Systematic Affiliation and Genome Analysis of Subtercola vilae DB165T with Particular Emphasis on Cold Adaptation of an Isolate from a High-Altitude Cold Volcano Lake
Abstract
1. Introduction
2. Materials and Methods
3. Results
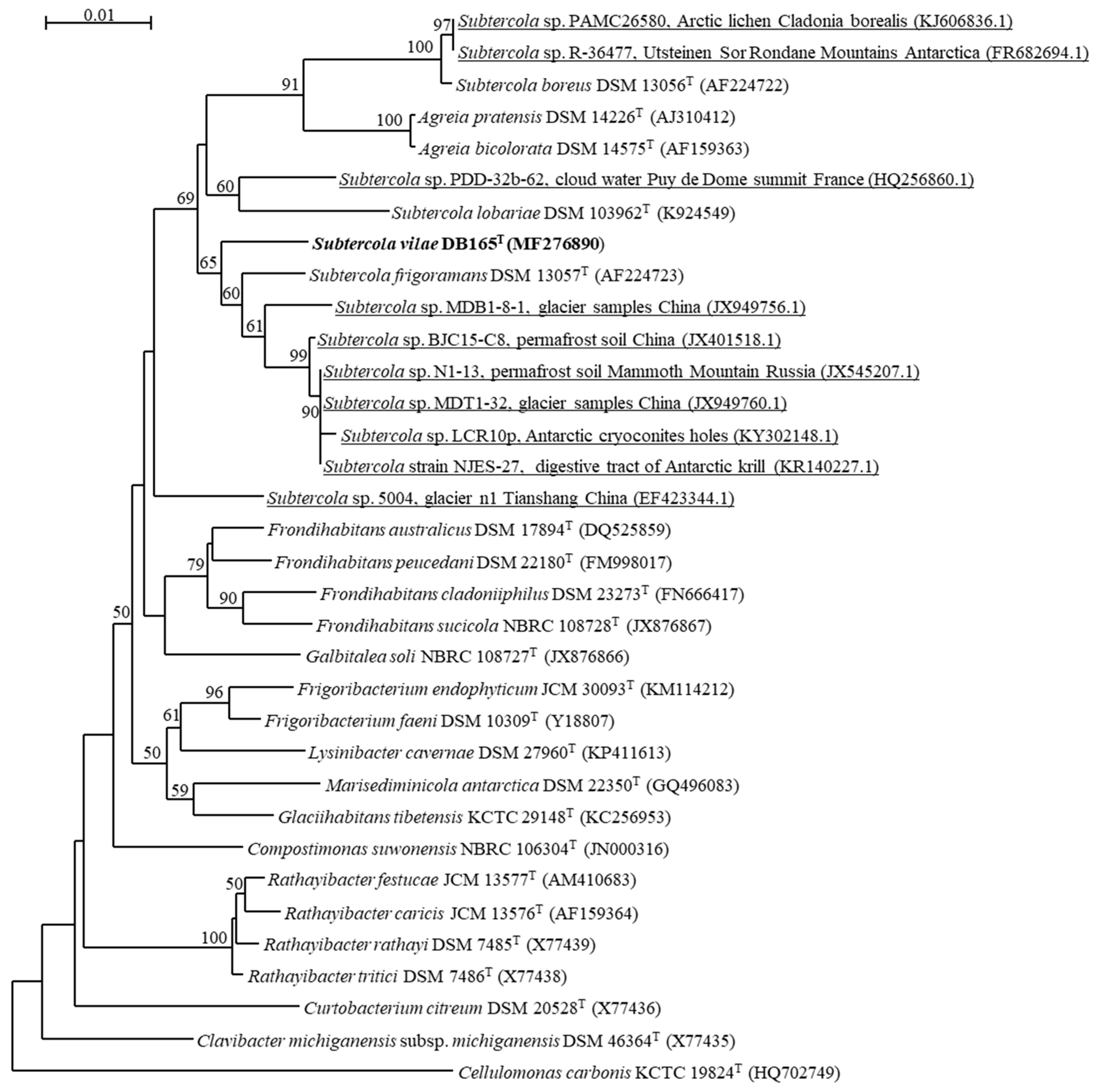
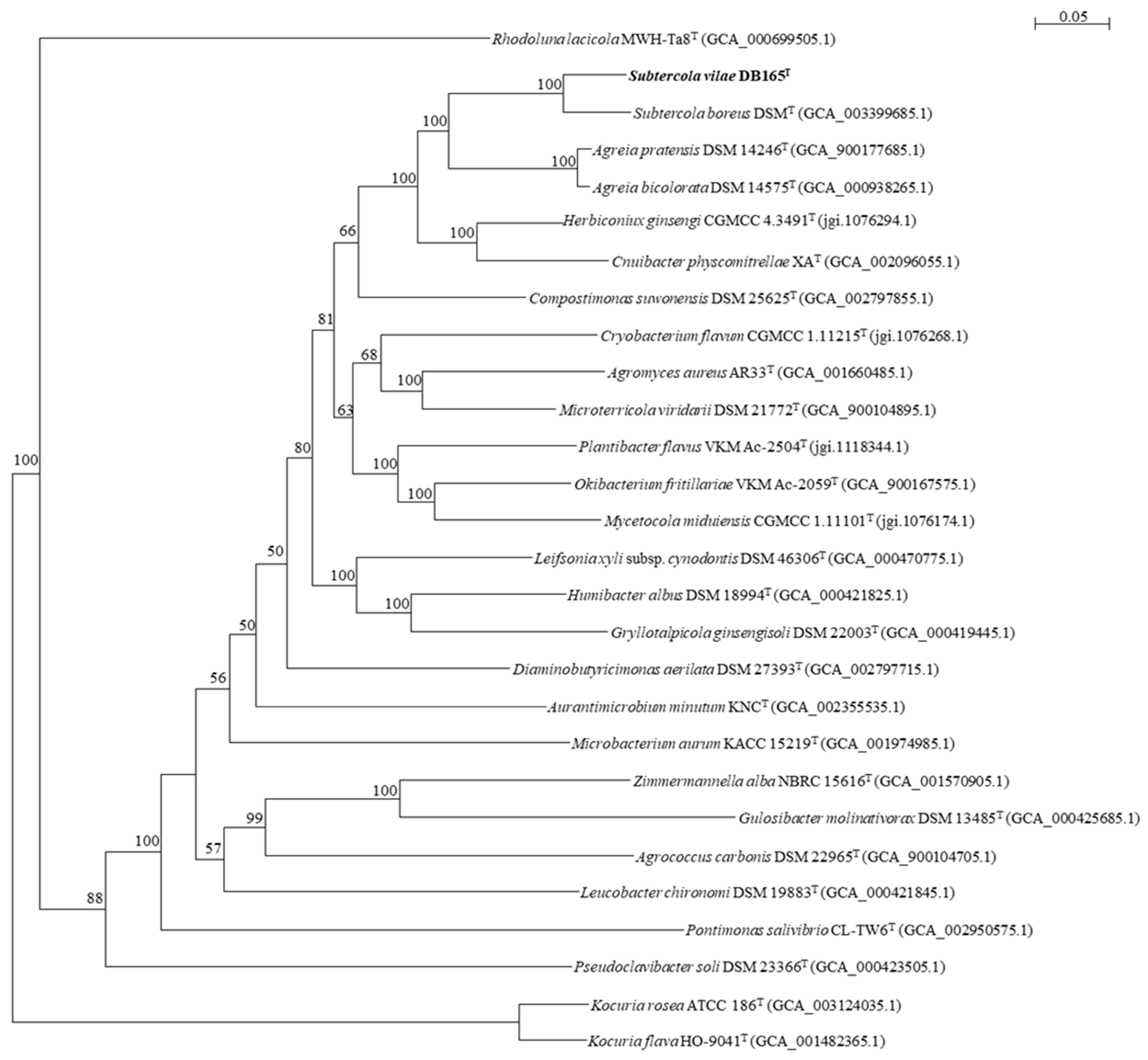
3.1. Diversity and Relations of Subtercola and Related Agreia Species
3.2. Genome Properties
3.2.1. General Properties
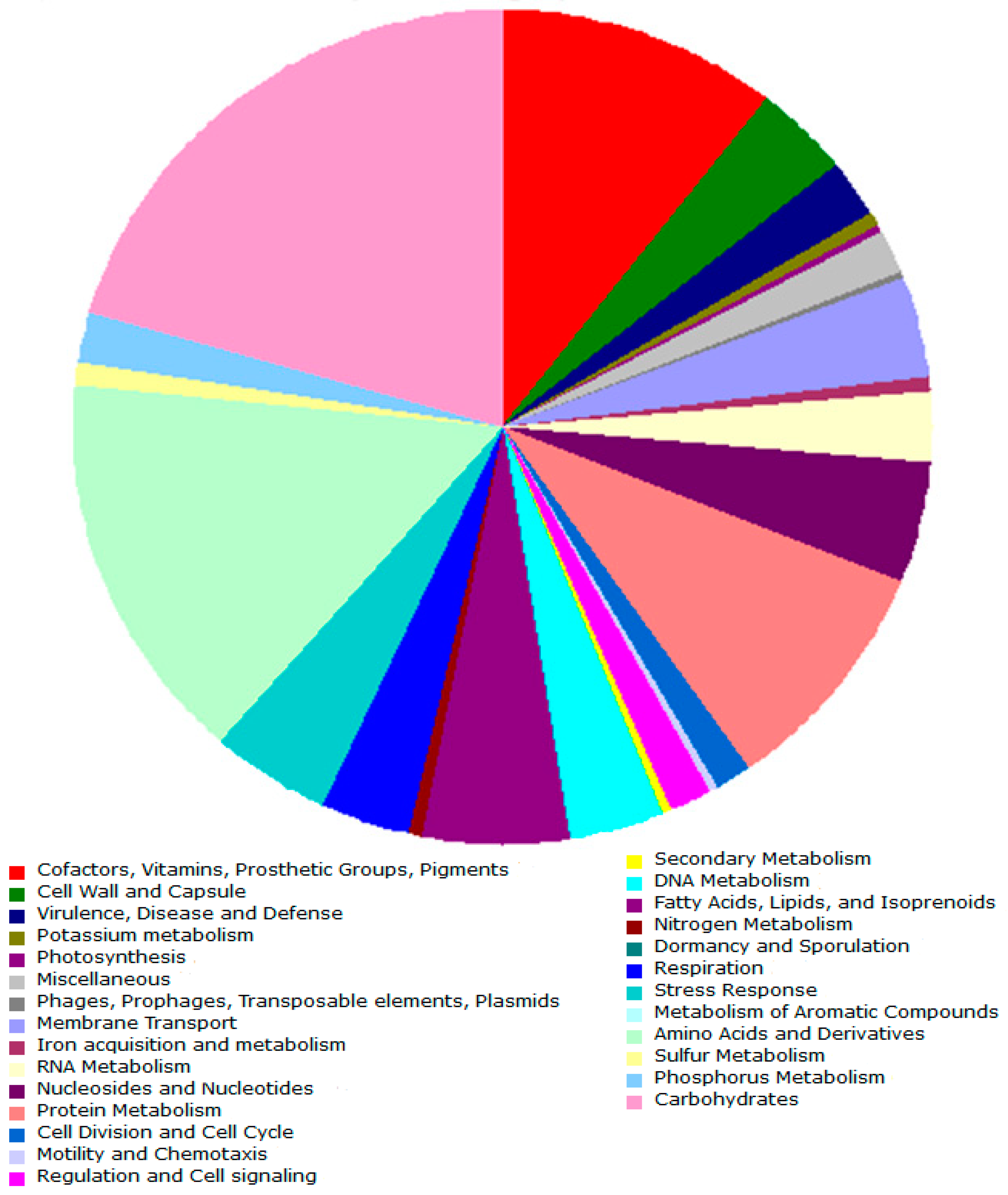
3.2.2. Carbon and Energy Metabolism
3.2.3. Secondary Metabolite Production
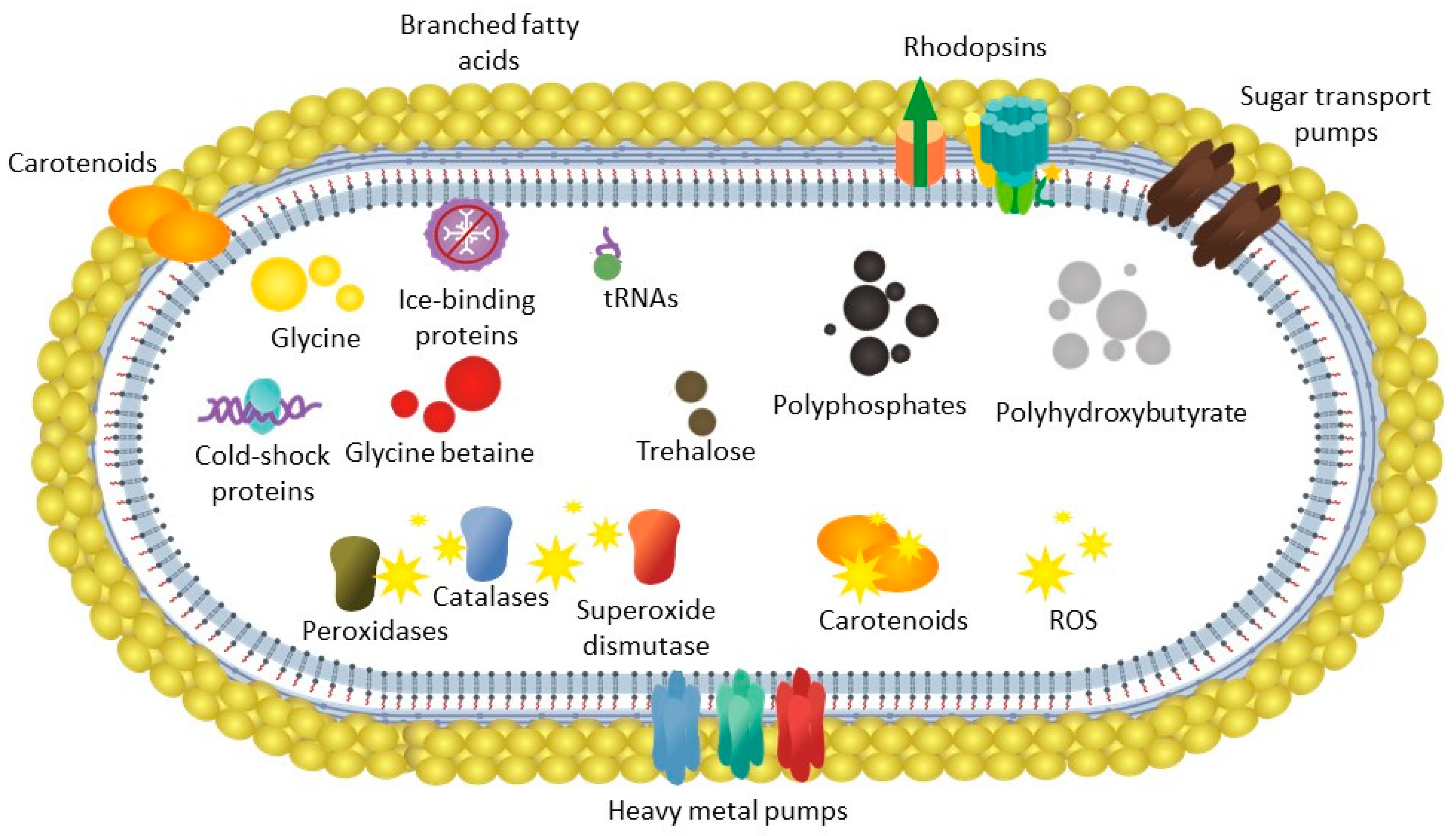
3.3. Cold stress Adaptation of Subtercola Vilae DB165T
3.3.1. Cryoprotectants
3.3.2. Temperature Shifts
3.3.3. Oxidative Stress
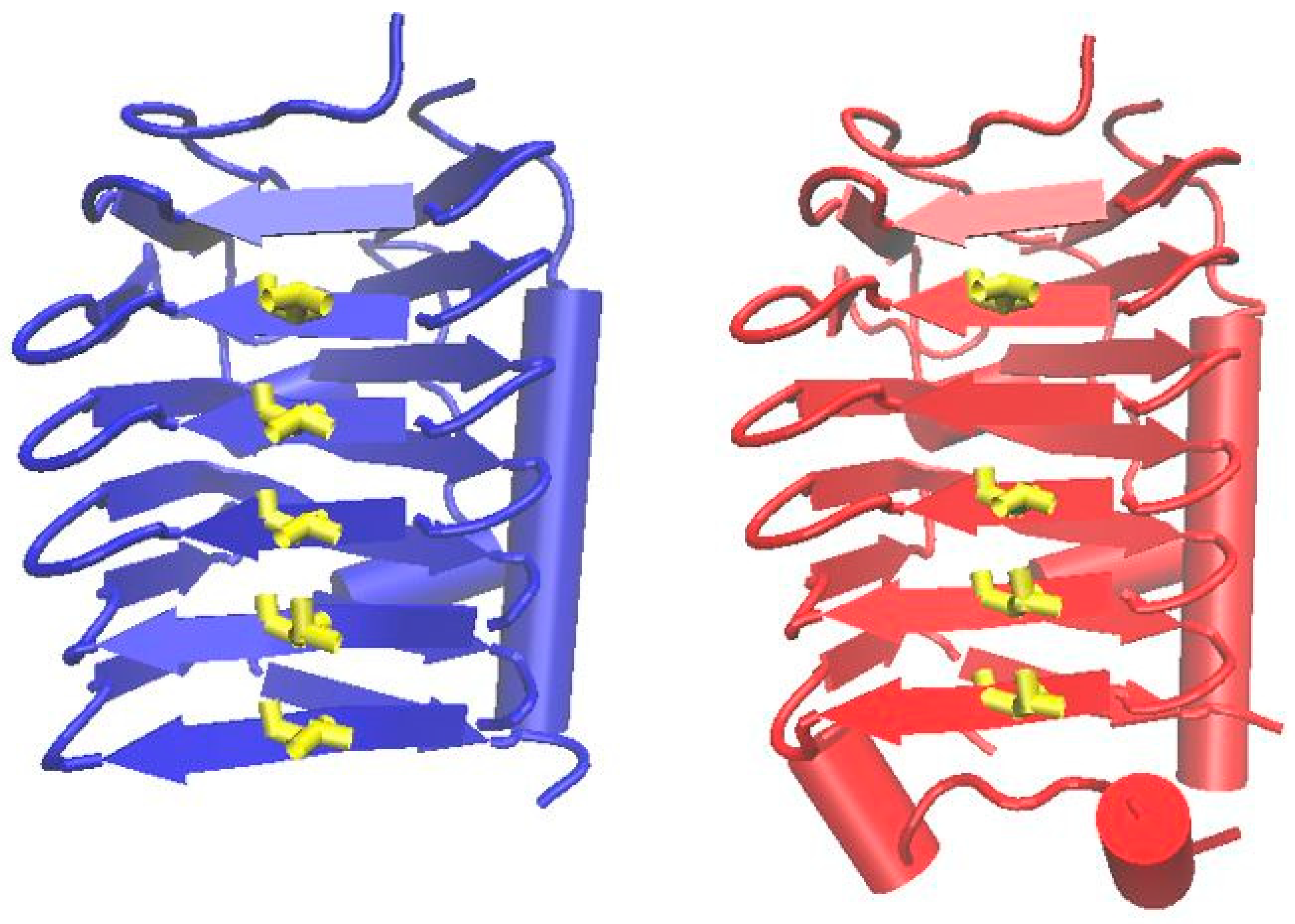
3.3.4. Ice-binding Proteins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evtushenko, L.I.; Family, X.I. Microbacteriaceae. In Bergey’s Manual of Systematic Bacteriology; Whitman, W., Goodfellow, M., Kämpfer, P., Busse, H.J., Trujillo, M., Ludwig, W., Suzuki, K.I., Parte, A., Eds.; Springer: Berlin, Germany, 2012; Volume 5, pp. 807–994. [Google Scholar]
- Männisto, M.K.; Schumann, P.; Rainey, F.A.; Kampfer, P.; Tsitko, I.; Tiirola, M.A.; Salkinoja-Salonen, M.S. Subtercola boreus gen. nov., sp. nov. and Subtercola frigoramans sp. nov., two new psychrophilic actinobacteria isolated from boreal groundwater. Int. J. Syst. Evol. Microbiol. 2000, 50, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, A.S.; Wiese, J.; Aguilar, P.; Dorador, C.; Imhoff, J.F. Subtercola vilae sp. nov., a novel actinobacterium from an extremely high-altitude cold volcano lake in Chile. Antonie Leeuwenhoek 2018, 111, 955–963. [Google Scholar] [CrossRef]
- Si, H.; Shi, F.; Zhang, L.; Yue, H.; Wang, H.; Zhao, Z. Subtercola lobariae sp. nov., an actinobacterium of the family Microbacteriaceae isolated from the lichen Lobaria retigera. Int. J. Syst. Evol. Microbiol. 2017, 67, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Vaïtilingom, M.; Attard, E.; Gaiani, N.; Sancelme, M.; Deguillaume, L.; Flossmann, A.I.; Amato, P.; Delort, A.M. Long-term features of cloud microbiology at the puy de Dôme (France). Atmos. Environ. 2012, 56, 88–100. [Google Scholar] [CrossRef]
- Peeters, K.; Ertz, D.; Willems, A. Culturable bacterial diversity at the Princess Elisabeth Station (Utsteinen, Sør Rondane Mountains, East Antarctica) harbours many new taxa. Syst. Appl. Microbiol. 2011, 34, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, U.; Ulrich, A.; Schumann, P.; Naumann, D.; Suzuki, K. Diversity of grass-associated Microbacteriaceae isolated from the phyllosphere and litter layer after mulching the sward; polyphasic characterization of Subtercola pratensis sp. nov., Curtobacterium herbarum sp. nov. and Plantibacter flavus gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1441–1454. [Google Scholar]
- Evtushenko, L.I.; Dorofeeva, L.V.; Dobrovolskaya, T.G.; Streshinskaya, G.M.; Subbotin, S.A.; Tiedje, J.M. Agreia bicolorata gen. nov., sp. nov., to accommodate actinobacteria isolated from narrow reed grass infected by the nematode Heteroanguina graminophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2073–2079. [Google Scholar] [CrossRef]
- Lynch, R.C.; King, A.J.; Farías, M.E.; Sowell, P.; Vitry, C.; Schmidt, S.K. The potential for microbial life in the highest-elevation (>6000 m.a.s.l.) mineral soils of the Atacama region. J. Geophys. Res. Biogeosci. 2012, 117, 1–10. [Google Scholar] [CrossRef]
- Lynch, R.C.; Darcy, J.L.; Kane, N.C.; Nemergut, D.R.; Schmidt, S.K. Metagenomic evidence for metabolism of trace atmospheric gases by high-elevation desert actinobacteria. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Rasuk, M.C.; Ferrer, G.M.; Kurth, D.; Portero, L.R.; Farías, M.E.; Albarracín, V.H. UV-resistant Actinobacteria from high-altitude Andean lakes: Isolation, characterization and antagonistic activities. Photochem. Photobiol. 2017, 93, 865–880. [Google Scholar] [CrossRef]
- Goodfellow, M.; Fiedler, H.P. A guide to successful bioprospecting: Informed by actinobacterial systematics. Antonie Leeuwenhoek 2010, 98, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013. [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Müller, R.; Wohlleben, W.; et al. AntiSMASH 3.0-A comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Pruesse, E.; Peplies, J.; Glöckner, F.O. SINA: Accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 2012, 28, 1823–1829. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Ankenbrand, M.J.; Keller, A. bcgTree: Automatized phylogenetic tree building from bacterial core genomes. Genome 2016, 59, 783–791. [Google Scholar] [CrossRef]
- Magrane, M.; UniProt Consortium. UniProt Knowledgebase: A hub of integrated protein data. Database 2011. [Google Scholar] [CrossRef]
- Kelly, L.A.; Mezulis, S.; Yates, C.; Wass, M.; Sternberg, M. The Phyre2 web portal for protein modelling, prediction, and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Tal, S.; Okon, Y. Production of the reserve material poly-ß-hydroxybutyrate and its function in Azospirillum brasilense Cd. Can. J. Microbiol. 1985, 31, 608–613. [Google Scholar] [CrossRef]
- Kadouri, D.; Jurkevitch, E.; Okon, Y. Involvement of the reserve material poly-β-hydroxybutyrate in Azospirillum brasilense stress endurance and root colonization. Appl. Environ. Microbiol. 2003, 69, 3244–3250. [Google Scholar] [CrossRef]
- Seufferheld, M.J.; Alvarez, H.M.; Farias, M.E. Role of polyphosphates in microbial adaptation to extreme environments. Appl. Environ. Microbiol. 2008, 74, 5867–5874. [Google Scholar] [CrossRef]
- Lanyi, J.K.; Balashov, S.P. Xanthorhodopsin: A bacteriorhodopsin-like proton pump with a carotenoid antenna. Biochim. Biophys. Acta 2008, 1777, 684–688. [Google Scholar] [CrossRef]
- Ernst, O.P.; Lodowski, D.T.; Elstner, M.; Hegemann, P.; Brown, L.S.; Kandori, H. Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chem. Rev. 2014, 114, 126–163. [Google Scholar] [CrossRef]
- Kozubek, A.; Tyman, J.H.P. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1998, 99, 1–26. [Google Scholar] [CrossRef]
- Kanda, N.; Ishizaki, N.; Inoue, N.; Oshima, M.; Handa, A.; Kitahara, T. DB-2073, a new alkylresorcinol antibiotic. I. Taxonomy, isolation and characterization. J. Antibiot. 1975, 28, 935–942. [Google Scholar] [CrossRef][Green Version]
- Chattopadhyay, M.K. Mechanism of bacterial adaptation to low temperature. J. Biosci. 2006, 31, 157–165. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Rocha, E.P.C. Codon usage bias from tRNA’s point of view: Redundancy, specialization, and efficient decoding for translation optimization. Genome Res. 2004, 14, 2279–2286. [Google Scholar] [CrossRef]
- Math, R.K.; Jin, H.M.; Kim, J.M.; Hahn, Y.; Park, W.; Madsen, E.L.; Jeon, C.O. Comparative genomics reveals adaptation by Alteromonas sp. SN2 to marine tidal-flat conditions: Cold tolerance and aromatic hydrocarbon metabolism. PLoS ONE 2012, 7, e35784. [Google Scholar] [CrossRef]
- Le Rudulier, D.; Strom, A.R.; Dandekar, A.M.; Smith, L.T.; Valentine, R.C. Molecular biology of osmoregulation. Science 1984, 224, 1064–1068. [Google Scholar] [CrossRef]
- Kandror, O.; DeLeon, A.; Goldberg, A.L. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 2002, 99, 9727–9732. [Google Scholar] [CrossRef]
- Phadtare, S.; Alsina, J.; Inouye, M. Cold-shock response and cold-shock proteins. Curr. Opin. Microbiol. 1999, 2, 175–180. [Google Scholar] [CrossRef]
- Chaikam, V.; Karlson, D.T. Comparison of structure, function and regulation of plant cold shock domain proteins to bacterial and animal cold shock domain proteins. BMB Rep. 2010, 43, 1–8. [Google Scholar] [CrossRef]
- Schumann, W. Regulation of bacterial heat shock stimulons. Cell Stress Chaperones 2016, 21, 959–968. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, S.; Collins, T.; Marx, J.C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Leinala, E.K.; Davies, P.L.; Jia, Z. Crystal structure of β-Helical antifreeze protein points to a general ice binding model. Structure 2002, 10, 619–627. [Google Scholar] [CrossRef]
- Imhoff, J.F.; Rodriguez-Valera, F. Betaine is the main compatible solute of halophilic eubacteria. J. Bacteriol. 1984, 160, 478–479. [Google Scholar] [PubMed]
- Imhoff, J.F. Minireview—True marine and halophilic anoxygenic phototrophic bacteria. Arch. Microbiol. 2001, 176, 243–254. [Google Scholar] [CrossRef]
- Imhoff, J.F. Osmoregulation and compatible solutes in eubacteria. FEMS Microbiol. Rev. 1986, 39, 57–66. [Google Scholar] [CrossRef]
| Attribute | Value | Percentage of total |
|---|---|---|
| Genomes Size (bp) | 4,043,135 | 100 |
| Contigs | 103 | |
| N50 | 87,665 | |
| DNA G+C content | 65.1 | |
| Total of genes | 3879 | 100 |
| Coding sequences | 3797 | 97.8 |
| Genes with function prediction | 2434 | 62.7 |
| Genes assigned to COGs | 1416 | 36.5 |
| RNA genes | 82 | 2.11 |
| rRNA genes | 5 | 0.1 |
| Pseudo genes | 0 | 0 |
| 5S rRNA | 3 | 0.07 |
| 16S rRNA | 1 | 0.02 |
| 23S rRNA | 1 | 0.02 |
| tRNA | 59 | 1.5 |
| Other RNA | 18 | 0.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos, A.S.; Wiese, J.; Imhoff, J.F.; Dorador, C.; Keller, A.; Hentschel, U. Systematic Affiliation and Genome Analysis of Subtercola vilae DB165T with Particular Emphasis on Cold Adaptation of an Isolate from a High-Altitude Cold Volcano Lake. Microorganisms 2019, 7, 107. https://doi.org/10.3390/microorganisms7040107
Villalobos AS, Wiese J, Imhoff JF, Dorador C, Keller A, Hentschel U. Systematic Affiliation and Genome Analysis of Subtercola vilae DB165T with Particular Emphasis on Cold Adaptation of an Isolate from a High-Altitude Cold Volcano Lake. Microorganisms. 2019; 7(4):107. https://doi.org/10.3390/microorganisms7040107
Chicago/Turabian StyleVillalobos, Alvaro S., Jutta Wiese, Johannes F. Imhoff, Cristina Dorador, Alexander Keller, and Ute Hentschel. 2019. "Systematic Affiliation and Genome Analysis of Subtercola vilae DB165T with Particular Emphasis on Cold Adaptation of an Isolate from a High-Altitude Cold Volcano Lake" Microorganisms 7, no. 4: 107. https://doi.org/10.3390/microorganisms7040107
APA StyleVillalobos, A. S., Wiese, J., Imhoff, J. F., Dorador, C., Keller, A., & Hentschel, U. (2019). Systematic Affiliation and Genome Analysis of Subtercola vilae DB165T with Particular Emphasis on Cold Adaptation of an Isolate from a High-Altitude Cold Volcano Lake. Microorganisms, 7(4), 107. https://doi.org/10.3390/microorganisms7040107







