Ample Arsenite Bio-Oxidation Activity in Bangladesh Drinking Water Wells: A Bonanza for Bioremediation?
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Hydrochemical Analysis
2.3. Enrichment of Aerobic Chemolithoautotrophic (CAO) Arsenite-Oxidizing Microorganisms
2.4. Enrichment of Anaerobic-Chemolithoautotrophic (AAO) and Heterotrophic (AHAO) Arsenite-Oxidizing Microorganisms
2.5. Enrichment of Aerobic Heterotrophic (HAO) Arsenite-Oxidizing Microorganisms
2.6. General Enrichment Strategy
2.7. DNA Extraction
2.8. DGGE Profiling of Enrichments
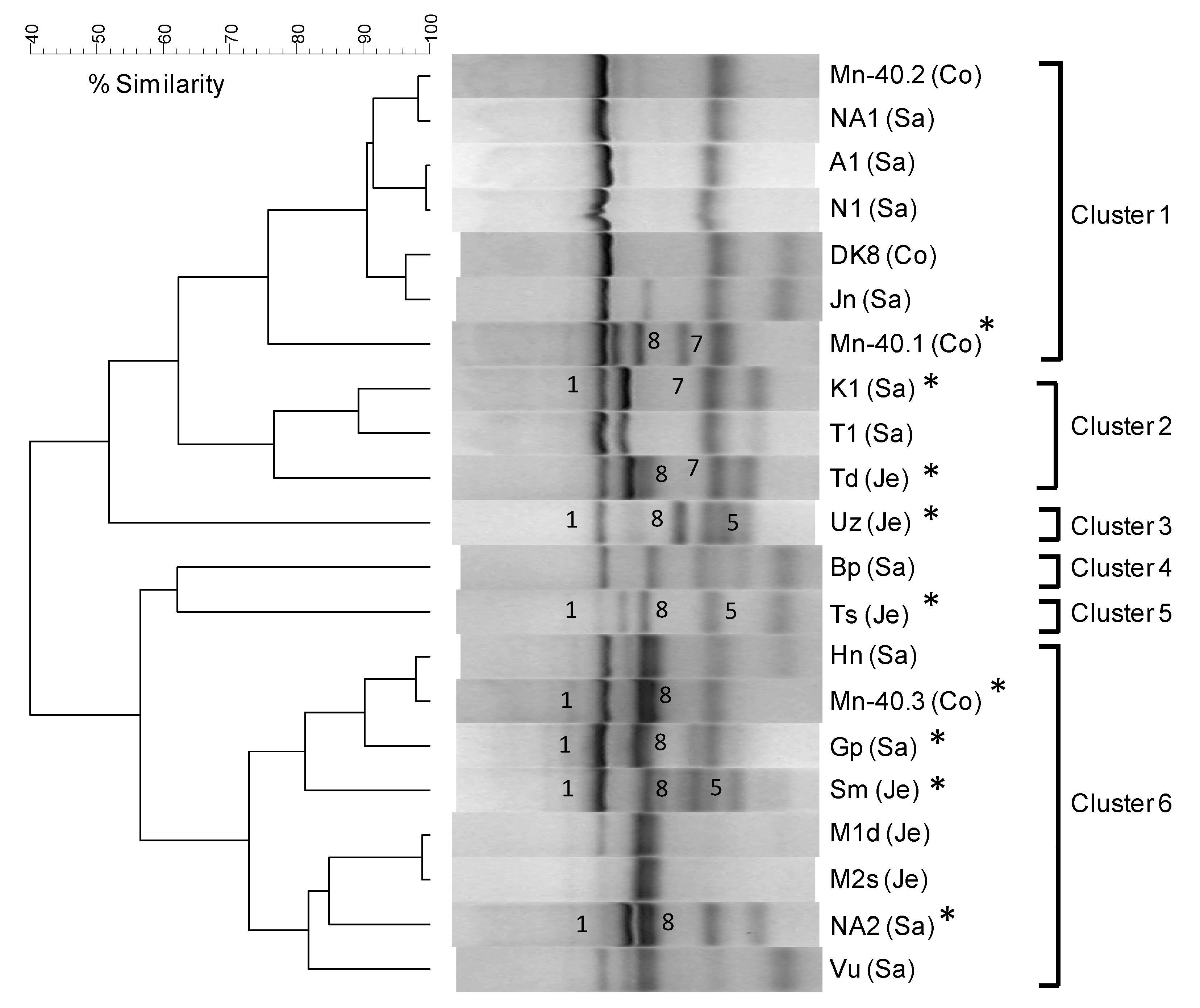
2.9. Biomarker-based Analysis of Arsenite-Oxidizing Communities
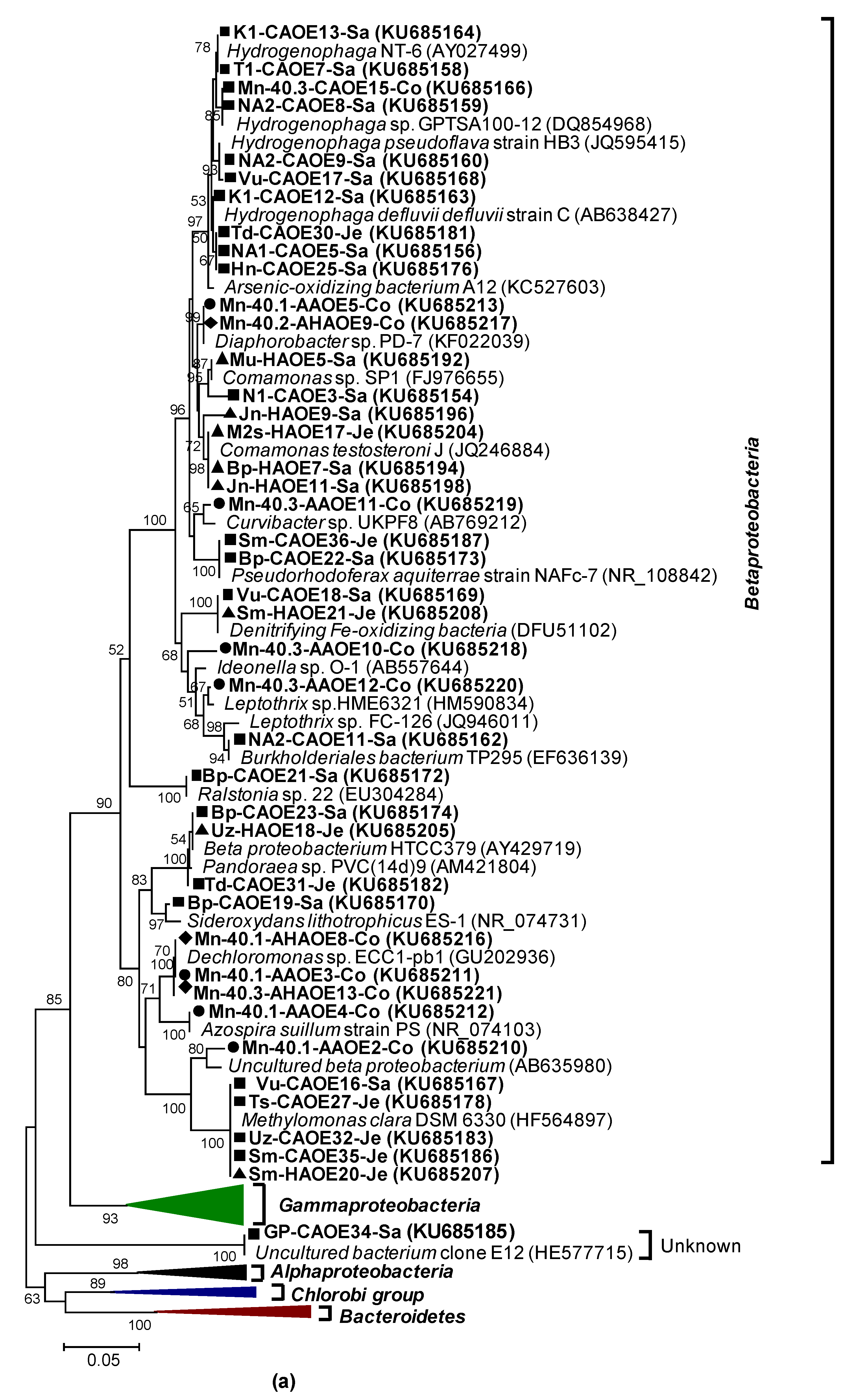
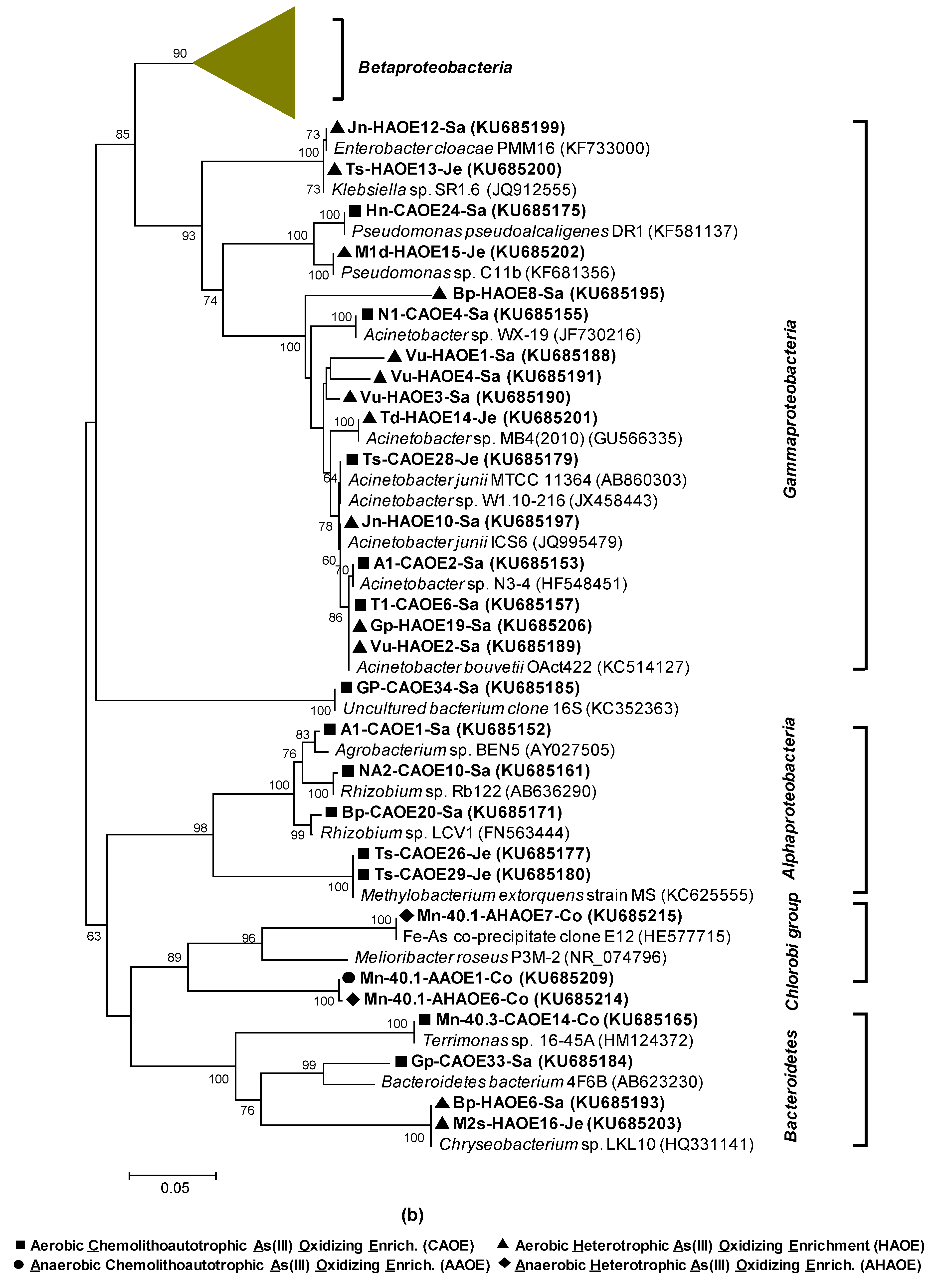
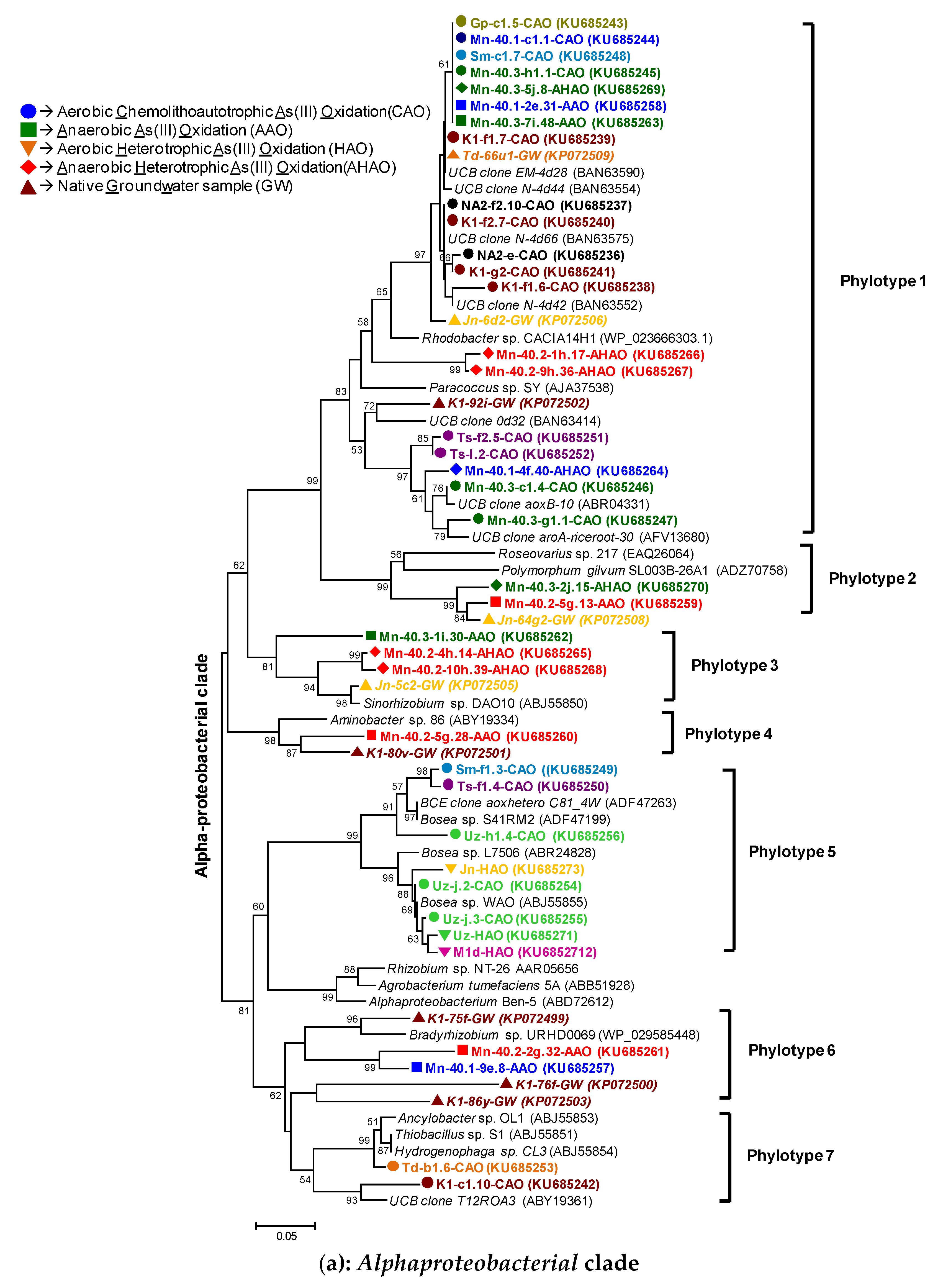
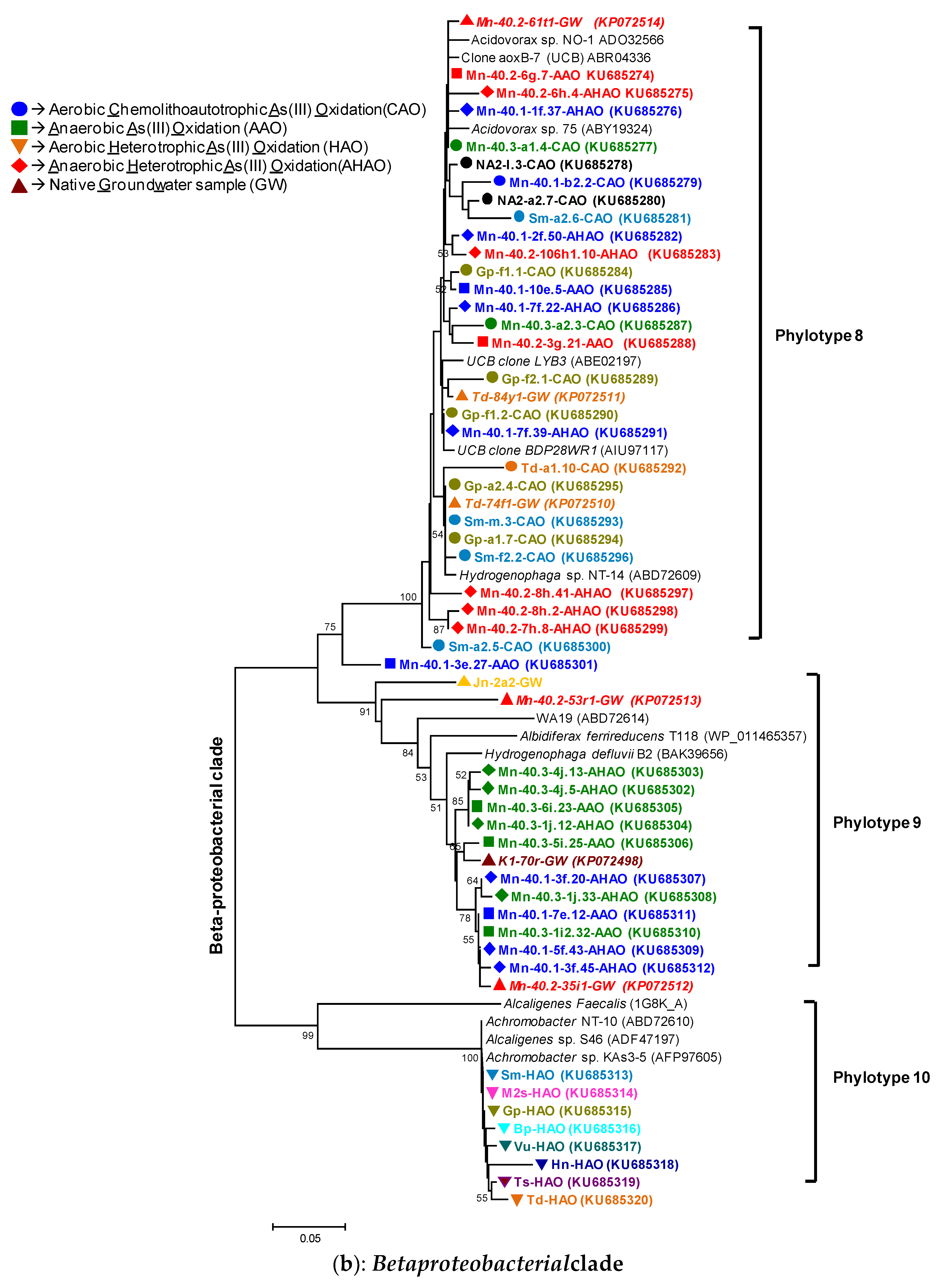
2.10. Phylogenetic Analyses
2.11. Statistical Analyses
3. Results
3.1. Growth on Arsenite and Presence of Arsenite Oxidase Genes in Enrichments
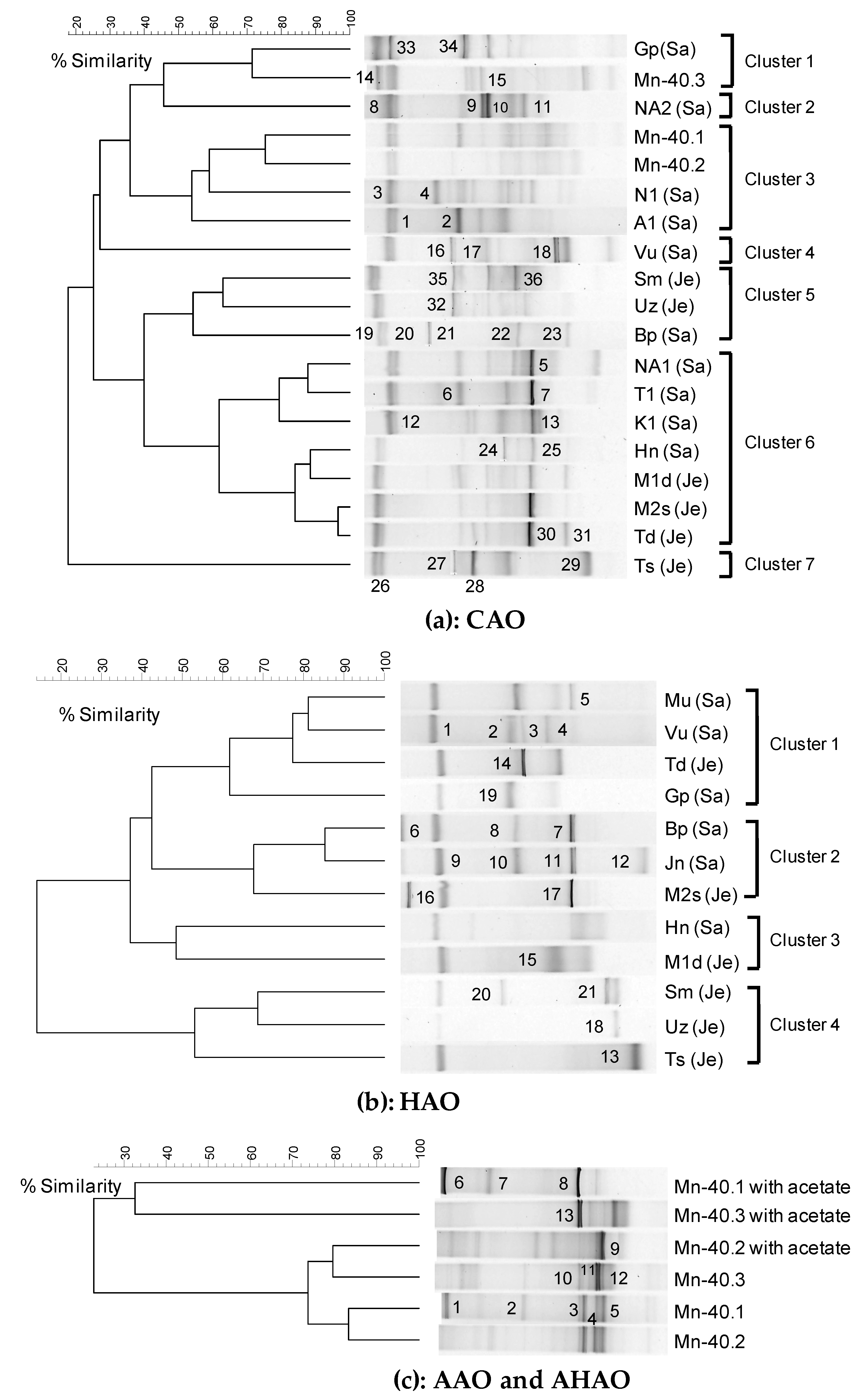
3.2. Diverse Aerobic Chemolithoautotrophic Arsenite-Oxidizing (CAO) Enrichments
3.2.1. rRNA Diversity in the CAO Enrichments and rRNA-Kinship to Known Organisms
3.2.2. AioA Sequence Diversity in the CAO Enrichments and aioA-Kinship to Known Organisms
3.3. Diverse Aerobic Heterotrophic Arsenite-Oxidizing (HAO) Enrichments and Kinship to Known Organisms
3.3.1. rRNA Diversity in the HAO Enrichments and rRNA-Kinship to Known Organisms
3.3.2. AioA Diversity in the HAO Enrichments and aioA-Kinship to Known Organisms
3.4. Diverse Anaerobic- Chemolithoautotrophic (AAO) and Heterotrophic (AHAO) Arsenite-Oxidizing Enrichments and Kinship to Known Organisms
3.4.1. rRNA Diversity in the AAO and AHAO Enrichments and rRNA-Kinship to Known Organisms
3.4.2. AioA Diversity in the AAO and AHAO Enrichments and aioA-Kinship to Known Organisms
4. Discussion
4.1. Microbial Communities in Bangladesh Groundwaters and Arsenite Oxidation: Sufficient Diversity
4.2. Implications for Possible Biology-Enhanced (Im)Mobilization of Arsenic: BioSAR
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kossoff, D.; Hudson-Edwards, K.A. Arsenic in the environment. In The Metabolism of Arsenite; Santini, J.M., Ward, S.A., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2012; pp. 1–23. [Google Scholar]
- Oremland, R.S.; Stolz, J.F. The ecology of arsenic. Science 2003, 300, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.V.; Johnston, R.B.; Zheng, Y. Arsenic in tube well water in Bangladesh: Health and economic impacts and implications for arsenic mitigation. Bull. World Health Organ. 2012, 90, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Saha, D.; Saha, R.; Ghosh, T.; Saha, B. A review on sources, toxicity and remediation technologies for removing arsenic from drinking water. Res. Chem. Intermed. 2014, 40, 447–485. [Google Scholar] [CrossRef]
- Van Halem, D.; Olivero, S.; de Vet, W.W.; Verberk, J.Q.; Amy, G.L.; van Dijk, J.C. Subsurface iron and arsenic removal for shallow tube well drinking water supply in rural Bangladesh. Water Res. 2010, 44, 5761–5769. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.C.B.; van Halem, D.; Rahman, M.M.; Verberk, J.Q.J.C.; Badruzzaman, A.B.M.; Van der Meer, W.G.J. Hand-pump subsurface arsenic removal: The effect of groundwater conditions and intermittent operation. Water Sci. Technol. 2014, 14, 119–126. [Google Scholar]
- Le Nguyen, A.; Sato, A.; Inoue, D.; Sei, K.; Soda, S.; Ike, M. Bacterial community succession during the enrichment of chemolithoautotrophic arsenite oxidizing bacteria at high arsenic concentrations. J. Environ. Sci. 2012, 24, 2133–2140. [Google Scholar]
- Tamaki, S.; Frankenberger, W.T. Environmental biochemistry of arsenic. Rev. Environ. Contam Toxicol. 1992, 124, 79–110. [Google Scholar]
- Stolz, J.F. Overview of microbial arsenic metabolism and resistance. In The Metabolism of Arsenite; Santini, J.M., Ward, S.A., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2012; pp. 55–60. [Google Scholar]
- Suhadolnik, M.L.S.; Salgado, A.P.C.; Scholte, L.L.S.; Bleicher, L.; Costa, P.S.; Reis, M.P.; Dias, M.F.; Ávila, M.P.; Barbosa, F.A.R.; Chartone-Souza, E.; et al. Novel arsenic-transforming bacteria and the diversity of their arsenic-related genes and enzymes arising from arsenic-polluted freshwater sediment. Sci. Rep. 2017, 7, 11231. [Google Scholar] [CrossRef]
- Cavalca, L.; Corsini, A.; Zaccheo, P.; Andreoni, V.; Muyzer, G. Microbial transformations of arsenic: Perspectives for biological removal of arsenic from water. Future Microbiol. 2013, 8, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Sultana, M.; van Breukelen, B.M.; Khan, S.I.; Röling, W.F. Diverse arsenic-and iron-cycling microbial communities in arsenic-contaminated aquifers used for drinking water in Bangladesh. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- Van Lis, R.; Nitschke, W.; Duval, S.; Schoepp-Cothenet, B. Arsenics as bioenergetic substrates. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Satyapal, G.; Rani, S.; Kumar, M.; Kumar, N. Potential role of arsenic resistant bacteria in bioremediation: Current status and future prospects. J. Microb. Biochem. Technol. 2016, 8, 256–258. [Google Scholar] [CrossRef]
- Glasser, N.R.; Oyala, P.H.; Osborne, T.H.; Santini, J.M.; Newman, D.K. Structural and mechanistic analysis of the arsenate respiratory reductase provides insight into environmental arsenic transformations. Proc. Natl. Acad. Sci. USA 2018, 115, E8614–E8623. [Google Scholar] [CrossRef] [PubMed]
- Andres, J.; Bertin, P.N. The microbial genomics of arsenic. FEMS Microbiol. Rev. 2016, 40, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Fendorf, S.; Michael, H.A.; van Geen, A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127. [Google Scholar] [CrossRef]
- Gnanaprakasam, E.T.; Lloyd, J.R.; Boothman, C.; Ahmed, K.M.; Choudhury, I.; Bostick, B.C.; van Geen, A.; Mailloux, B.J. Microbial community structure and arsenic biogeochemistry in two arsenic-impacted aquifers in Bangladesh. Mbio 2017, 8, e01326-17. [Google Scholar] [CrossRef]
- Mailloux, B.J.; Alexandrova, E.; Keimowitz, A.R.; Wovkulich, K.; Freyer, G.A.; Herron, M.; Stolz, J.F.; Kenna, T.C.; Pichler, T.; Polizzotto, M.L. Microbial mineral weathering for nutrient acquisition releases arsenic. Appl.Environ. Microbiol. 2009, 75, 2558–2565. [Google Scholar] [CrossRef]
- Neumann, R.B.; Ashfaque, K.N.; Badruzzaman, A.; Ali, M.A.; Shoemaker, J.K.; Harvey, C.F. Anthropogenic influences on groundwater arsenic concentrations in Bangladesh. Nat. Geosci. 2010, 3, 46–52. [Google Scholar] [CrossRef]
- Lin, B.; Braster, M.; Röling, W.F.M.; van Breukelen, B.M. Iron-reducing microorganisms in a landfill leachate-polluted aquifer: Complementing culture-independent information with enrichments and isolations. Geomicrobiol. J. 2007, 24, 283–294. [Google Scholar] [CrossRef]
- Cai, L.; Rensing, C.; Li, X.; Wang, G. Novel gene clusters involved in arsenite oxidation and resistance in two arsenite oxidizers: Achromobacter sp. SY8 and Pseudomonas sp. TS44. Appl. Microbiol. Biotechnol. 2009, 83, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Santini, J.M.; Sly, L.I.; Schnagl, R.D.; Macy, J.M. A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: Phylogenetic, physiological, and preliminary biochemical studies. Appl. Environ. Microbiol. 2000, 66, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Gihring, T.M.; Banfield, J.F. Arsenite oxidation and arsenate respiration by a new Thermus isolate. FEMS Microbiol. Lett. 2001, 204, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Su, C.; Wang, Y.; Yao, J.; Zhao, K.; Wang, G. Sedimentary arsenite-oxidizing and arsenate-reducing bacteria associated with high arsenic groundwater from Shanyin, Northwestern China. J. Appl. Microbiol. 2008, 105, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16s rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar]
- Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. Rapid determination of 16s ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 1985, 82, 6955–6959. [Google Scholar] [CrossRef]
- Zargar, K.; Conrad, A.; Bernick, D.L.; Lowe, T.M.; Stolc, V.; Hoeft, S.; Oremland, R.S.; Stolz, J.; Saltikov, C.W. Arxa, a new clade of arsenite oxidase within the DMSO reductase family of molybdenum oxidoreductases. Environ. Microbiol. 2012, 14, 1635–1645. [Google Scholar] [CrossRef]
- Mead, D.A.; Szczesna-Skorupa, E.; Kemper, B. Single-stranded DNA ‘blue’T7 promoter plasmids: A versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986, 1, 67–74. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. Mega4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Van Verseveld, H.W.; Röling, W.F.M. Cluster analysis and statistical comparison of molecular community profile data. In Molecular Microbial Ecology Manual; Springer: Berlin, Germany, 2004; pp. 3275–3298. [Google Scholar]
- Jia, Y.; Huang, H.; Chen, Z.; Zhu, Y.-G. Arsenic uptake by rice is influenced by microbe-mediated as redox changes in the rhizosphere. Environ. Sci. Technol. 2014, 48, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, W.; Liu, B.; He, J.; Shen, Q.; Zhao, F.-J. Anaerobic arsenite oxidation by an autotrophic arsenite-oxidizing bacterium from an arsenic-contaminated paddy soil. Environ. Sci. Technol. 2015, 49, 5956–5964. [Google Scholar] [CrossRef] [PubMed]
- Quéméneur, M.; Cebron, A.; Billard, P.; Battaglia-Brunet, F.; Garrido, F.; Leyval, C.; Joulian, C. Population structure and abundance of arsenite-oxidizing bacteria along an arsenic pollution gradient in waters of the upper isle river basin, France. Appl. Environ. Microbiol. 2010, 76, 4566–4570. [Google Scholar] [CrossRef] [PubMed]
- Vanden Hoven, R.N.; Santini, J.M. Arsenite oxidation by the heterotroph Hydrogenophagasp. str. NT-14: The arsenite oxidase and its physiological electron acceptor. Biochim. Biophys. Acta 2004, 1656, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.; Vogler, S.; Zargar, K.; Schmidt, A.C.; Saltikov, C.; Seifert, J.; Schlömann, M. New clusters of arsenite oxidase and unusual bacterial groups in enrichments from arsenic-contaminated soil. Arch. Microbiol. 2012, 194, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Inskeep, W.P.; Macur, R.E.; Hamamura, N.; Warelow, T.P.; Ward, S.A.; Santini, J.M. Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ. Microbiol. 2007, 9, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Santini, J.M.; Sly, L.I.; Wen, A.; Comrie, D.; Wulf-Durand, P.D.; Macy, J.M. New arsenite-oxidizing bacteria isolated from Australian gold mining environments–phylogenetic relationships. Geomicrobiol. J. 2002, 19, 67–76. [Google Scholar] [CrossRef]
- Liao, V.H.; Chu, Y.J.; Su, Y.C.; Hsiao, S.Y.; Wei, C.C.; Liu, C.W.; Liao, C.M.; Shen, W.C.; Chang, F.J. Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J. Contam. Hydrol. 2011, 123, 20–29. [Google Scholar] [CrossRef]
- Sun, W.; Sierra-Alvarez, R.; Milner, L.; Field, J.A. Anaerobic oxidation of arsenite linked to chlorate reduction. Appl. Environ. Microbiol. 2010, 76, 6804–6811. [Google Scholar] [CrossRef]
- Sun, W.; Sierra-Alvarez, R.; Fernandez, N.; Sanz, J.L.; Amils, R.; Legatzki, A.; Maier, R.M.; Field, J.A. Molecular characterization and in situ quantification of anoxic arsenite-oxidizing denitrifying enrichment cultures. FEMS Microbiol. Ecol. 2009, 68, 72–85. [Google Scholar] [CrossRef]
- Rhine, E.D.; Phelps, C.D.; Young, L.Y. Anaerobic arsenite oxidation by novel denitrifying isolates. Environ. Microbiol. 2006, 8, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; Amalfitano, S.; Casentini, B.; Fazi, S.; Petruccioli, M.; Rossetti, S. Arsenic-related microorganisms in groundwater: A review on distribution, metabolic activities and potential use in arsenic removal processes. Rev. Environ. Sci. Biotechnol. 2017, 16, 647–665. [Google Scholar] [CrossRef]
- Osborne, T.; Santini, J. Prokaryotic aerobic oxidation of arsenite. In The Metabolism of Arsenite; Santini, J.M., Ward, S.A., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2012; pp. 61–72. [Google Scholar]
- Ai, L.N.; Sato, A.; Inoue, D.; Sei, K.; Soda, S.; Ike, M. Enrichment of arsenite oxidizing bacteria under autotrophic conditions and the isolation and characterization of facultative chemolithoautotrophic arsenite oxidizing bacteria for removal of arsenic from groundwater. Water Sci. Technol. 2012, 12, 707–714. [Google Scholar] [CrossRef]
- Hassan, Z.; Sultana, M.; Westerhoff, H.V.; Khan, S.I.; Röling, W.F. Iron cycling potentials of arsenic-contaminated groundwater in Bangladesh as revealed by enrichment cultivation. Geomicrobiol. J. 2016, 33, 779–792. [Google Scholar] [CrossRef]
- Price, R.E.; Lesniewski, R.; Nitzsche, K.S.; Meyerdierks, A.; Saltikov, C.; Pichler, T.; Amend, J.P. Archaeal and bacterial diversity in an arsenic-rich shallow-sea hydrothermal system undergoing phase separation. Front. Microbiol. 2013, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Edwardson, C.F.; Hollibaugh, J.T. Composition and activity of microbial communities along the redox gradient of an alkaline, hypersaline, lake. Front. Microbiol. 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Amachi, S. Microbiology of inorganic arsenic: From metabolism to bioremediation. J. Biosci. Bioeng. 2014, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bachate, S.P.; Khapare, R.M.; Kodam, K.M. Oxidation of arsenite by two β-proteobacteria isolated from soil. Appl. Microbiol. Biotechnol. 2012, 93, 2135–2145. [Google Scholar] [CrossRef]
- Corsini, A.; Zaccheo, P.; Muyzer, G.; Andreoni, V.; Cavalca, L. Arsenic transforming abilities of groundwater bacteria and the combined use of Aliihoeflea sp. strain 2WW and goethite in metalloid removal. J. Hazard. Mater. 2014, 269, 89–97. [Google Scholar] [CrossRef]
- Zargar, K.; Hoeft, S.; Oremland, R.; Saltikov, C.W. Identification of a novel arsenite oxidase gene, arxA, in the haloalkaliphilic, arsenite-oxidizing bacterium Alkalilimnicola ehrlichii strain MLHE-1. J. Bacteriol. 2010, 192, 3755–3762. [Google Scholar] [CrossRef]
- Oremland, R.S.; Saltikov, C.W.; Stolz, J.F.; Hollibaugh, J.T. Autotrophic microbial arsenotrophy in arsenic-rich soda lakes. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Oremland, R.; Stolz, J.; Saltikov, C. Anaerobic oxidation of arsenite by autotrophic bacteria: The view from mono lake, california. In The Metabolism of Arsenic; Santini, J.M., Ward, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 73–80. [Google Scholar]
- Heinrich-Salmeron, A.; Cordi, A.; Brochier-Armanet, C.; Halter, D.; Pagnout, C.; Abbaszadeh-fard, E.; Montaut, D.; Seby, F.; Bertin, P.N.; Bauda, P.; et al. Unsuspected diversity of arsenite-oxidizing bacteria as revealed by widespread distribution of the aoxB gene in prokaryotes. Appl. Environ. Microbiol. 2011, 77, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Quéméneur, M.; Heinrich-Salmeron, A.; Muller, D.; Lièvremont, D.; Jauzein, M.; Bertin, P.N.; Garrido, F.; Joulian, C. Diversity surveys and evolutionary relationships of aoxB genes in aerobic arsenite-oxidizing bacteria. Appl. Environ. Microbiol. 2008, 74, 4567–4573. [Google Scholar] [CrossRef] [PubMed]
- Herrgård, M.J.; Swainston, N.; Dobson, P.; Dunn, W.B.; Arga, K.Y.; Arvas, M.; Blüthgen, N.; Borger, S.; Costenoble, R.; Heinemann, M. A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology. Nat. Biotechnol. 2008, 26, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Lièvremont, D.; N’negue, M.A.; Behra, P.; Lett, M.C. Biological oxidation of arsenite: Batch reactor experiments in presence of kutnahorite and chabazite. Chemosphere 2003, 51, 419–428. [Google Scholar] [CrossRef]
- Simeonova, D.D.; Micheva, K.; Muller, D.A.E.; Lagarde, F.; Lett, M.C.; Groudeva, V.I.; Lievremont, D. Arsenite oxidation in batch reactors with alginate-immobilized ULPAs1 strain. Biotechnol. Bioeng. 2005, 91, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Gude, J.; Rietveld, L.; van Halem, D. Biological As (III) oxidation in rapid sand filters. J. Water Process. Eng. 2018, 21, 107–115. [Google Scholar] [CrossRef]
| A | Location | Physicochemical Parameters | Cultivation Condition | |||||
|---|---|---|---|---|---|---|---|---|
| CAO | ||||||||
| Sample ID | Name of the Village | District | Depth (meter) | pH | As (μM) | NO3 (mg/L) | Dilution Factor | aioA |
| A1 | Assasuni sadar | Satkhira | 14 | 6.8 | 1.6 | 24.0 | 1 (Gr+, Ox+) | + |
| N1 | Nagda | 146 | 8.0 | 0.06 | 0.05 | 1 (Gr+, Ox+) | + | |
| NA1 | Nawapara1 | 23 | 6.9 | 0.09 | 0.08 | 1 (Gr+, Ox+) | + | |
| T1 | Tarali | 49 | 6.7 | 3.3 | 0.06 | 1 (Gr+, Ox+) | + | |
| NA2 | Nawapara2 | 49 | 6.8 | 3.5 | 3.0 | 1 (Gr+, Ox+) | + | |
| K1 | Kaliganj sadar | 29 | 6.8 | 0.1 | 25.0 | 1 (Gr+, Ox+) | + | |
| DK-8 | Daudkandi | Comilla | 24 | 6.1 | 3.2 | 0.06 | 1 (Gr+, Ox+) | + |
| B | Location | Physicochemical Parameters | Cultivation Condition | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAO | HAO | |||||||||
| Sample ID | Name of the Village | District | Depth (meter) | pH | As (μM) | NO3 (mg/L) | Dilution Factor | aioA | Dilution Factor | aioA |
| Vu | Vurulia | Satkhira | 27 | 8.0 | 3.1 | 0.4 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox+) | + |
| Mu | Munshiganj | 81 | 6.6 | 0.0003 | 0.2 | 1 (Gr‒, Ox‒) | ‒ | 1 (Gr‒, Ox‒) | ‒ | |
| Bp | Boropukut | 62 | 7.0 | 0.035 | 0.05 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox+) | + | |
| Hn | Henchi | 55 | 6.6 | 1.0 | 0.05 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox+) | + | |
| Jn | Jaynagar | 14 | 6.6 | 2.7 | 0.05 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox+) | + | |
| Gp | Gopalpur | 52 | 7.8 | 8.3 | 0.07 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox‒) | + | |
| Ts | Tirerhat | Jessore | 177 | 6.7 | 6.1 | 0.5 | 3 (Gr+, Ox+) | + | 3 (Gr+, Ox‒) | + |
| Td | Tirerhat-deep | 207 | 6.3 | 1.4 | 0.4 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox‒) | + | |
| M1d | Magura-deep | 26 | 6.6 | 1.0 | 0.1 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox‒) | + | |
| M2s | Magura | 24 | 6.1 | 4.0 | 0.14 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox+) | + | |
| Uz | Uzzalpur | 36 | 6.2 | 1.8 | 0.11 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox+) | + | |
| Sm | Samta | 21 | 6.2 | 2.2 | 1.04 | 1 (Gr+, Ox+) | + | 3 (Gr+, Ox‒) | + | |
| C | Location | Physicochemical Parameters | Cultivation Condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Comilla | CAO | AAO | AHAO | ||||||||
| Sample ID | Name of the Village | Depth (meter) | pH | As (μM) | NO3 (mg/L) | Dilution Factor | aioA | Dilution Factor | aioA | Dilution Factor | aioA |
| Mn-40.1 | Payob | 14 | 6.6 | 2.8 | 20.58 | 1 (Gr+, Ox+) | + | 1 (Gr+, Ox+) | + | 1 (Gr+, Ox+) | + |
| Mn-40.2 | Payob | 21 | 6.3 | 1.1 | 14.67 | 1 (Gr+, Ox+) | + | 1 (Gr+, Ox+) | + | 1 (Gr+, Ox+) | + |
| Mn-40.3 | Payob | 23 | 6.1 | 1.1 | 14.24 | 1 (Gr+, Ox+) | + | 1 (Gr+, Ox+) | + | 1 (Gr+, Ox+) | + |
| Enrichment ID | Percentage (Number) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Types of Enrichment | Phylotypes | NA2 | K1 | Gp | Mn-40.1 | Mn-40.3 | Sm | Ts | Td | Uz | % (TOTAL) | Most closely isolates | ||
| Aerobic: CAO | 1 | 65(20) | 86(25) | 29(6) | 30(8) | 27(6) | 65(15) | 7(2) | 36(82) | Paracoccus sp. SY, Rhodobacter sp. | ||||
| 5 | 14(3) | 26(6) | 86(25) | 15(34) | Bosea sp. WAO | |||||||||
| 7 | 14(4) | 14(4) | 60(9) | 8(17) | Hydrogenophaga sp. CL3, Ancylobacter sp. OL1 | |||||||||
| 8 | 35(11) | 71(15) | 86(25) | 70(19) | 59(13) | 9(2) | 40(6) | 7(2) | 41(93) | Hydrogenophaga NT-14, Acidovorax sp. strains NO-1, 75 | ||||
| Total | 100(31) | 100(29) | 100(21) | 100(29) | 100(27) | 100(22) | 100(23) | 100(15) | 100(29) | 100(226) | ||||
| Anaerobic: AAO and *AHAO | Phylotypes | Mn-40.1 | Mn-40.3 | Mn-40.2 | % (TOTAL) | Most closely isolates | ||||||||
| 1 | 16(2, 4*) | 32(7, 5*) | 16(6*) | 21(9, 15*) | Paracoccus sp. SY, Rhodobacter sp. | |||||||||
| 2 | 13(5*) | 16(6) | 10(6, 5*) | Roseovarius sp. 217, Polymorphum gilvum SL003 | ||||||||||
| 3 | 21(8) | 10(4*) | 11(4, 8*) | Sinorhizobium sp. DAO10 | ||||||||||
| 4 | 5(2) | 2(2) | Aminobacter sp. 86 | |||||||||||
| 6 | 16(6) | 16(6) | 11(12) | Bradyrhizobium sp. | ||||||||||
| 8 | 38(5, 9*) | 37(5, 9*) | 25(10, 18*) | Hydrogenophaga NT-14, Acidovorax sp. strains NO-1, 75 | ||||||||||
| 9 | 30(5, 6*) | 34(4, 9*) | 21(9, 15*) | Hydrogenophaga defluvii B2, Acinetobacter sp. WA19, Albidiferax ferrireducens T118 | ||||||||||
| Total | 100(37) | 100(38) | 100(38) | 100(113) | ||||||||||
| Aerobic: HAO | Phylotypes | Vu | Bp | Gp | Hn | Jn | Sm | Ts | Td | Uz | M1d | M2s | % (TOTAL) | Most closely isolates |
| 5 | 100(1) | 100(1) | 100(1) | 27(3) | Bosea sp. WAO | |||||||||
| 10 | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 73(8) | Achromobacter sp. NT-10, Alcaligenes sp. S46 | ||||
| Total | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(1) | 100(11) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, Z.; Sultana, M.; Khan, S.I.; Braster, M.; Röling, W.F.M.; Westerhoff, H.V. Ample Arsenite Bio-Oxidation Activity in Bangladesh Drinking Water Wells: A Bonanza for Bioremediation? Microorganisms 2019, 7, 246. https://doi.org/10.3390/microorganisms7080246
Hassan Z, Sultana M, Khan SI, Braster M, Röling WFM, Westerhoff HV. Ample Arsenite Bio-Oxidation Activity in Bangladesh Drinking Water Wells: A Bonanza for Bioremediation? Microorganisms. 2019; 7(8):246. https://doi.org/10.3390/microorganisms7080246
Chicago/Turabian StyleHassan, Zahid, Munawar Sultana, Sirajul I. Khan, Martin Braster, Wilfred F.M. Röling, and Hans V. Westerhoff. 2019. "Ample Arsenite Bio-Oxidation Activity in Bangladesh Drinking Water Wells: A Bonanza for Bioremediation?" Microorganisms 7, no. 8: 246. https://doi.org/10.3390/microorganisms7080246
APA StyleHassan, Z., Sultana, M., Khan, S. I., Braster, M., Röling, W. F. M., & Westerhoff, H. V. (2019). Ample Arsenite Bio-Oxidation Activity in Bangladesh Drinking Water Wells: A Bonanza for Bioremediation? Microorganisms, 7(8), 246. https://doi.org/10.3390/microorganisms7080246







