Biocidal Effectiveness of Selected Disinfectants Solutions Based on Water and Ozonated Water against Listeria monocytogenes Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Ozonated Water
2.3. Disinfectants
2.4. Preparation of Bacterial Supensions
2.5. Assessment of Biocidal Effectiveness of Ozonated and Non-Ozonated Water
2.6. Evaluation of the Effectiveness of Disinfectants
2.7. Assessment of the Stability of QAC 2, OA 3, and ChC1 Solutions
2.8. Statistical Analysis
2.8.1. Biocidal Effectiveness of Ozonated and Non-Ozonated Water
2.8.2. Evaluation of the Effectiveness of Disinfectants
2.8.3. Reduction in Bacteria Number
2.8.4. Maximal Effectiveness Coefficients for Disinfectants
3. Results
3.1. Assessment of Biocidal Effectiveness of Ozonated and Non-Ozonated Water
3.2. Evaluation of Effectiveness of Disinfectant
3.3. Coefficients of Effectiveness of Disinfectants
3.4. Assessment of the Stability of QAC 2, OA 3, and ChC 1 Solutions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Orsi, R.H.; Wiedmann, M. Characteristics and distribution of Listeria spp., including Listeria species newly described since 2009. Appl. Microbiol. Biotechnol. 2016, 100, 5273–5287. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- Książczyk, M.; Krzyżewska, E.; Futoma-Kołoch, B.; Bugla-Płoskońska, G. Oddziaływanie związków dezynfekcyjnych na komórki bakteryjne w kontekście bezpieczeństwa higieny i zdrowia publicznego. Postępy Hig. Med. Dośw. 2015, 69, 1042–1055. [Google Scholar]
- Demir, F.; Atguden, A. Experimental Investigation on the Microbial Inactivation of Domestic Well Drinking Water using Ozone under Different Treatment Conditions. Ozone-Sci. Eng. 2016, 38, 25–35. [Google Scholar] [CrossRef]
- Wysok, B.; Uradziński, J.; Gomółka-Pawlicka, M. Ozone as an alternative disinfectant—A review. Pol. J. Food Nutr. Sci. 2006, 15, 3–8. [Google Scholar]
- Alexopoulos, A.; Plessas, S.; Ceciu, S.; Lazar, V.; Mantzourani, I.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Evaluation of ozone efficacy on the reduction of microbial population of fresh cut lettuce (Lactuca sativa) and green bell pepper (Capsicum annuum). Food Control 2013, 30, 491–496. [Google Scholar] [CrossRef]
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef]
- Thanomsub, B.; Anupunpisit, V.; Chanphetch, S.; Watcharachaipong, T.; Poonkhum, R.; Srisukonth, C. Effects of ozone treatment on cell growth and ultrastructural changes in bacteria. J. Gen. Appl. Microbiol. 2002, 48, 193–199. [Google Scholar] [CrossRef]
- Khadere, M.A.; Yousef, A.E.; Kim, J.-G. Microbiological aspects of ozone applications in food: A review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Seki, M.; Ishikawa, T.; Terada, H.; Nashimoto, M. Microbidical Effects of Stored Aqueous Ozone Solution Generated by Nano-bubble Technology. In Vivo 2017, 31, 579–583. [Google Scholar] [PubMed]
- Arayan, L.T.; Reyes, A.W.B.; Hop, H.T.; Xuan, H.T.; Yang, H.S.; Chang, H.H.; Kim, S. Optimized applications of ozonated water as an effective disinfectant for Staphylococcus aureus clearance in an abattoir setting. J. Prev. Vet. Med. 2017, 41, 71–74. [Google Scholar] [CrossRef]
- Białoszewski, D.; Bocian, E.; Bukowska, B.; Czajkowska, M.; Sokół-Leszczyńska, B.; Tyski, S. Antimicrobial activity of ozonated water. Med. Sci. Monit. 2010, 16, 71–75. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (2018) Breakpoints Tables for Interpretation of MICs and Zones Diameters. Version 8.0. Available online: http://www.eucast.org (accessed on 1 March 2019).
- PN-EN 1276:2010. Chemiczne środki dezynfekcyjne i antyseptyczne—Ilościowa zawiesinowa metoda określania działania bakteriobójczego chemicznych środków dezynfekcyjnych i antyseptycznych stosowanych w sektorze żywnościowym, warunkach przemysłowych i domowych oraz zakładach użyteczności publicznej - metoda badania i wymagania (faza 2, etap 1). (In Polish). Available online: http://sklep.pkn.pl/pn-en-1276-2010e.html (accessed on 1 March 2019).
- International Organization for Standardization (ISO). Water Quality—Determination of Free Chlorine and Total Chlorine—Part 2: Colorimetric Method Using N,N-dialkyl-1,4-phenylenediamine, for Routine Control Purposes; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Technical Committee on Microbiological Methods for Foods. In Compendium of Methods for the Microbiological Examination of Foods; APHA: Washington, DC, USA, 1984. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Bacteriological Analytical Manual; AOAC: Arlington, VA, USA, 2017.
- Skowron, K.; Grudlewska, K.; Krawczyk, A.; Gospodarek-Komkowska, E. The effectiveness of radiant catalytic ionization in inactivation of Listeria monocytogenes planktonic and biofilm cells from food and food contact surfaces as a method of food preservation. J. Appl. Microbiol. 2018, 124, 1493–1505. [Google Scholar] [CrossRef]
- Muthukumar, A.; Muthuchamy, M. Optimization of ozone in gaseous phase to inactive Listeria monocytogenes on raw chicken samples. Food Res. Int. 2013, 54, 1128–1130. [Google Scholar] [CrossRef]
- Sheelamary, M.; Muthukumar, M. Effectiveness of Ozone in Inactivating Listeria monocytogenes from Milk Samples. World J. Young Res. 2011, 1, 40–44. [Google Scholar]
- Patil, S.; Valdramidis, V.; Frias, J.M.; Cullen, P.; Bourke, P. Ozone inactivation of acid stressed Listeria monocytogenes and Listeria innocua in orange juice using a bubble column. Food Control 2010, 21, 1723–1730. [Google Scholar] [CrossRef]
- Sung, H.J.; Song, W.J.; Kim, K.W.; Ryu, S.; Kang, D.H. Combination effect of ozone and heat treatments for the inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in apple juice. Int. J. Food Microbiol. 2013, 171, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Shin, J.Y.; Ryu, S.; Kang, D.H. Inactivation of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes in apple juice at different pH levels by gaseous ozone treatment. J. Appl. Microbiol. 2015, 119, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.D.; Williams, R.C.; Sumner, S.S.; Eifert, J.D. Effect of ozone and ultraviolet light on Listeria monocytogenes populations in fresh and spent chill brines. Food Control 2016, 59, 172–177. [Google Scholar] [CrossRef]
- Kang, S.N.; Kim, K.J.; Park, J.H.; Lee, O.H. Effect of a combination of low level ozone and metal ions on reducing Escherichia coli O157:H7 and Listeria monocytogenes. Molecules 2013, 18, 4018–4025. [Google Scholar] [CrossRef]
- Marino, M.; Maifreni, M.; Baggio, A.; Innocente, N. Inactivation of Foodborne Bacteria Biofilms by Aqueous and Gaseous Ozone. Front. Microbiol. 2018, 9, 2024. [Google Scholar] [CrossRef]
- Korany, A.M.; Hua1, Z.; Green, T.; Hanrahan, I.; El-Shinawy, S.H.; El-Kholy, A.; Hassan, G.; Zhu, M.J. Efficacy of Ozonated Water, Chlorine, Chlorine Dioxide, Quaternary Ammonium Compounds and Peroxyacetic Acid Against Listeria monocytogenes Biofilm on Polystyrene Surfaces. Front. Microbiol. 2018, 9, 2296. [Google Scholar] [CrossRef]
- Fishburn, J.D.; Tang, Y.; Frank, J.F. Efficacy of Various Consumer-Friendly Produce Washing Technologies in Reducing Pathogens on Fresh Produce. Food Protect. Trends 2011, 32, 456–466. [Google Scholar]
- Lariviere-Gauthier, G.; Letellier, A.; Quessy, S.; Fournaise, S.; Fravalo, P. Assessment of the efficiency of ozonated water as bacterial contamination reduction tool in a pork cutting plant. SafePork 2013, 42, 143–146. [Google Scholar]
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef]
- Leggett, M.J.; Schwarz, J.S.; Burke, P.A.; McDonnell, G.; Denyer, S.P.; Maillard, J.-Y. Mechanism of sporicidal activity for the synergistic combination of peracetic acid and hydrogen peroxide. Appl. Environ. Microbiol. 2016, 82, 1035–1039. [Google Scholar] [CrossRef]
- Chavant, P.; Gaillard- Martine, B.; Hébraud, M. Antimicrobial effects of sanitizers against planktonic and sessile Listeria monocytogenes cells according to the growth phase. FEMS Microbiol. Lett. 2004, 236, 241–248. [Google Scholar] [CrossRef]
- Aarnisalo, K.; Lundén, J.; Korkeala, H.; Wirtanen, G. Susceptibility of Listeria monocytogenes strains to disinfectants and chlorinated alkaline cleaners at cold temperatures. LWT 2008, 40, 1041–1048. [Google Scholar] [CrossRef]
- Heir, E.; Lindstedt, B.A.; Røtterud, O.J.; Vardund, T.; Kapperud, G.; Nesbakken, T. Molecular epidemiology and disinfectant susceptibility of Listeria monocytogenes from meat processing plants and human infections. Int. J. Food Microbiol. 2004, 96, 85–96. [Google Scholar] [CrossRef]
- Popowska, M.; Olszak, M.; Markiewicz, Z. Susceptibility of Listeria monocytogenes strains isolated from dairy products and frozen vegetables to antibiotics inhibiting murein synthesis and to disinfectants. Pol. J. Microbiol. 2006, 55, 279–288. [Google Scholar] [PubMed]
- Fouladkhah, A.; Geornaras, J.; Sofos, J.N. Biofilm formation of O157 and Non-O157 Shiga toxin-producing Escherichia coli and multidrug-resistant and susceptible Salmonella Typhimurium and Newport and their inactivation by sanitizers. J. Food Sci. 2013, 78, 880–886. [Google Scholar] [CrossRef] [PubMed]

| Group of Disinfectants | Name | Active Substance | Working Solution Concentration (g/mL) |
|---|---|---|---|
| Quaternary ammonium compounds | QAC 1 | benzyl-C12-18-alkydimethyl ammonium chlorides | 2.0 × 10−3 |
| QAC 2 | benzyl-C12-16 alkyldimethyl chlorides | 2.55 × 100 | |
| QAC 3 | didecyldimethylammonium chloride, benzyl-C12-16-alkyldimethyl chlorides | 2.97 × 100 | |
| Oxidizing agents | OA 1 | hydrogen peroxide, silver nitrate | 1.20 × 101 |
| OA 2 | perlactic acid | 4.90 × 100 | |
| OA 3 | peracetic acid, hydrogen peroxide | 6.15 × 10−3 | |
| OA 4 | bis (sulphate) bis (peroxymonosulfate) pentapotassium, benzenesulfonic acid, C10-13 alkyl derivatives, sodium salts, malic acid, sulfamic acid | 2.00 × 10−2 | |
| Chlorine compounds | ChC 1 | chlorine dioxide | 1.00 × 10−5 |
| ChC 2 | hypochlorous acid calcium salt | 2.00 × 10−3 | |
| Iodine compounds | IC 1 | iodine | 6.15 × 100 |
| IC 2 | iodine | 1.17 × 101 | |
| Nanoparticles | NANO 1 | nanocopper | 1.50 × 10−4 |
| NANO 2 | nanosilver | 1.50 × 10−4 |
| Group of Disinfectants | Name | Initial Concentration (g/mL) | Final Concentration (g/mL) |
|---|---|---|---|
| Quaternary ammonium compounds | QAC 1 | 4.00 × 10−3; 3.60 × 10−3; 3.20 × 10−3; 2.80 × 10−3; 2.40 × 10−3; 2.00 × 10−3; 1.60 × 10−3; 1.20 × 10−3; 8.00 × 10−4; 4.00 × 10−4; 2.00 × 10−4; 4.00 × 10−5; 2.00 × 10−5 | 2.00 × 10−3; 1.80 × 10−3; 1.60 × 10−3; 1.40 × 10−3; 1.20 × 10−3; 1.00 × 10−3; 8.00 × 10−4; 6.00 × 10−4; 4.00 × 10−4; 2.00 × 10−4; 1.00 × 10−4; 2.00 × 10−5; 1.00 × 10−5 |
| QAC 2 | 5.1 × 100; 4.59 × 100; 4.08 × 100; 3.57 × 100; 3.06 × 100; 2.55 × 100; 2.04 × 100; 1.53 × 100; 1.02 × 100; 5.1 × 10−1; 2.6 × 10−1; 5.00 × 10−2; 2.6 × 10−2 | 2.55 × 100; 2.30 × 100; 2.04 × 100; 1.79 × 100; 1.53 × 100; 1.28 × 100; 1.02 × 100; 7.70 × 10−1; 5.10 × 10−1; 2.60 × 10−1; 1.30 × 10−1; 2.60 × 10−2; 1.00 × 10−2 | |
| QAC 3 | 5.94 × 100; 5.35 × 100; 4.75 × 100; 4.16 × 100; 3.56 × 100; 2.97 × 100; 2.38 × 100; 1.78 × 100; 1.19 × 100; 5.90 × 10−1; 3.00 × 10−1; 5.90 × 10−2; 3.00 × 10−2 | 2.97 × 100; 2.67 × 100; 2.38 × 100; 2.08 × 100; 1.78 × 100; 1.49 × 100; 1.19 × 100; 8.90 × 10−1; 5.90 × 10−1; 2.30 × 10−1; 1.50 × 10−1; 3.00 × 10−2; 1.50 × 10−2 | |
| Oxidizing agents | OA 1 | 2.40 × 101; 2.16 × 101; 1.92 × 101; 1.68 × 101; 1.44 × 101; 1.20 × 101, 9.60 × 100; 7.20 × 100; 4.80 × 100; 2.40 × 100; 1.20 × 100; 2.40 × 10−1; 1.20 × 10−1 | 1.20 × 101; 1.08 × 101; 9.60 × 100; 8.40 × 100; 7.20 × 100; 6.00 × 100; 4.80 × 100; 3.60 × 100; 2.40 × 100; 1.20 × 100; 6.00 × 10−1; 1.20 × 10−1; 6.00 × 10−2 |
| OA 2 | 9.80 × 100; 8.82 × 100; 7.84 × 100; 6.86 × 100; 5.88 × 100, 4.90 × 100; 3.92 × 100; 2.94 × 100; 1.96 × 100; 9.80 × 10−1; 4.90 × 10−1; 9.80 × 10−2; 4.90 × 10−2 | 4.90 × 100; 4.41 × 100; 3.92 × 100; 3.43 × 100; 2.45 × 100; 2.40 × 100; 1.96 × 100; 1.47 × 100; 9.80 × 10−1; 4.90 × 10−1; 2.50 × 10−1; 5.00 × 10−2; 2.50 × 10−2 | |
| OA 3 | 1.20 × 10−2; 1.00 × 10−2; 9.80 × 10−3; 8.60 × 10−3; 7.40 × 10−3; 6.15 × 10−3; 4.92 × 10−3; 3.69 × 10−3; 2.46 × 10−3; 1.23 × 10−3; 6.15 × 10−4; 1.23 × 10−4; 6.15 × 10−5 | 6.15 × 10−3; 5.50 × 10−3; 4.90 × 10−3; 4.30 × 10−3; 3.70 × 10−3; 3.10 × 10−3; 2.46 × 10−3; 1.90 × 10−3; 1.23 × 10−3; 6.15 × 10−4; 3.10 × 10−4; 6.15 × 10−5; 3.10 × 10−5 | |
| OA 4 | 4.00 × 10−2; 3.60 × 10−2; 3.20 × 10−2; 2.80 × 10−2; 2.40 × 10−2; 2.00 × 10−2; 1.60 × 10−2; 1.20 × 10−2; 8.00 × 10−3; 4.00 × 10−3; 2.00 × 10−3; 4.00 × 10−4; 2.00 × 10−4 | 2.00 × 10−2; 1.80 × 10−2; 1.60 × 10−2; 1.40 × 10−2; 1.20 × 10−2; 1.00 × 10−2; 8.00 × 10−3; 6.00 × 10−3; 4.00 × 10−3; 2.00 × 10−3; 1.00 × 10−3; 2.00 × 10−4; 1.00 × 10−4 | |
| Chlorine compounds | ChC 1 | 2.00 × 10−5; 1.8 × 10−5; 1.6 × 10−5; 1.4 × 10−5; 1.20 × 10−5; 1.00 × 10−5; 8.00 × 10−6; 6.00 × 10−6; 4.00 × 10−6; 2.00 × 10−6; 1.00 × 10−6; 2.00 × 10−7; 1.00 × 10−7 | 1.00 × 10−5; 9.00 × 10−6; 8.00 × 10−6; 7.00 × 10−6; 6.00 × 10−6;5.00 × 10−6; 4.00 × 10−6; 3.00 × 10−6; 2.00 × 10−6; 1.00 × 10−6; 2.00 × 10−7; 1.00 × 10−7 |
| ChC 2 | 4.00 × 10−3; 3.60 × 10−3; 3.20 × 10−3; 2.80 × 10−3; 2.40 × 10−3; 2.00 × 10−3; 1.60 × 10−3; 1.20 × 10−3; 8.00 × 10−4; 4.00 × 10−4; 2.00 × 10−4; 4.00 × 10−5; 2.00 × 10−5 | 2.00 × 10−3; 1.80 × 10−3; 1.60 × 10−3; 1.40 × 10−3; 1.20 × 10−3; 1.00 × 10−3; 8.00 × 10−4; 6.00 × 10−4; 4.00 × 10−4; 2.00 × 10−4; 1.00 × 10−4; 2.00 × 10−5; 1.00 × 10−5 | |
| Iodine compounds | IC 1 | 1.23 × 101; 1.11 × 101; 9.84 × 100; 8.61 × 100; 7.38 × 100; 6.15 × 100; 4.92 × 100; 3.69 × 100; 2.46 × 100; 1.23 × 100; 6.20 × 10−1; 1.20 × 10−1; 6.20 × 10−2 | 6.15 × 100; 5.54 × 100; 4.92 × 100; 4.31 × 100; 3.69 × 100; 3.08 × 100; 2.46 × 100; 1.85 × 100; 1.23 × 100; 6.20 × 10−1; 3.10 × 10−1; 6.20 × 10−2; 3.10 × 10−2 |
| IC 2 | 2.34 × 101; 2.11 × 101; 1.87 × 101; 1.64 × 101; 1.40 × 101; 1.17 × 101; 9.36 × 100; 7.02 × 100; 4.68 × 100; 2.34 × 100; 1.17 × 100; 2.30 × 10−1; 1.20 × 10−1 | 1.17 × 101; 1.05 × 101; 9.36 × 100; 8.19 × 100; 7.02 × 100; 5.85 × 100; 4.68 × 100; 3.51 × 100; 2.34 × 100; 1.17 × 100; 5.90 × 10−1; 1.20 × 10−1; 5.90 × 10−2 | |
| Nanoparticles | NANO 1 | 3.00 × 10−3; 2.70 × 10−3; 2.40 × 10−3; 2.10 × 10−3; 1.80 × 10−3; 1.50 × 10−3; 1.20 × 10−3; 9.00 × 10−4; 6.00 × 10−4; 3.00 × 10−4; 1.50 × 10−4; 3.00 × 10−5; 1.50 × 10−5 | 1.50 × 10−4; 1.35 × 10−4; 1.20 × 10−4; 1.05 × 10−4; 9.00 × 10−5; 7.50 × 10−5; 6.00 × 10−5; 4.50 × 10−5; 3.00 × 10−5; 1.50 × 10−5; 7.50 × 10−6; 1,50 × 10−6; 7.50 × 10−7 |
| NANO 2 | 3.00 × 10−3; 2.70 × 10−3; 2.40 × 10−3; 2.10 × 10−3; 1.80 × 10−3; 1.50 × 10−3; 1.20 × 10−3; 9.00 × 10−4; 6.00 × 10−4; 3.00 × 10−4; 1.50 × 10−4; 3.00 × 10−5; 1.50 × 10−5 | 1.50 × 10−4; 1.35 × 10−4; 1.20 × 10−4; 1.05 × 10−4; 9.00 × 10−5; 7.50 × 10−5; 6.00 × 10−5; 4.50 × 10−5; 3.00 × 10−5; 1.50 × 10−5; 7.50 × 10−6; 1,50 × 10−6; 7.50 × 10−7 |
| Group of Disinfectants | Disinfectant | Water Type | Minimal Bactericidal Concentration of Disinfectant (g/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Strain | ||||||||
| LMO-ATCC | LMO-W | LMO-M | LMO-N | LMO-R | LMO-K | |||
| Quaternary ammonium compounds | QAC 1 | Nonozonated | 1.00 × 10−4 a | 1.00 × 10−4 a | 4.00 × 10−5 b | 4.00 × 10−5 b | 4.00 × 10−5 b | 1.00 × 10−4 a |
| Ozonated | 4.00 × 10−5 b | 2.00 × 10−5 b | 1.00 × 10−5 b | 1.00 × 10−5 b | 1.00 × 10−5 b | 4.00 × 10−5 b | ||
| QAC 2 | Nonozonated | 1.28 × 10−1 c | 1.28 × 10−1 c | 1.28 × 10−1 c | 1.28 × 10−1 c | 1.28 × 10−1 c | 1.28 × 10−1 c | |
| Ozonated | 1.28 × 10−2 d | 1.28 × 10−2 d | 1.28 × 10−2 d | 1.28 × 10−2 d | 1.28 × 10−2 d | 1.28 × 10−2 d | ||
| QAC 3 | Nonozonated | 1.49 × 10−1 c | 1.49 × 10−1 c | 1.49 × 10−1 c | 1.49 × 10−1 c | 1.49 × 10−1 c | 1.49 × 10−1 c | |
| Ozonated | 1.49 × 10−2 d | 2.97 × 10−2 d | 1.49 × 10−2 d | 1.49 × 10−2 d | 1.49 × 10−2 d | 1.49 × 10−2 d | ||
| Oxidizing agents | OA 1 | Nonozonated | 1.20 × 101 e | 1.20 × 101 e | 1.20 × 101 e | 1.20 × 101 e | 1.20 × 101 e | 1.20 × 101 e |
| Ozonated | 1.01 × 101 e | 1.01 × 101 e | 1.01 × 101 e | 1.01 × 101 e | 1.01 × 101 e | 1.01 × 101 e | ||
| OA 2 | Nonozonated | 4.90 × 100 k | Ineffective | Ineffective | 4.90 × 100 k | Ineffective | 4.90 × 100 k | |
| Ozonated | 2.70 × 100 f | 2.79 × 100 f | 3.38 × 100 f,k | 2.70 × 100 f | 3.68 × 100 f,k | 2.70 × 100 f | ||
| OA 3 | Nonozonated | 1.42 × 10−3 g | 1.42 × 10−3 g | 1.85 × 10−3 g | 1.85 × 10−3 g | 3.69 × 10−3 g | 1.42 × 10−3 g | |
| Ozonated | 3.08 × 10−4 a | 3.08 × 10−4 a | 4.31 × 10−4 a | 3.08 × 10−4 a | 5.54 × 10−4 a | 3.08 × 10−4 a | ||
| OA 4 | Nonozonated | 4.20 × 10−3 h | 4.20 × 10−3 h | 4.20 × 10−3 h | 4.20 × 10−3 h | 4.20 × 10−3 h | 4.20 × 10−3 h | |
| Ozonated | 1.60 × 10−3 g | 1.60 × 10−3 g | 1.60 × 10−3 g | 1.60 × 10−3 g | 1.60 × 10−3 g | 1.60 × 10−3 g | ||
| Chlorine compounds | ChC 1 | Nonozonated | 2.00 × 10−7 i | 4.00 × 10−7 i | 7.00 × 10−7 i | 4.00 × 10−7 i | 6.00 × 10−7 i | 4.00 × 10−7 i |
| Ozonated | 5.00 × 10−8 j | 1.00 × 10−7 i | 3.00 × 10−7 i | 1.00 × 10−7 i | 2.00 × 10−7 i | 1.00 × 10−7 i | ||
| ChC 2 | Nonozonated | 2.40 × 10−1 c | 3.20 × 10−1 c | 3.60 × 10−1 c | 3.20 × 10−1 c | 2.80 × 10−1 c | 4.0 × 10−1 c | |
| Ozonated | 2.00 × 10−1 c | 2.00 × 10−1 c | 2.00 × 10−1 c | 2.00 × 10−1 c | 2.00 × 10−1 c | 2.80 × 10−1 c | ||
| Iodine compounds | IC 1 | Nonozonated | 2.15 × 100 f | 2.15 × 100 f | 2.15 × 100 f | 2.15 × 100 f | 2.15 × 100 f | 2.15 × 100 f |
| Ozonated | 1.05 × 100 l | 1.29 × 100 l | 1.29 × 100 l | 1.29 × 100 l | 1.29 × 100 l | 1.05 × 100 l | ||
| IC 2 | Nonozonated | 3.63 × 100 f,k | 3.63 × 100 f,k | 3.86 × 100 f,k | 3.63 × 100 f,k | 3.63 × 100 f,k | 3.86 × 100 f,k | |
| Ozonated | 1.64 × 100 l | 1.64 × 100 l | 1.76 × 100 l | 1.64 × 100 l | 1.69 × 100 l | 1.76 × 100 l | ||
| Nanoparticles | NANO 1 | Nonozonated | 1.37 × 10−4 a | 1.37 × 10−4 a | 1.41 × 10−4 a | 1.37 × 10−4 a | 1.47 × 10−4 a | 1.37 × 10−4 a |
| Ozonated | 1.08 × 10−4 a | 1.14 × 10−4 a | 1.14 × 10−4 a | 1.08 × 10−4 a | 1.19 × 10−4 a | 1.08 × 10−4 a | ||
| NANO 2 | Nonozonated | Ineffective | Ineffective | Ineffective | Ineffective | Ineffective | Ineffective | |
| Ozonated | 1.20 × 10−4 a | 1.20 × 10−4 a | 1.20 × 10−4 a | 1.20 × 10−4 a | 1.20 × 10−4 a | 1.20 × 10−4 a | ||
| Group of Disinfectants | Disinfectant | Water Type | Reduction in Bacteria Number (log CFU) | |||||
|---|---|---|---|---|---|---|---|---|
| Strain | ||||||||
| LMO-ATCC | LMO-W | LMO-M | LMO-N | LMO-R | LMO-K | |||
| Quaternary ammonium compounds | QAC 1 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 |
| Nonozonated | 7.23 a | 6.96 a | 6.84 a | 6.92 a | 6.76 a | 7.07 a | ||
| Ozonated | 8.28 b | 8.00 b | 7.88 b | 7.97 b | 7.80 b | 8.12 b | ||
| QAC 2 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 | |
| Nonozonated | 7.05 a | 6.78 a | 6.62 a | 6.75 a | 6.50 a | 6.90 a | ||
| Ozonated | 7.93 b | 7.65 b | 7.62 b | 7.49 a | 7.36 b | 7.77 b | ||
| QAC 3 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 | |
| Nonozonated | 6.70 a | 6.43 a | 6.27 a | 6.40 a | 6.15 a | 6.55 a | ||
| Ozonated | 7.49 a | 7.22 a | 7.06 a | 7.19 a | 6.93 a | 7.34 a | ||
| Oxidizing agents | OA 1 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 |
| Nonozonated | 6.52 a | 6.26 a | 6.10 a | 6.22 a | 5.98 a | 6.37 a | ||
| Ozonated | 7.23 b | 6.96 a | 6.79 a | 6.93 a | 6.67 a | 7.07 a | ||
| OA 2 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 | |
| Nonozonated | 2.64 a | 2.43 a | 2.37 a | 2.17 a | 2.26 a | 2.53 a | ||
| Ozonated | 5.11 b | 4.87 b | 4.59 b | 4.82 b | 4.70 b | 4.98 b | ||
| OA 3 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 | |
| Nonozonated | 6.61 a | 6.35 a | 6.18 a | 6.31 a | 6.06 a | 6.46 a | ||
| Ozonated | 7.40 a | 7.13 a | 6.97 a | 7.08 a | 6.80 a | 7.25 a | ||
| OA 4 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 | |
| Nonozonated | 6.43 a | 6.18 a | 6.02 a | 6.12 a | 5.83 a | 6.29 a | ||
| Ozonated | 7.35 b | 7.04 b | 6.90 b | 7.00 b | 6.75 b | 7.10 b | ||
| Chlorine compounds | ChC 1 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 |
| Nonozonated | 7.67 a | 7.39 a | 7.23 a | 7.33 a | 7.06 a | 7.52 a | ||
| Ozonated | 8.55 b | 8.30 b | 8.24 b | 8.10 a | 7.91 b | 8.33 b | ||
| ChC 2 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 | |
| Nonozonated | 7.31 a | 6.95 a | 6.71 a | 6.93 a | 6.58 a | 7.08 a | ||
| Ozonated | 7.85 a | 7.56 a | 7.30 a | 7.55 a | 7.41 b | 7.65 a | ||
| Iodine compounds | IC 1 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 |
| Nonozonated | 6.48 a | 6.22 a | 6.05 a | 6.16 a | 5.93 a | 6.37 a | ||
| Ozonated | 7.54 b | 7.26 b | 7.17 b | 7.31 b | 7.03 b | 7.38 b | ||
| IC 2 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 | |
| Nonozonated | 6.49 a | 6.21 a | 6.03 a | 6.15 a | 5.90 a | 6.33 a | ||
| Ozonated | 7.63 b | 7.34 b | 7.16 b | 7.30 b | 7.04 b | 7.47 b | ||
| Nanoparticles | NANO 1 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 |
| Nonozonated | 6.17 a | 5.91 a | 5.75 a | 5.87 a | 5.69 a | 6.03 a | ||
| Ozonated | 7.05 b | 6.79 b | 6.62 b | 6.75 b | 6.50 b | 6.90 b | ||
| NANO 2 | Initial no. | 8.81 | 8.69 | 8.71 | 8.76 | 8.66 | 8.73 | |
| Nonozonated | 1.76 a | 1.57 a | 1.30 a | 1.49 a | 1.37 a | 1.66 a | ||
| Ozonated | 6.03 b | 5.75 b | 5.87 b | 5.91 b | 5.63 b | 6.17 b | ||
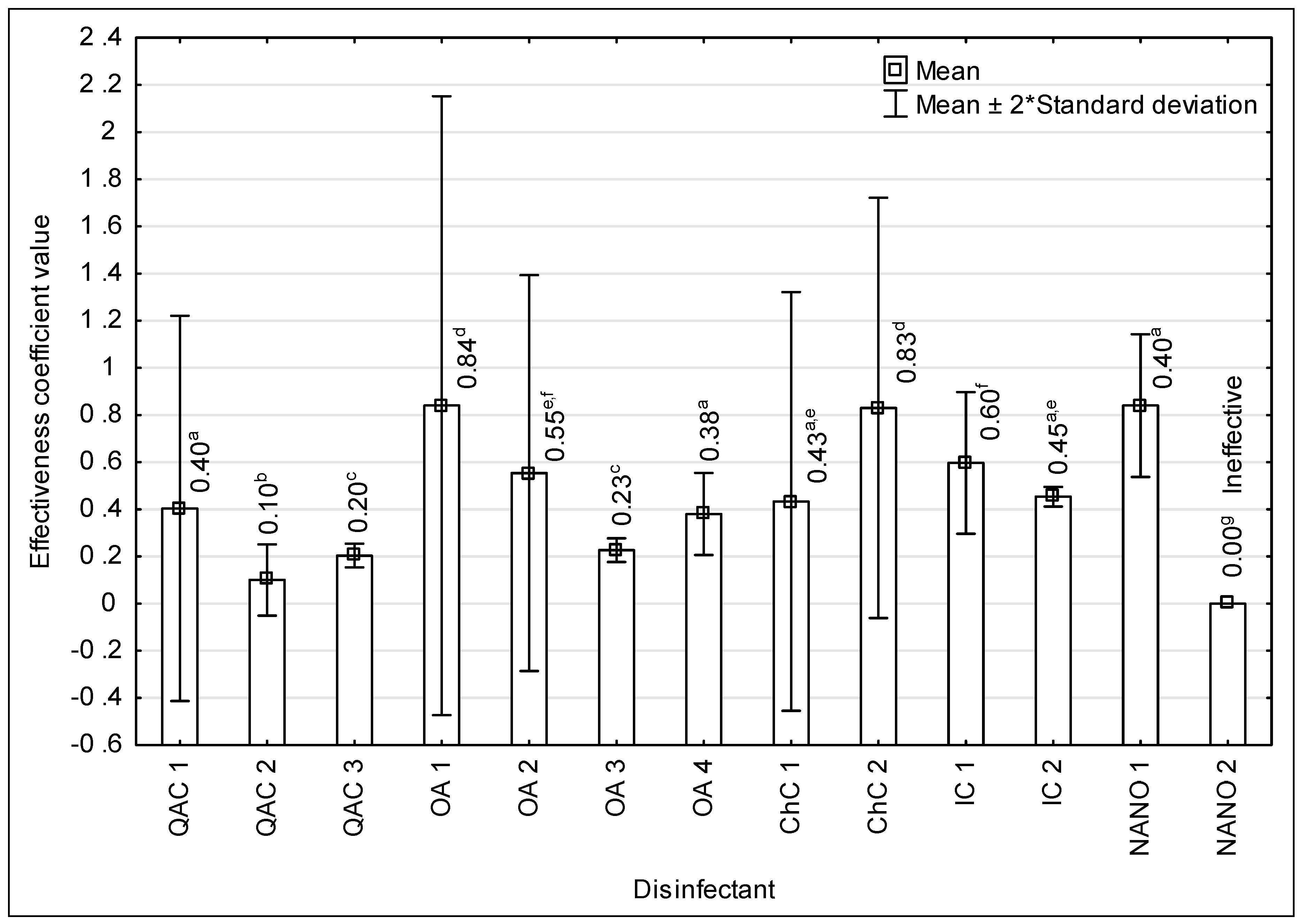
| Disinfectant | Efficiency Coefficient (A) | |||||
|---|---|---|---|---|---|---|
| Strain | ||||||
| LMO-ATCC | LMO-W | LMO-M | LMO-N | LMO-R | LMO-K | |
| QAC 1 | 0.40 a | 0.20 b | 0.25 b,g | 0.25 b,g | 0.25 b,g | 0.40 a |
| QAC 2 | 0.10 c,f | 0.10 c,f | 0.10 c,f | 0.10 c,f | 0.10 c,f | 0.10 c,f |
| QAC 3 | 0.10 c,f | 0.20 b | 0.10 c,f | 0.10 c,f | 0.10 c,f | 0.10 c,f |
| OA 1 | 0.84 d | 0.84 d | 0.84 d | 0.84 d | 0.84 d | 0.84 d |
| OA 2 | 0.55 e,i | Ineffective | Ineffective | 0.55 e,i | Ineffective | 0.55 e,i |
| OA 3 | 0.22 b | 0.22 b | 0.23 b | 0.17 b,f | 0.15 b,f | 0.22 b |
| OA 4 | 0.38 a | 0.38 a | 0.38 a | 0.38 a | 0.38 a | 0.38 a |
| ChC 1 | 0.25 b,g | 0.25 b,g | 0.43 a | 0.25 b,g | 0.33 a,g | 0.25 b,g |
| ChC 2 | 0.83 d | 0.63 e,h | 0.56 e | 0.63 e,h | 0.71 h,j | 0.70 h,j |
| IC 1 | 0.49 a,i | 0.60 e,h | 0.60 e,h | 0.60 e,h | 0.60 e,h | 0.49 a,i |
| IC 2 | 0.45 a,i | 0.45 a,i | 0.45 a,i | 0.45 a,i | 0.45 a,i | 0.45 a,i |
| NANO 1 | 0.70 h,j | 0.84 d | 0.81 d | 0.79 d,j | 0.81 d | 0.79 d,j |
| NANO 2 | Ineffective | Ineffective | Ineffective | Ineffective | Ineffective | Ineffective |
| Disinfectant | Water Type | Storage Time (h) | Minimal Bactericidal Concentration of Disinfectant [g/cm3] | |||||
|---|---|---|---|---|---|---|---|---|
| Strain | ||||||||
| LMO-ATCC | LMO-W | LMO-M | LMO-N | LMO-R | LMO-K | |||
| QAC 2 (working solution: 2.55 × 100 g/mL) | Nonozonated | 0 | 1.28 × 10−4 a | 1.28 × 10−4 a | 1.28 × 10−4 a | 1.28 × 10−4 a | 1.28 × 10−4 a | 1.28 × 10−4 a |
| 12 | 1.28 × 10−4 a | 1.28 × 10−4 a | 1.28 × 10−4 a | 1.28 × 10−4 a | 1.28 × 10−4 a | 1.28 × 10−4 a | ||
| 24 | 1.53 × 10−4 a | 1.53 × 10−4 a | 1.53 × 10−4 a | 1.53 × 10−4 a | 1.53 × 10−4 a | 1.53 × 10−4 a | ||
| Ozonated | 0 | 1.28 × 10−5 b | 1.28 × 10−5 b | 1.28 × 10−5 b | 1.28 × 10−5 b | 1.28 × 10−5 b | 1.28 × 10−5 b | |
| 12 | 1.02 × 10−4 a | 1.28 × 10−5 b | 1.28 × 10−5 b | 1.28 × 10−5 b | 1.28 × 10−5 b | 1.02 × 10−4 a | ||
| 24 | 1.53 × 10−4 a | 1.53 × 10−4 a | 1.53 × 10−4 a | 1.53 × 10−4 a | 1.79 × 10−4 a | 1.53 × 10−4 a | ||
| OA 3 (working solution: 6.15 × 10−3 g/mL) | Nonozonated | 0 | 1.42 × 10−3 c | 1.42 × 10−3 c | 1.42 × 10−3 c | 1.80 × 10−3 c | 3.81 × 10−3 d,f | 1.42 × 10−3 c |
| 12 | 1.66 × 10−3 c | 1.72 × 10−3 c | 2.40 × 10−3 c,d | 2.20 × 10−3 c,d | 4.30 × 10−3 e,f | 1.60 × 10−3 c | ||
| 24 | 2.40 × 10−3 c,d | 2.58 × 10−3 c,d | 3.40 × 10−3 d | 3.20 × 10−3 d | 5.23 × 10−3 e | 2.50 × 10−3 c,d | ||
| Ozonated | 0 | 3.10 × 10−4 g | 3.10 × 10−4 g | 4.30 × 10−4 g | 3.10 × 10−4 g | 6.15 × 10−4 h | 2.46 × 10−4 g | |
| 12 | 1.60 × 10−3 c | 1.66 × 10−3 c | 2.40 × 10−3 c,d | 2.15 × 10−3 c,d | 4.12 × 10−3 e,f | 1.60 × 10−3 c | ||
| 24 | 2.40 × 10−3 c,d | 2.65 × 10−3 c,d | 3.40 × 10−3 d | 3.9 × 10−3 d,f | 5.10 × 10−3 e | 2.50 × 10−3 c,d | ||
| ChC 1 (working solution: 1.00 × 10−5 g/mL) | Nonozonated | 0 | 2.00 × 10−7 i | 4.00 × 10−7 i,k | 7.00 × 10−7 j | 4.00 × 10−7 i,k | 6.00 × 10−7 j,k | 4.00 × 10−7 i,k |
| 12 | 3.00 × 10−7 i | 6.00 × 10−7 j,k | 8.00 × 10−7 j | 5.00 × 10−7 i,k | 8.00 × 10−7 j | 5.00 × 10−7 i,k | ||
| 24 | 5.00 × 10−7 i,k | 8.00 × 10−7 j | 1.00 × 10−6 l | 7.00 × 10−7 j | 1.10 × 10−6 l | 7.00 × 10−7 j | ||
| Ozonated | 0 | 5.00 × 10−8 m | 1.00 × 10−7 i | 3.00 × 10−7 i | 1.00 × 10−7 i | 2.00 × 10−7 i | 1.00 × 10−7 i | |
| 12 | 2.00 × 10−7 i | 5.00 × 10−7 i,k | 8.00 × 10−7 j | 5.00 × 10−7 i,k | 8.00 × 10−7 j | 4.00 × 10−7 i,k | ||
| 24 | 5.00 × 10−7 i,k | 8.00 × 10−7 j | 1.00 × 10−6 l | 8.00 × 10−7 j | 1.10 × 10−6 l | 7.00 × 10−7 j | ||
| Disinfectant | Storage Time (h) | Efficiency Coefficient (A) | |||||
|---|---|---|---|---|---|---|---|
| Strain | |||||||
| LMO-ATCC | LMO-W | LMO-M | LMO-N | LMO-R | LMO-K | ||
| QAC 2 (working solution: 2.55 × 100 g/mL) | 0 12 24 | 0.10 a 0.80 b 1.00 b,c | 0.10 a 0.10 a 1.00 b,c | 0.10 a 0.10 a 1.00 b,c | 0.10 a 0.10 a 1.00 b,c | 0.10 a 0.10 a 1.17 c | 0.10 a 0.80 b 1.00 b,c |
| OA 3 (working solution: 6.15 × 10−3 g/mL) | 0 12 24 | 0.22 a 0.96 b 1.00 b,c | 0.22 a 0.96 b 1.02 b,c | 0.23 a 1.00 b,c 1.00 b,c | 0.17 a 0.97 b 1.06 b,c | 0.16 a 0.96 b 0.98 b,c | 0.17 a 1.00 b,c 1.00 b,c |
| ChC 1 (working solution: 1.00 × 10−5 g/mL) | 0 12 24 | 0.25 a,e 0.67 f 1.00 b,c | 0.25 a 0.83 b 1.00 b,c | 0.43 d 1.00 b,c 1.00 b,c | 0.25 a,e 1.00 b,c 1.14 b,c | 0.33 d,e 1.00 b,c 1.00 b,c | 0.25 a,e 0.80 b 1.00 b,c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowron, K.; Wałecka-Zacharska, E.; Grudlewska, K.; Białucha, A.; Wiktorczyk, N.; Bartkowska, A.; Kowalska, M.; Kruszewski, S.; Gospodarek-Komkowska, E. Biocidal Effectiveness of Selected Disinfectants Solutions Based on Water and Ozonated Water against Listeria monocytogenes Strains. Microorganisms 2019, 7, 127. https://doi.org/10.3390/microorganisms7050127
Skowron K, Wałecka-Zacharska E, Grudlewska K, Białucha A, Wiktorczyk N, Bartkowska A, Kowalska M, Kruszewski S, Gospodarek-Komkowska E. Biocidal Effectiveness of Selected Disinfectants Solutions Based on Water and Ozonated Water against Listeria monocytogenes Strains. Microorganisms. 2019; 7(5):127. https://doi.org/10.3390/microorganisms7050127
Chicago/Turabian StyleSkowron, Krzysztof, Ewa Wałecka-Zacharska, Katarzyna Grudlewska, Agata Białucha, Natalia Wiktorczyk, Agata Bartkowska, Maria Kowalska, Stefan Kruszewski, and Eugenia Gospodarek-Komkowska. 2019. "Biocidal Effectiveness of Selected Disinfectants Solutions Based on Water and Ozonated Water against Listeria monocytogenes Strains" Microorganisms 7, no. 5: 127. https://doi.org/10.3390/microorganisms7050127
APA StyleSkowron, K., Wałecka-Zacharska, E., Grudlewska, K., Białucha, A., Wiktorczyk, N., Bartkowska, A., Kowalska, M., Kruszewski, S., & Gospodarek-Komkowska, E. (2019). Biocidal Effectiveness of Selected Disinfectants Solutions Based on Water and Ozonated Water against Listeria monocytogenes Strains. Microorganisms, 7(5), 127. https://doi.org/10.3390/microorganisms7050127





