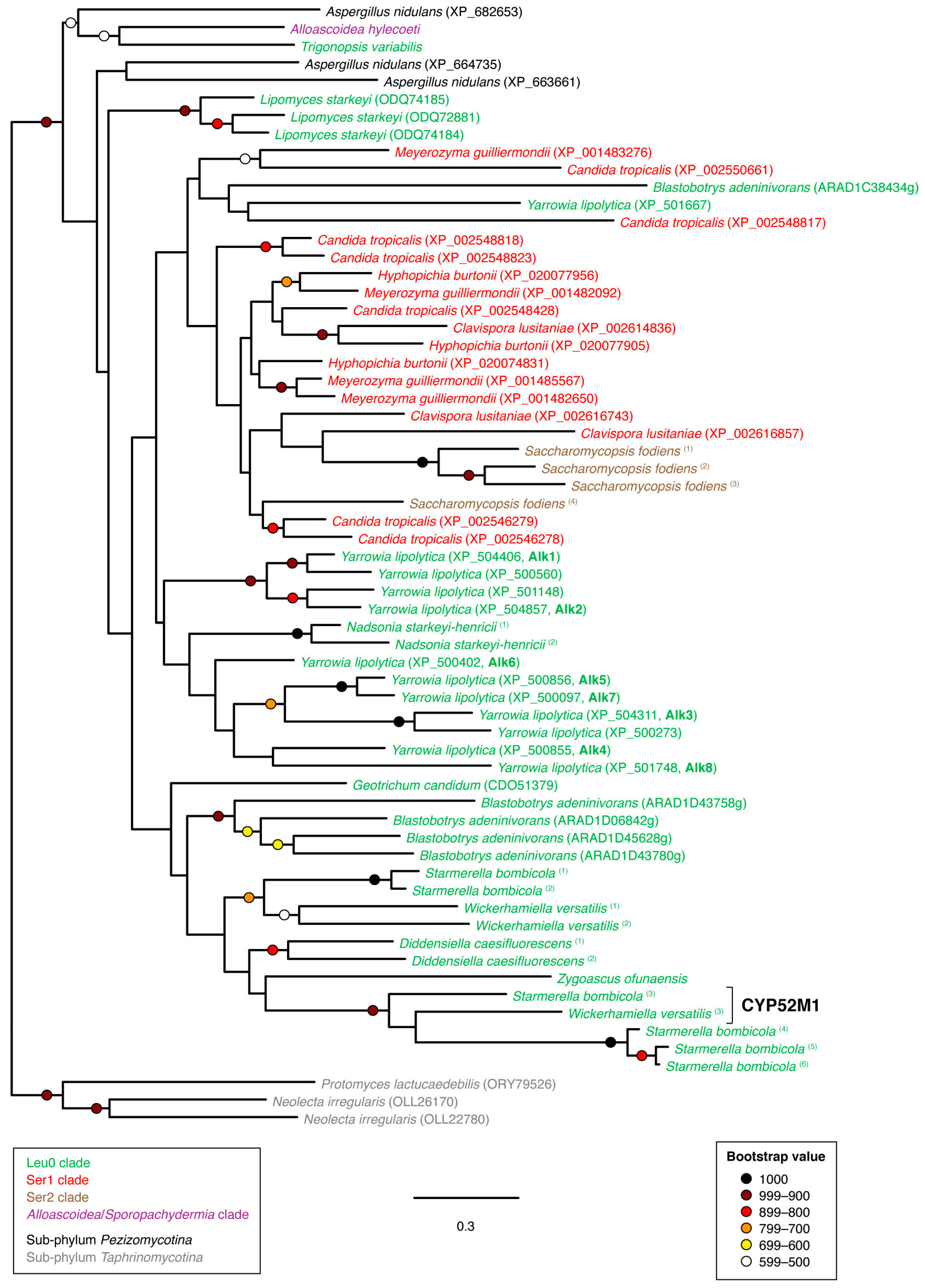
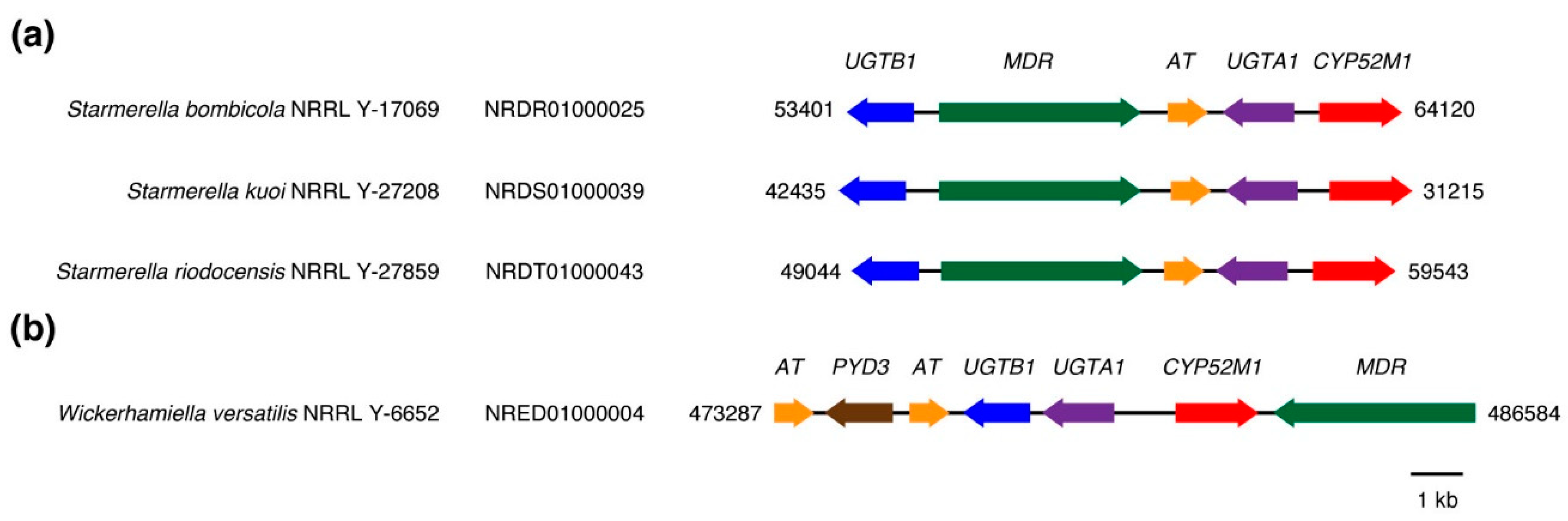

| CYP Family | Species | Accession Number |
|---|---|---|
| 51 (ERG11) | Saccharomyces cerevisiae | NP_011871 |
| 52 (ALK) | Candida tropicalis | XP_002546278 |
| 56 (DIT2) | Saccharomyces cerevisiae | NP_010690 |
| 61 (ERG5) | Saccharomyces cerevisiae | NP_013728 |
| 501 | Meyerozyma guillermondii | XP_001485863 |
| 504 | Meyerozyma guillermondii | XP_001483214 |
| 548 | Yarrowia lipolytica | XP_501196 |
| 5217 | Candida albicans | XP_715414 |
| 5223 | Yarrowia lipolytica | XP_503945 |
| 5251 (PUL2) | Kluyveromyces lactis | XP_453057 |
| 5252 | Cyberlindnera fabianii | CDR39009 |
| Species | Protein Accession | Top Assigned Asp. nidulans CYP Hit (Identity/Similarity) |
|---|---|---|
| Group I (CYP617-like) | ||
| Lipomyces starkeyi | ODQ70065 | XP_659488 (31%/50%) |
| Lipomyces starkeyi | ODQ69649 | XP_659488 (32%/50%) |
| Lipomyces starkeyi | ODQ71312 | XP_659488 (32%/51%) |
| Lipomyces starkeyi | ODQ70063 | XP_659488 (34%/55%) |
| Lipomyces starkeyi | ODQ72725 | XP_659488 (34%/51%) |
| Tortispora caseinolytica | ODV92859 | XP_659488 (27%/47%) |
| Group II (CYP540) | ||
| Lipomyces starkeyi | ODQ75312 | XP_682188 (46%/65%) 1 |
| Group III (CYP59-like) | ||
| Lipomyces starkeyi | ODQ74007 2 | XP_681077 (38%/55%) 3 |
| Group IV (CYP677) | ||
| Lipomyces starkeyi | ODQ75272 | XP_681884 (44%/60%) |
| Group V | ||
| Geotrichum candidum | CDO53625 | XP_661721 (22%/41%) |
| Lipomyces starkeyi | ODQ69471 | XP_661721 (23%/40%) |
| Lipomyces starkeyi | ODQ74469 | XP_661721 (23%/36%) |
| Lipomyces starkeyi | ODQ69396 | XP_660953 (27%/45%) |
| Lipomyces starkeyi | ODQ72272 | XP_661721 (22%/40%) |
| Lipomyces starkeyi | ODQ72285 | XP_682522 (31%/48%) |
| Group VI (CYP505) | ||
| Lipomyces starkeyi | ODQ75335 | XP_664439 (46%/58%) |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linder, T. Taxonomic Distribution of Cytochrome P450 Monooxygenases (CYPs) among the Budding Yeasts (Sub-Phylum Saccharomycotina). Microorganisms 2019, 7, 247. https://doi.org/10.3390/microorganisms7080247
Linder T. Taxonomic Distribution of Cytochrome P450 Monooxygenases (CYPs) among the Budding Yeasts (Sub-Phylum Saccharomycotina). Microorganisms. 2019; 7(8):247. https://doi.org/10.3390/microorganisms7080247
Chicago/Turabian StyleLinder, Tomas. 2019. "Taxonomic Distribution of Cytochrome P450 Monooxygenases (CYPs) among the Budding Yeasts (Sub-Phylum Saccharomycotina)" Microorganisms 7, no. 8: 247. https://doi.org/10.3390/microorganisms7080247
APA StyleLinder, T. (2019). Taxonomic Distribution of Cytochrome P450 Monooxygenases (CYPs) among the Budding Yeasts (Sub-Phylum Saccharomycotina). Microorganisms, 7(8), 247. https://doi.org/10.3390/microorganisms7080247





