Necrotic Enteritis in Broiler Chickens: The Role of Tight Junctions and Mucosal Immune Responses in Alleviating the Effect of the Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Housing, and Diets
2.2. Necrotic Enteritis Challenge
2.3. Mortality
2.4. Lesion Scores
1 = Thin-walled or friable
2 = Focal necrosis or ulceration
3 = Multifocal coalescing areas (large patches) of necrosis
4 = Severe extensive necrosis
2.5. Performance Parameters
2.6. Gene Expression of Tight Junction Proteins, Cytokines, and Nutrient Transporters
2.7. Statistical Analysis
Yij = measured value for each observation (data)
μ = grand mean
Ai = treatment effect
eij = experimental error
3. Results
3.1. Mortality
3.2. Necrotic Enteritis Lesion Scores
3.3. Performance Parameters
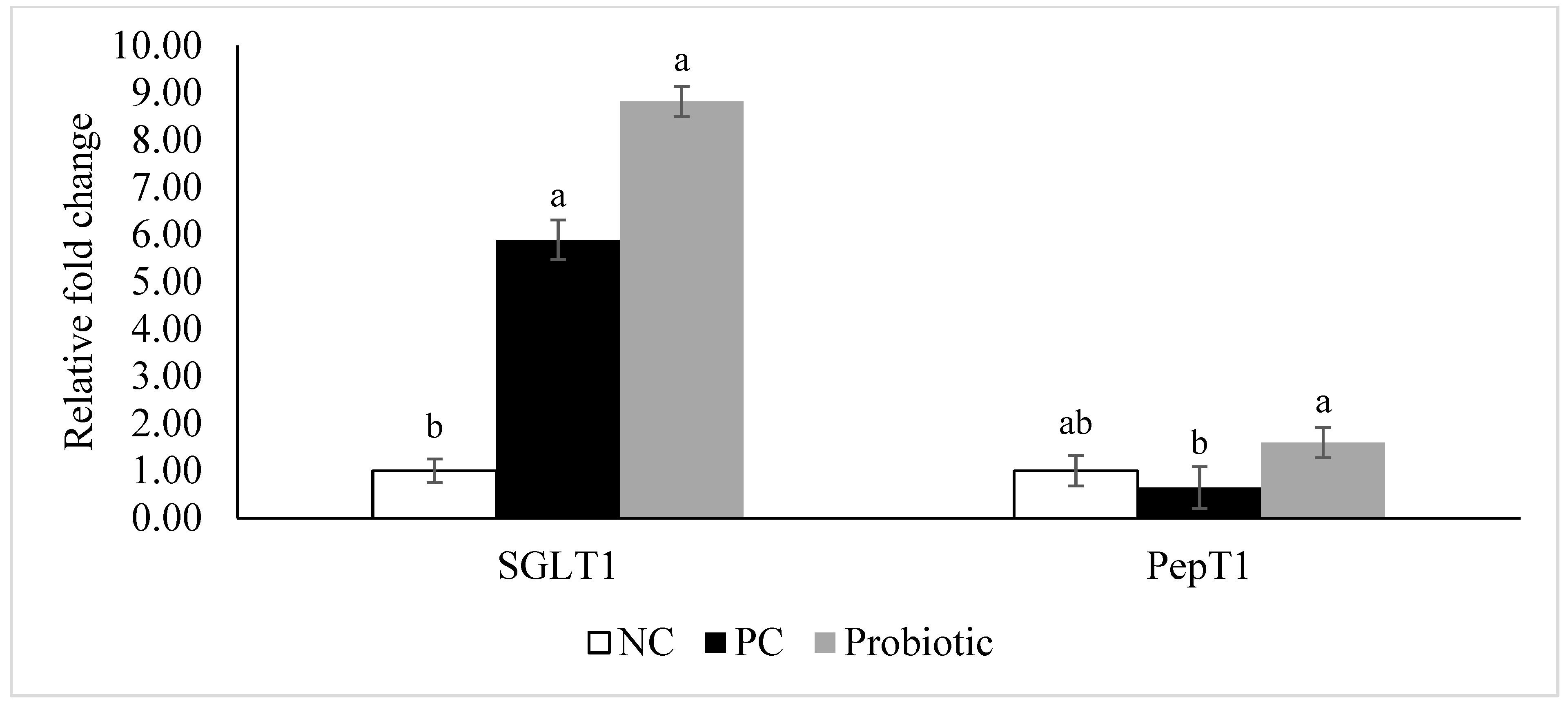
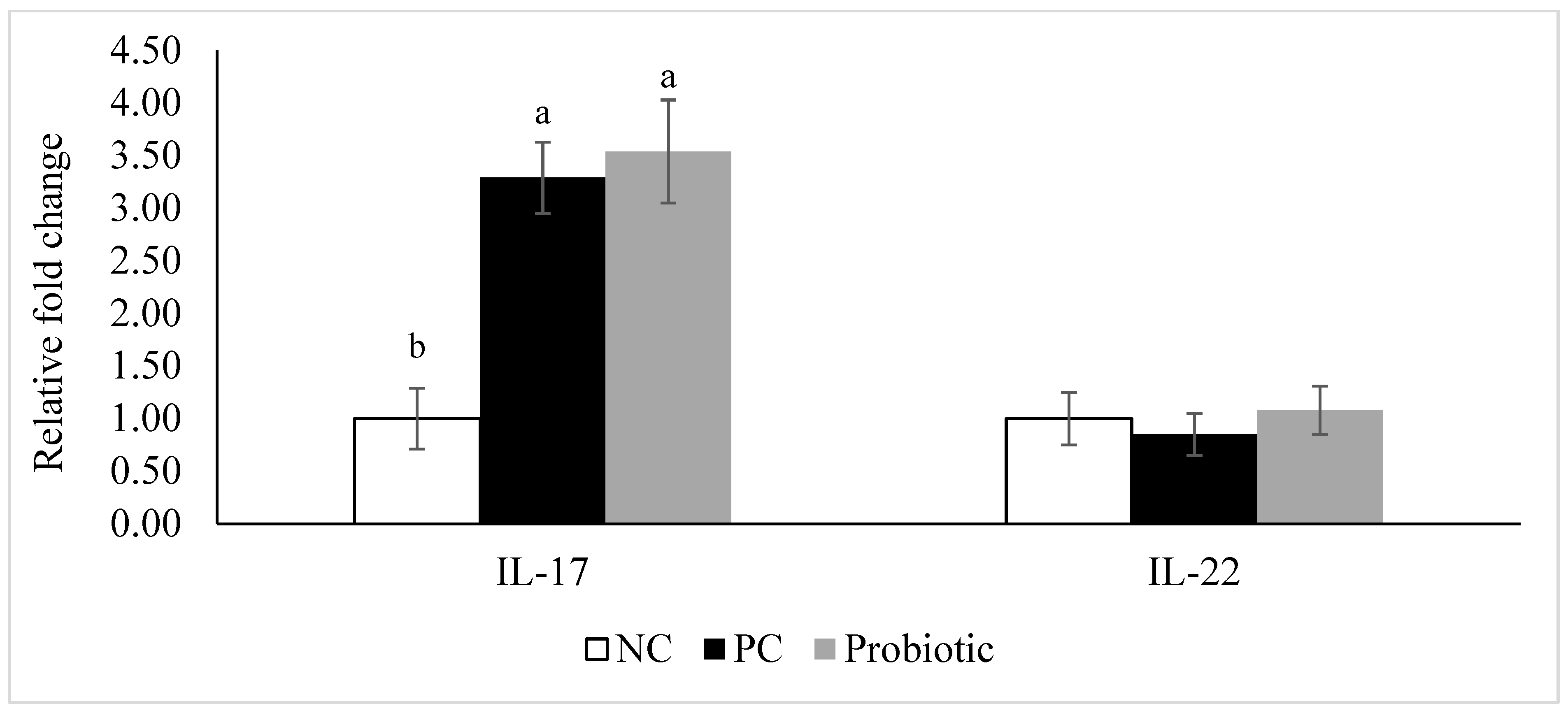
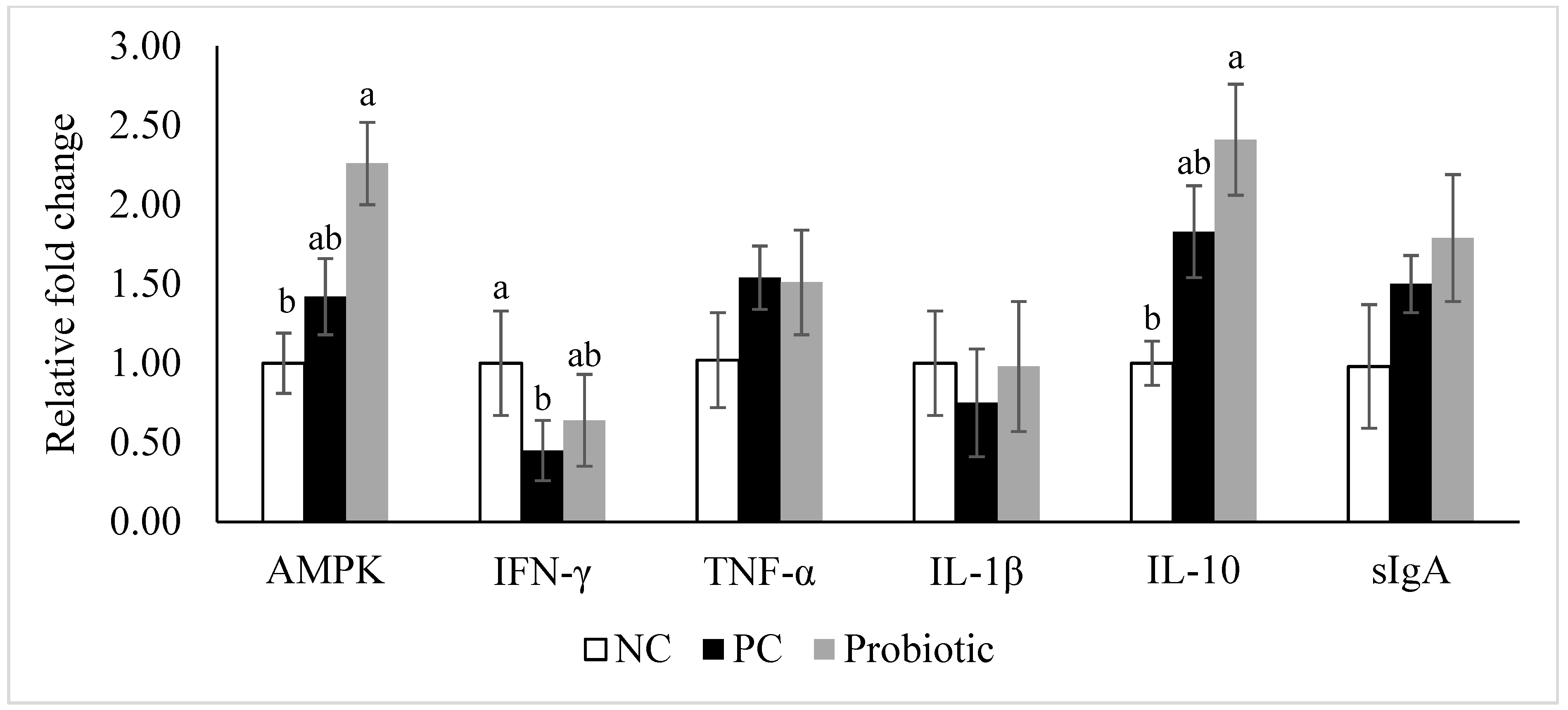
3.4. Gene Expression of Tight Junction Proteins, Cytokines, and Nutrient Transporters
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wade, B.; Keyburn, A. The true cost of necrotic enteritis. World Poult. 2015, 31, 16–17. [Google Scholar]
- Flynn, D. USDA: US foodborne illnesses cost more than $15.6 billion annually. Food Safety News, 9 October 2014. [Google Scholar]
- M’Sadeq, S.A.; Wu, S.; Swick, R.A.; Choct, M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim. Nutr. 2015, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluis, W. Clostridial enteritis is an often underestimated problem. World Poult. 2000, 16, 42–43. [Google Scholar]
- Van Immerseel, F.; Rood, J.I.; Moore, R.J.; Titball, R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009, 17, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-B.; Rodgers, N.; Choct, M. Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Dis. 2010, 54, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Uzal, F.A.; McClane, B.A. Clostridium perfringens sialidases: Potential contributors to intestinal pathogenesis and therapeutic targets. Toxins 2016, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Shojadoost, B.; Vince, A.R.; Prescott, J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: A critical review. Vet. Res. 2012, 43, 74. [Google Scholar] [CrossRef]
- Stanley, D.; Wu, S.-B.; Rodgers, N.; Swick, R.A.; Moore, R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS ONE 2014, 9, e104739. [Google Scholar] [CrossRef]
- Antonissen, G.; Eeckhaut, V.; Van Driessche, K.; Onrust, L.; Haesebrouck, F.; Ducatelle, R.; Moore, R.J.; Van Immerseel, F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016, 45, 308–312. [Google Scholar] [CrossRef]
- Moore, R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016, 45, 275–281. [Google Scholar] [CrossRef]
- Wu, S.-B.; Stanley, D.; Rodgers, N.; Swick, R.A.; Moore, R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014, 169, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ritzi, M.M.; Abdelrahman, W.; Mohnl, M.; Dalloul, R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014, 93, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.X.; Chen, H.; Yu, S.; Zhang, L.; Caplan, M.J.; Chan, H.C. Lymphocytes accelerate epithelial tight junction assembly: Role of AMP-activated protein kinase (AMPK). PLoS ONE 2010, 5, e12343. [Google Scholar] [CrossRef] [PubMed]
- Guttman, J.A.; Finlay, B.B. Tight junctions as targets of infectious agents. BBA Biomembr. 2009, 1788, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Suzuki, H.; Tani, K.; Nishikawa, K.; Irie, K.; Ogura, Y.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 2015, 347, 775. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.A.; Koval, M. Specificity of interaction between Clostridium perfringens enterotoxin and claudin-family tight junction proteins. Toxins 2010, 2, 1595–1611. [Google Scholar] [CrossRef]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef]
- Bland, M.L.; Lee, R.J.; Magallanes, J.M.; Foskett, J.K.; Birnbaum, M.J. AMPK supports growth in Drosophila by regulating muscle activity and nutrient uptake in the gut. Dev. Biol. 2010, 344, 293–303. [Google Scholar] [CrossRef]
- Kogut, M.H.; Genovese, K.J.; He, H.; Arsenault, R.J. AMPK and mTOR: Sensors and regulators of immunometabolic changes during Salmonella infection in the chicken. Poult. Sci. 2015, 95, 345–353. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.-J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef]
- Pender, C.M.; Kim, S.; Sumners, L.H.; Ritzi, M.M.; Young, M.; Dalloul, R.A. In ovo and dietary supplementation of probiotics affects post-hatch expression of immune-related genes in broiler chicks. J. Immunobiol. 2017, 2, 126–134. [Google Scholar] [CrossRef]
- Prescott, J.F.; Sivendra, R.; Barnum, D.A. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. Can. Vet. J. 1978, 19, 181–183. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Cheng, Y.; Li, Y.; Wen, C.; Zhou, Y. Dietary L-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018, 119, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, N.A.; Wong, E.A. Differential mRNA expression of nutrient transporters in male and female chickens. Poult. Sci. 2017, 97, 313–318. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Li, H.; Emmerson, D.A.; Webb, K.E., Jr.; Wong, E.A. Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult. Sci. 2007, 86, 1739–1753. [Google Scholar] [CrossRef]
- Shimizu, K.; Komaki, Y.; Fukano, N.; Bungo, T. Transporter gene expression and transference of fructose in broiler chick intestine. J. Poult. Sci. 2018, 55, 137–141. [Google Scholar] [CrossRef]
- Nighot, M.; Nighot, P. Pathophysiology of avian intestinal ion transport. Worlds Poult. Sci. J. 2018, 74, 347–360. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Eichner, M.; Protze, J.; Piontek, A.; Krause, G.; Piontek, J. Targeting and alteration of tight junctions by bacteria and their virulence factors such as Clostridium perfringens enterotoxin. Pflügers Arch. Eur. J. Physiol. 2017, 469, 77–90. [Google Scholar] [CrossRef]
- Park, M.; Rafii, F. Effects of bile acids and nisin on the production of enterotoxin by Clostridium perfringens in a nutrient-rich medium. Int. J. Microbiol. 2018, 2018, 7276523. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Leung, H.; Akhtar, N.; Li, J.; Barta, J.R.; Wang, Y.; Yang, C.; Kiarie, E. Growth performance and gastrointestinal responses of broiler chickens fed corn-soybean meal diet without or with exogenous epidermal growth factor upon challenge with Eimeria. Poult. Sci. 2017, 96, 3676–3686. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, K.; Dimartino, V.; Cavani, A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Dann, S.M.; Manthey, C.F.; Le, C.; Miyamoto, Y.; Gima, L.; Abrahim, A.; Cao, A.T.; Hanson, E.M.; Kolls, J.K.; Raz, E.; et al. IL-17A promotes protective IgA responses and expression of other potential effectors against the lumen-dwelling enteric parasite Giardia. Exp. Parasitol. 2015, 156, 68–78. [Google Scholar] [CrossRef]
- Cai, C.W.; Blase, J.R.; Zhang, X.; Eickhoff, C.S.; Hoft, D.F. Th17 Cells are more protective than Th1 cells against the intracellular parasite Trypanosoma cruzi. PLOS Pathog. 2016, 12, e1005902. [Google Scholar] [CrossRef]
- Park, S.S.; Lillehoj, H.S.; Allen, P.C.; Park, D.W.; FitzCoy, S.; Bautista, D.A.; Lillehoj, E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008, 52, 14–22. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Qing, X.; Liu, L.; Lai, J.; Khalique, A.; Li, G.; Pan, K.; Jing, B.; Zeng, D. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front. Immunol. 2017, 8, 1592. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Lillehoj, H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019, 98, 188–198. [Google Scholar] [CrossRef]
- Nazli, A.; Yang, P.-C.; Jury, J.; Howe, K.; Watson, J.L.; Söderholm, J.D.; Sherman, P.M.; Perdue, M.H.; McKay, D.M. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am. J. Pathol. 2004, 164, 947–957. [Google Scholar] [CrossRef]
- Aznar, N.; Patel, A.; Rohena, C.C.; Dunkel, Y.; Joosen, L.P.; Taupin, V.; Kufareva, I.; Farquhar, M.G.; Ghosh, P. AMP-activated protein kinase fortifies epithelial tight junctions during energetic stress via its effector GIV/Girdin. Elife 2016, 5, e20795. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, M.-J. AMP-activated protein kinase: A therapeutic target in intestinal diseases. Open Biol. 2017, 7, 170104. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, W.H.; Lee, S.-J.; Lillehoj, H.S. Detection of chicken interleukin-10 production in intestinal epithelial cells and necrotic enteritis induced by Clostridium perfringens using capture ELISA. Vet. Immunol. Immunopathol. 2018, 204, 52–58. [Google Scholar] [CrossRef]
- Bremner, A. Innate Responses and Biomarkers of Resistance to Eimeria Infection in the Chicken. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 2018. [Google Scholar]
- Morhardt, T.L.; Hayashi, A.; Ochi, T.; Quirós, M.; Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Kao, J.Y.; et al. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019, 9, 1223. [Google Scholar] [CrossRef]

| Feeding Phase (Days) | |||
|---|---|---|---|
| Ingredients (%) | Starter (0–14) | Grower 1 + 2 (15–28) | Finisher (29–42) |
| Corn (7.81% crude protein) | 59.53 | 64.12 | 65.70 |
| Soybean meal (48% crude protein) | 33.5 | 28.80 | 26.86 |
| Soybean Oil (9000 kcal/kg) | 2.18 | 2.60 | 3.50 |
| Dicalcium Phosphate (18.5% Phosphorus, 22% Calcium) | 2.05 | 1.92 | 1.70 |
| Calcium Carbonate (37% Calcium) | 1.11 | 1.00 | 0.90 |
| Sodium Chloride | 0.37 | 0.37 | 0.35 |
| DL-Methionine (990 g/kg) 2 | 0.38 | 0.34 | 0.29 |
| L-Lysine Hydrochloride (788 g L-Lysine/kg) 3 | 0.37 | 0.35 | 0.24 |
| L-Threonine (985 g/kg) 4 | 0.15 | 0.14 | 0.10 |
| Vitamin/Trace Mineral Premix 5 | 0.36 | 0.36 | 0.36 |
| Calculated Analysis (% unless specified) | |||
| ME (kcal/kg) | 3007 | 3087 | 3168 |
| Crude protein | 21.81 | 19.90 | 18.94 |
| Total phosphorus | 0.76 | 0.71 | 0.66 |
| Available phosphorus | 0.45 | 0.42 | 0.38 |
| Calcium | 0.90 | 0.84 | 0.76 |
| Chlorine | 0.33 | 0.33 | 0.29 |
| Sodium | 0.16 | 0.16 | 0.15 |
| Potassium | 0.85 | 0.77 | 0.73 |
| Methionine | 0.67 | 0.61 | 0.55 |
| Methionine + Cysteine | 0.98 | 0.89 | 0.82 |
| Lysine | 1.32 | 1.19 | 1.05 |
| Threonine | 0.86 | 0.78 | 0.71 |
| Linoleic acid | 1.44 | 1.52 | 1.55 |
| Dietary cation-anion balance (mEq) | 194 | 174 | 170 |
| Gene | Primer Sequence | Size | Acc (Reference) |
|---|---|---|---|
| Claudin-1 | GTGTTCAGAGGCATCAGGTATC GTCAGGTCAAACAGAGGTACAA | 107 | NM_001013611.2 |
| Claudin-3 | CCCGTCCCGTTGTTGTTTTG CCCCTTCAACCTTCCCGAAA | 126 | NM_204202.1 [25] |
| Zonula occluden-1 | GGAGTACGAGCAGTCAACATAC GAGGCGCACGATCTTCATAA | 101 | XM_413773 |
| Zonula occluden-2 | GCGTCCCATCCTGAGAAATAC CTTGTTCACTCCCTTCCTCTTC | 89 | NM_204918 |
| Interferon-γ | GCTCCCGATGAACGACTTGA TGTAAGATGCTGAAGAGTTCATTCG | 63 | NM_205149.1 |
| TNF-α 1 | CCCATCCCTGGTCCGTAAC ATACGAAGTAAAGGCCGTCCC | 77 | MF000729.1 |
| sIgA 2 | GTCACCGTCACCTGGACTACA ACCGATGGTCTCCTTCACATC | 192 | S40610 |
| Interleukin-1β | CCCGCCTTCCGCTACA CACGAAGCACTTCTGGTTGATG | 66 | XM_015297469.1 |
| Interleukin-10 | CGCTGTCACCGCTTCTTCA CGTCTCCTTGATCTGCTTGATG | 63 | NM_001004414.2 |
| Interleukin-17A | AGCTGGACCACAGCGTCAAC GGCGGAGGACGAGGATCT | 57 | NM_204460.1 |
| AMPK-α1 3 | ATCTGTCTCGCCCTCATCCT CCACTTCGCTCTTCTTACACCTT | 125 | NM_001039603 |
| SGLT1 4 | GCCATGGCCAGGGCTTA CAATAACCTGATCTGTGCACCAGTA | 71 | NM_0,012,93240.1 [26] |
| PepT1 5 | CCCCTGAGGAGGATCACTGTT CAAAAGAGCAGCAGCAACGA | 66 | NM_204,365.1 [26] |
| GAPDH 6 | CCTAGGATACACAGAGGACCAGGTT GGTGGAGGAATGGCTGTCA | 64 | NM_204305 |
| Treatments 1 | Time Period (day) | ||||||
|---|---|---|---|---|---|---|---|
| 0–8 | 7–9 | 9–14 | 15–21 | 22–28 | 29–42 | 0–42 | |
| NC | 5.28 a | 4.44 a | 2.23 | 0.00 | 1.64 | 3.60 a | 11.39 a |
| PC | 0.28 b | 0.55 b | 1.50 | 0.31 | 0.00 | 0.00 b | 1.94 b |
| Probiotic | 4.72 a | 1.51 b | 0.95 | 0.64 | 0.73 | 0.42 b | 6.94 ab |
| SEM2 | 1.39 | 0.94 | 0.61 | 0.31 | 0.53 | 0.73 | 1.79 |
| p-value | 0.030 | 0.018 | 0.347 | 0.347 | 0.106 | 0.002 | 0.003 |
| Treatments 2 | Small Intestine Section | ||
|---|---|---|---|
| Duodenum | Jejunum | Ileum | |
| NC | 2.25 a | 1.32 a | 0.32 |
| PC | 1.77 ab | 0.85 b | 0.12 |
| Probiotic | 1.41 b | 0.86 b | 0.34 |
| SEM3 | 0.17 | 0.14 | 0.16 |
| p-value | 0.006 | 0.040 | 0.582 |
| Dietary Treatments 2 | |||||
|---|---|---|---|---|---|
| Item 3 | NC | PC | Probiotic | SEM4 | p-Value |
| d 0–8 | |||||
| ADFI, g | 23.28 | 23.50 | 23.31 | 0.24 | 0.784 |
| ADG, g | 17.1 | 16.59 | 17.29 | 0.25 | 0.137 |
| FCR, g/g | 1.36 ab | 1.41 a | 1.35 b | 0.02 | 0.053 |
| d 9-14 | |||||
| ADFI, g | 57.21 | 56.09 | 56.84 | 0.64 | 0.474 |
| ADG, g | 41.97 | 40.38 | 40.59 | 0.59 | 0.134 |
| FCR | 1.36 b | 1.39 ab | 1.40 a | 0.01 | 0.043 |
| d 15–21 | |||||
| ADFI, g | 106.10 | 105.41 | 104.55 | 1.34 | 0.708 |
| ADG, g | 71.15 | 70.65 | 68.84 | 1.42 | 0.468 |
| FCR | 1.49 | 1.49 | 1.52 | 0.02 | 0.414 |
| d 22–28 | |||||
| ADFI, g | 161.89 a | 159.72 a | 154.75 b | 1.75 | 0.018 |
| ADG, g | 103.07 | 105.47 | 103.78 | 1.62 | 0.567 |
| FCR | 1.57 a | 1.51 b | 1.49 b | 0.01 | ˂0.001 |
| d 29–42 | |||||
| ADFI, g | 202.19 | 203.59 | 199.85 | 2.17 | 4.74 |
| ADG, g | 113.00 b | 119.53 a | 116.00 ab | 1.55 | 0.020 |
| FCR | 1.79 a | 1.70 b | 1.72 b | 0.01 | 0.001 |
| d 0–42 | |||||
| ADFI, g | 119.45 ab | 120.62 a | 116.80 b | 1.11 | 0.050 |
| ADG, g | 74.99 b | 77.69 a | 75.72 ab | 0.85 | 0.048 |
| FCR | 1.59 a | 1.55 b | 1.54 b | 0.01 | ˂0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emami, N.K.; Calik, A.; White, M.B.; Young, M.; Dalloul, R.A. Necrotic Enteritis in Broiler Chickens: The Role of Tight Junctions and Mucosal Immune Responses in Alleviating the Effect of the Disease. Microorganisms 2019, 7, 231. https://doi.org/10.3390/microorganisms7080231
Emami NK, Calik A, White MB, Young M, Dalloul RA. Necrotic Enteritis in Broiler Chickens: The Role of Tight Junctions and Mucosal Immune Responses in Alleviating the Effect of the Disease. Microorganisms. 2019; 7(8):231. https://doi.org/10.3390/microorganisms7080231
Chicago/Turabian StyleEmami, Nima K., Ali Calik, Mallory B. White, Mark Young, and Rami A. Dalloul. 2019. "Necrotic Enteritis in Broiler Chickens: The Role of Tight Junctions and Mucosal Immune Responses in Alleviating the Effect of the Disease" Microorganisms 7, no. 8: 231. https://doi.org/10.3390/microorganisms7080231
APA StyleEmami, N. K., Calik, A., White, M. B., Young, M., & Dalloul, R. A. (2019). Necrotic Enteritis in Broiler Chickens: The Role of Tight Junctions and Mucosal Immune Responses in Alleviating the Effect of the Disease. Microorganisms, 7(8), 231. https://doi.org/10.3390/microorganisms7080231





