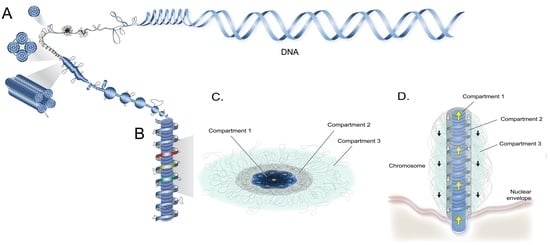
Architectural Organization of Dinoflagellate Liquid Crystalline Chromosomes
Abstract
1. Introduction
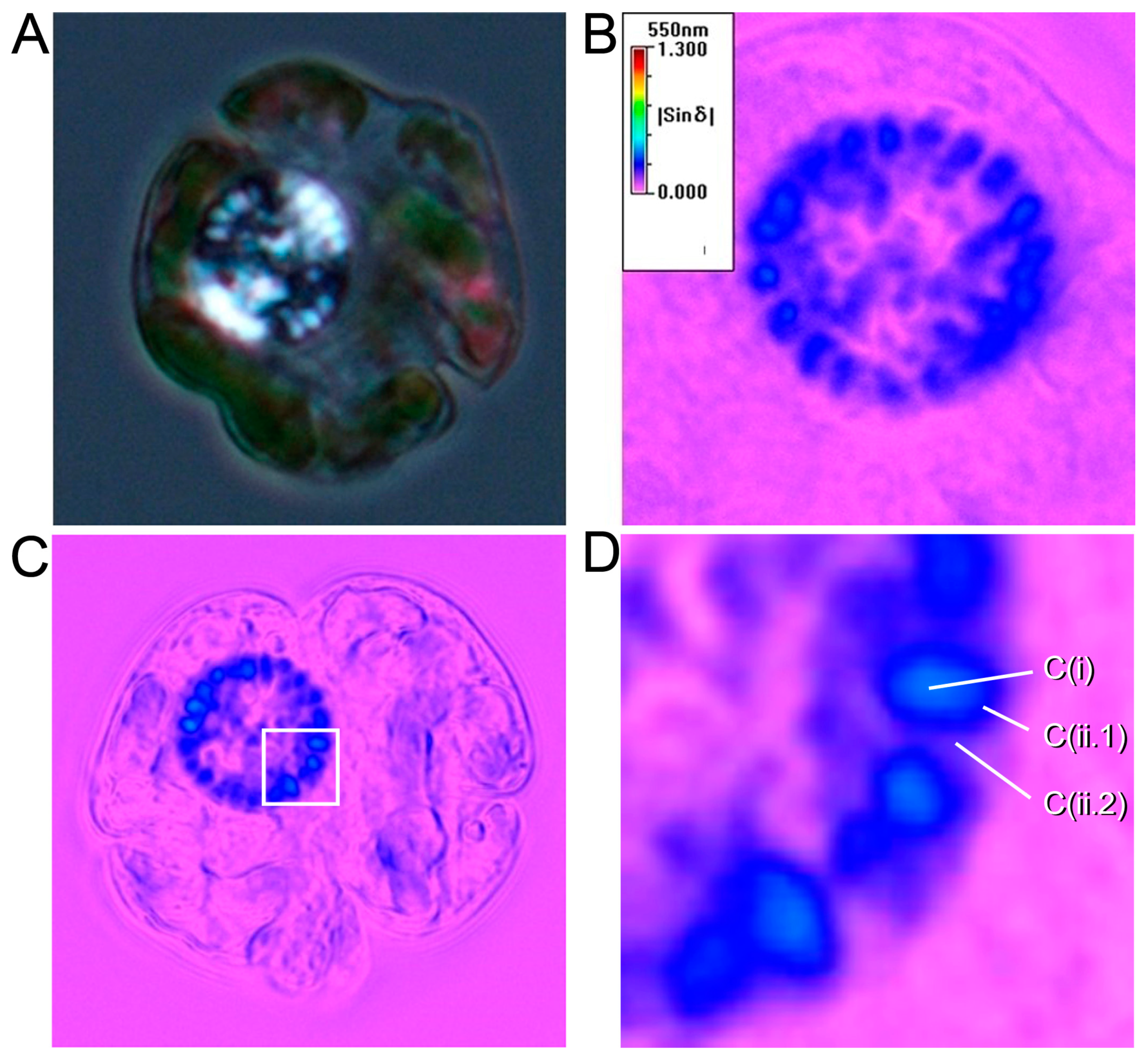
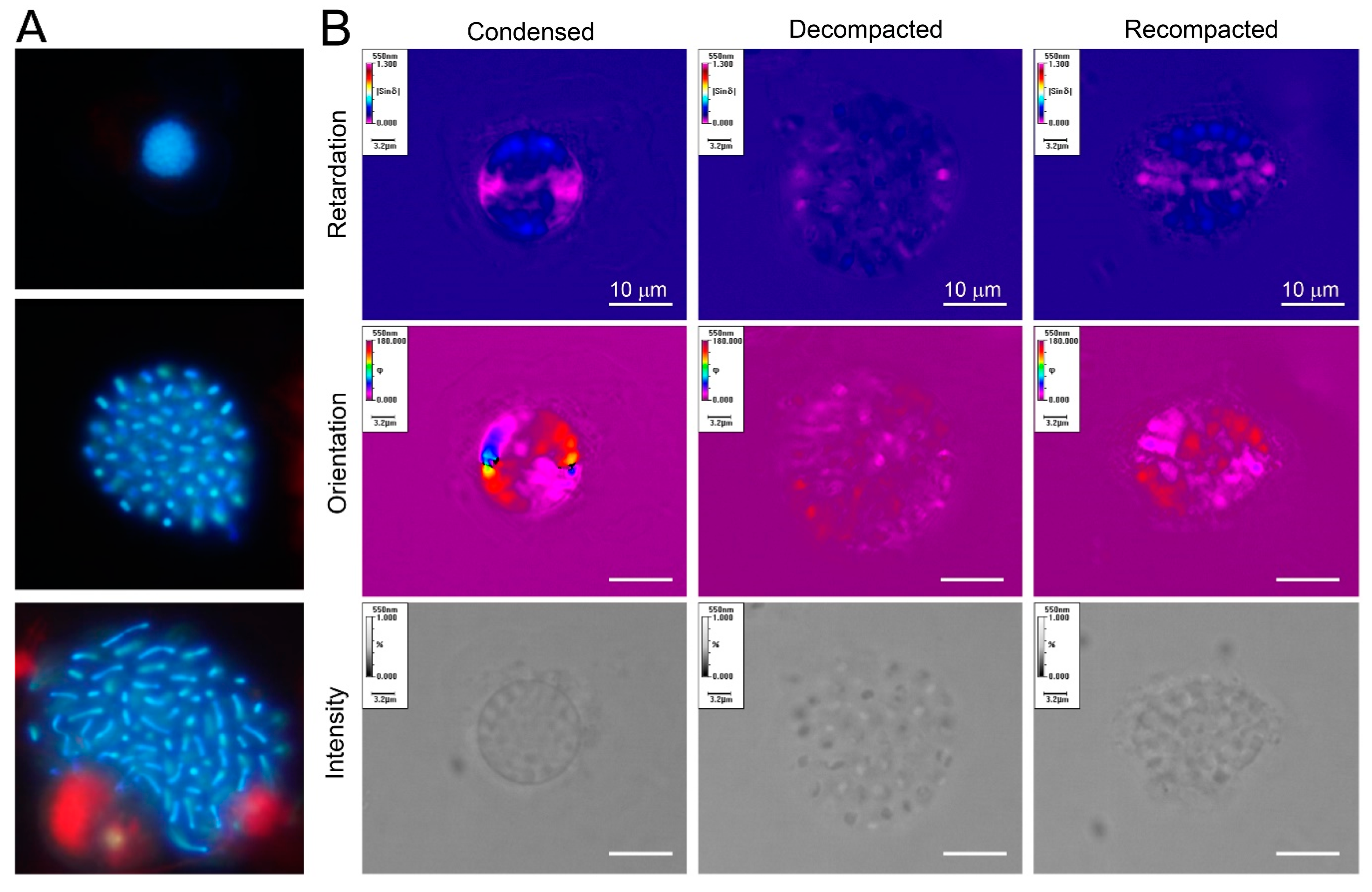
2. Superhelical Plectonemic Modules (SPMs) and Compartmentation
3. Physical Genomic Karyotype (PGK) of Liquid Crystalline Chromosomes
4. 5-Hydroxymethyluracil and Z-DNA
5. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | Anisotropic Cluster Formation |
| ChoM | Cholesteric Mesophase |
| CH-M | Columnar-Hexagonal Mesophase |
| CSLCM | Compartmental Superhelical Liquid Crystalline Model |
| đ | interaxial space between DNA, or interstitial space between DNA in lc-mesophases |
| PGK | Physical Genomic Karyotypes |
| 5hmu | 5-hydroxymethyluracil |
| BTIL | Biphasic Transitions Between Isotropic and Liquid Crystalline Phases |
| lc | liquid crystalline |
| LCCs | Liquid Crystalline Chromosomes |
| Nc | Nucleosomal |
| NE | Nuclear Envelope |
| Nl | Nuclear Lamina |
| ƥ | Helical Pitch of DNA Double Helix |
| Ƥ | Helical Pitch of Liquid Crystalline Phases |
| PCL | Peripheral Chromosomal Loop |
| SPMs | Superhelical Plectonemic Modules |
| cwaters | structural waters |
References
- Cachon, J.; Sato, H.; Cachon, M.; Sato, Y. Analysis by polarzing microscopy of chromosomal structure among dinoflagellates and its phylogenetic involvement. Biol. Cell 1989, 65, 51–60. [Google Scholar] [CrossRef]
- Chow, M.H.; Yan, K.T.H.; Bennett, M.J.; Wong, J.T.Y. Birefringence and DNA Condensation of Liquid Crystalline Chromosomes. Eukaryot. Cell 2010, 9, 1577–1587. [Google Scholar] [CrossRef]
- Bodansky, S.; Mintz, L.B.; Holmes, D.S. The mesokaryote Gyrodinium cohnii lacks nucleosomes. Biochem. Biophys. Res. Commun. 1979, 88, 1329–1336. [Google Scholar] [CrossRef]
- Herzog, M.; Soyer, M.O. Distinctive features of dinoflagellate chromatin. Absence of nucleosomes in a primitive species Prorocentrum micans E. Eur. J. Cell Biol. 1981, 23, 295–302. [Google Scholar]
- Shupe, K.; Rizzo, P.J. Nuclease Digestion of Chromatin from the Eukaryotic Algae Olisthodiscus luteus, Peridinium balticum, and Crypthecodinium cohnii. J. Protozool. 1983, 30, 599–606. [Google Scholar] [CrossRef]
- Rizzo, P.J.; Nooden, L.D. Isolation and chemical composition of dinoflagellate nuclei. J. Protozool. 1973, 20, 666–672. [Google Scholar] [CrossRef]
- Hou, Y.; Lin, S. Distinct Gene Number-Genome Size Relationships for Eukaryotes and Non-Eukaryotes: Gene Content Estimation for Dinoflagellate Genomes. PLoS ONE 2009, 4, e6978. [Google Scholar] [CrossRef] [PubMed]
- Haapala, O.K.; Soyer, M.O. Structure of dinoflagellate chromosomes. Nat. New Biol. 1973, 244, 195–197. [Google Scholar] [CrossRef]
- Oakley, B.R.; Dodge, J.D. Evidence for a double-helically coiled toroidal chromonema in the dinoflagellate chromosome. Chromosoma 1979, 70, 277–291. [Google Scholar] [CrossRef]
- Livolant, F.; Bouligand, Y. Double helical arrangement of spread dinoflagellate chromosomes. Chromosoma 1980, 80, 97–118. [Google Scholar] [CrossRef]
- Sun, S.; Liu, M.; Dai, Q.; Dong, F.; Liu, L.; Huo, T. The analysis of microscopy imaging on liquid crystalline components of the cell nucleus. J. Biomed. Sci. Eng. 2012, 5, 307–314. [Google Scholar] [CrossRef]
- Geday, M.A.; Kaminsky, W.; Lewis, J.G.; Glazer, A.M. Images of absolute retardance L.Deltan, using the rotating polariser method. J. Microsc. 2000, 198, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Glazer, A.M.; Lewis, J.G.; Kaminsky, W. An automatic optical imaging system for birefringent media. Proc. R. Soc. Lond. A Math. Phys. Sci. 1996, 452, 2751–2765. [Google Scholar] [CrossRef]
- Sun, S.; Wong, J.T.; Liu, M.; Dong, F. Counterion-mediated decompaction of liquid crystalline chromosomes. DNA Cell Biol. 2012, 31, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Strick, R.; Strissel, P.L.; Gavrilov, K.; Levi-Setti, R. Cation-chromatin binding as shown by ion microscopy is essential for the structural integrity of chromosomes. J. Cell Biol. 2001, 155, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Levi-Setti, R.; Gavrilov, K.L.; Rizzo, P.J. Divalent cation distribution in dinoflagellate chromosomes imaged by high-resolution ion probe mass spectrometry. Eur. J. Cell Biol. 2008, 87, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Heggeler-Bordier, B.; Wahli, W.; Stasiak, A.Z.; Stasiak, A.; Dubochet, J. Direct visualization of supercoiled DNA molecules in solution. EMBO J. 1990, 9, 4551–4554. [Google Scholar] [CrossRef]
- Zinchenko, A.; Berezhnoy, N.V.; Wang, S.; Rosencrans, W.M.; Korolev, N.; van der Maarel, J.R.C.; Nordenskiöld, L. Single-molecule compaction of megabase-long chromatin molecules by multivalent cations. Nucleic Acids Res. 2018, 46, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.K.; Denton, A.R. Influence of polymer shape on depletion potentials and crowding in colloid-polymer mixtures. Soft Matter 2016, 12, 2247–2252. [Google Scholar] [CrossRef]
- Strick, T.; Allemand, J.-F.; Croquette, V.; Bensimon, D. Twisting and stretching single DNA molecules. Prog. Biophys. Mol. Biol. 2000, 74, 115–140. [Google Scholar] [CrossRef]
- Zakharova, S.S.; Jesse, W.; Backendorf, C.; Egelhaaf, S.U.; Lapp, A.; van der Maarel, J.R.C. Dimensions of Plectonemically Supercoiled DNA. Biophys. J. 2002, 83, 1106–1118. [Google Scholar] [CrossRef]
- Zakharova, S.S.; Jesse, W.; Backendorf, C.; van der Maarel, J.R.C. Liquid Crystal Formation in Supercoiled DNA Solutions. Biophys. J. 2002, 83, 1119–1129. [Google Scholar] [CrossRef]
- Ziv, R.; Levin-Zaidman, S.; Gutman, S.B.; Arad, T.; Minsky, A. Supercoiling-Regulated Liquid-Crystalline Packaging of Topologically-Constrained, Nucleosome-Free DNA Molecules. Biochemistry 2002, 33, 14177–14184. [Google Scholar] [CrossRef]
- Reich, Z.; Wachtel, E.J.; Minsky, A. Liquid-crystalline mesophases of plasmid DNA in bacteria. Science 1994, 264, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, P.J.; Nooden, L.D. Isolation and partial characterization of dinoflagellate chromatin. Biochim. Biophys. Acta 1974, 349, 402–414. [Google Scholar] [CrossRef]
- Sigee, D.C. Structural DNA and genetically active DNA in dinoflagellate chromosomes. Biosystems 1983, 16, 203–210. [Google Scholar] [CrossRef]
- Babillot, C. Etude de’incorporation d’uridine-H3 dans Ie noyau chez l’Amphidinium carteri, Dinoflagelle. C. R. Acad Sci. (Paris) 1970, 271, 828–831. [Google Scholar]
- Livolant, F.; Maestre, M.F. Circular dichroism microscopy of compact forms of DNA and chromatin in vivo and in vitro: Cholesteric liquid-crystalline phases of DNA and single dinoflagellate nuclei. Biochemistry 1988, 27, 3056–3068. [Google Scholar] [CrossRef] [PubMed]
- Livolant, F.; Levelut, A.M.; Doucet, J.; Benoit, J.P. The highly concentrated liquid-crystalline phase of DNA is columnar hexagonal. Nature 1989, 339, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Grabher, A.; Herzog, M. Separation of coding sequences from structural DNA in the dinoflagellate Crypthecodinium cohnii. Mol. Mar. Biol. Biotechnol. 2002, 1, 89–96. [Google Scholar]
- Sun, S. Assembly of Liquid Crystalline Chromosomes. Ph.D. Thesis, Nanoscience and Naotechnology Program. Hong Kong University of Science and Technology, Hong Kong, China, 2010. [Google Scholar]
- Kellenberger, E.; Johansen, R.; Maeder, M.; Bohrmann, B.; Stauffer, E.; Villiger, W. Artefacts and morphological changes during chemical fixation. J. Microsc. 1992, 168, 181–201. [Google Scholar] [CrossRef] [PubMed]
- Grassé, P.P.; Dragesco, J. L’ultrastructure du chromosome des péridiens et ses conséquences génétiques. C. R. Hebd. Seances Acad. Sci. 1957, 245, 2447–2452. [Google Scholar] [PubMed]
- Gautier, A.; Michel-Salamin, L.; Tosi-Couture, E.; McDowall, A.W.; Dubochet, J. Electron microscopy of the chromosomes of dinoflagellates in situ: Confirmation of Bouligand’s liquid crystal hypothesis. J Ultrastruct. Mol. Struct. Res. 1986, 97, 10–30. [Google Scholar] [CrossRef]
- Fang, Y.; Hoh, J.H.; Spisz, T.S. Ethanol-induced structural transitions of DNA on mica. Nucleic Acids Res. 1999, 27, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, P.J. Biochemistry of the dinoflagellate nucleus. In The Biology of the Dinoflagellates; Taylor, F.J.R., Ed.; Blackwell Scientific Publications: Oxford, UK; London, UK; Edinburgh, UK; Boston, MA, USA; Palo Alto, CA, USA; Melbourne, Australian, 1987; pp. 143–173. [Google Scholar]
- Golyshev, S.; Berdieva, M.; Musinova, Y.; Sheval1, E.; Skarlato, S. Ultrastructural organization of the chromatin elements in chromosomes of the dinoflagellate Prorocentrum minimum. Protistology 2018, 12, 163–172. [Google Scholar]
- Roy, S.; Morse, D. Transcription and Maturation of mRNA in Dinoflagellates. Microorganisms 2013, 1, 71–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hou, Y.; Miranda, L.; Campbell, D.A.; Sturm, N.R.; Gaasterland, T.; Lin, S. Spliced leader RNA trans-splicing in dinoflagellates. Proc. Natl. Acad. Sci. USA 2007, 104, 4618–4623. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, S.; Song, B.; Zhong, X.; Lin, X.; Li, W.; Li, L.; Zhang, Y.; Zhang, H.; Ji, Z.; et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science 2015, 350, 691–694. [Google Scholar] [CrossRef]
- Shoguchi, E.; Shinzato, C.; Kawashima, T.; Gyoja, F.; Mungpakdee, S.; Koyanagi, R.; Takeuchi, T.; Hisata, K.; Tanaka, M.; Fujiwara, M.; et al. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr. Biol. 2013, 23, 1399–1408. [Google Scholar] [CrossRef]
- Song, B.; Morse, D.; Song, Y.; Fu, Y.; Lin, X.; Wang, W.; Cheng, S.; Chen, W.; Liu, X.; Lin, S. Comparative genomics reveals two major bouts of gene retroposition coinciding with crucial periods of Symbiodinium evolution. Genome Biol. Evolut. 2017, 9, 2037–2047. [Google Scholar] [CrossRef]
- Song, B.; Chen, S.; Chen, W. Dinoflagellates, a Unique Lineage for Retrogene Research. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Polymenidou, M. The RNA face of phase separation. Science 2018, 360, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Maharana, S.; Wang, J.; Papadopoulos, D.K.; Richter, D.; Pozniakovsky, A.; Poser, I.; Bickle, M.; Rizk, S.; Guillén-Boixet, J.; Franzmann, T.M.; et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 2018, 360, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Langdon, E.M.; Qiu, Y.; Ghanbari Niaki, A.; McLaughlin, G.A.; Weidmann, C.A.; Gerbich, T.M.; Smith, J.A.; Crutchley, J.M.; Termini, C.M.; Weeks, K.M.; et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science 2018, 360, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, M.; Roy, S.; Daoust, P.; Dagenais-Bellefeuille, S.; Bertomeu, T.; Letourneau, L.; Lang, B.F.; Morse, D. Dinoflagellate tandem array gene transcripts are highly conserved and not polycistronic. Proc. Natl. Acad. Sci. USA 2012, 109, 15793–15798. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Murchie, A.I.H.; Lilley, D.M.J. RNA bulges and the helical periodicity of double-stranded RNA. Nature 1990, 343, 484. [Google Scholar] [CrossRef]
- Ma, C.; Bloomfield, V.A. Condensation of supercoiled DNA induced by MnCl2. Biophys. J. 1994, 67, 1678–1681. [Google Scholar] [CrossRef]
- Herzog, M.; Soyer, M.O.; Daney de Marcillac, G. A high level of thymine replacement by 5-hydroxymethyluracil in nuclear DNA of the primitive dinoflagellate Prorocentrum micans E. Eur. J. Cell Biol. 1982, 27, 151–155. [Google Scholar]
- Rae, P.M. 5-Hydroxymethyluracil in the DNA of a dinoflagellate. Proc. Natl. Acad. Sci. USA 1973, 70, 1141–1145. [Google Scholar] [CrossRef]
- Rae, P.M.; Steele, R.E. Modified bases in the DNAs of unicellular eukaryotes: An examination of distributions and possible roles, with emphasis on hydroxymethyluracil in dinoflagellates. Biosystems 1978, 10, 37–53. [Google Scholar] [CrossRef]
- Vu, H.M.; Pepe, A.; Mayol, L.; Kearns, D.R. NMR-derived solution structure of a 17mer hydroxymethyluracil-containing DNA. Nucleic Acids Res. 1999, 27, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, L.B.; Bramham, J.; Mayol, L.; Galeone, A.; Jia, X.; Kearns, D.R. 1 H NMR Studies of the 5-(Hydroxymethyl)-2′-Deoxyuridine Containing TF1 Binding Site. Nucleic Acids Res. 1996, 24, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.; Jakobson, K.S.; Norby, Ø. Characterization of DNA from the dinoflagellate Woloszynskia bostoniensis1. J. Protozool. 1988, 35, 418–422. [Google Scholar] [CrossRef]
- Fuller, W.; Forsyth, T.; Mahendrasingam, A. Water-DNA interactions as studied by X-ray and neutron fibre diffraction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1237–1247; discussion 1247–1238. [Google Scholar] [CrossRef]
- Scipioni, C.; Anselmi, C.; Zuccheri, G.; Samori, B.; Santis, P. De Sequence-Dependent DNA Curvature and Flexibility from Scanning Force Microscopy Images. Biophys. J. 2002, 83, 2408–2418. [Google Scholar] [CrossRef]
- Kawasaki, F.; Beraldi, D.; Hardisty, R.E.; McInroy, G.R.; van Delft, P.; Balasubramanian, S. Genome-wide mapping of 5-hydroxymethyluracil in the eukaryote parasite Leishmania. Genome Biol. 2017, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Van de Sande, J.H.; McIntosh, L.P.; Jovin, T.M. Mn2+ and other transition metals at low concentration induce the right-to-left helical transformation of poly[d(G-C)]. EMBO J. 1982, 1, 777–782. [Google Scholar] [CrossRef]
- Zacharias, W.; Jaworski, A.; Wells, R.D. Cytosine methylation enhances Z-DNA formation in vivo. J. Bacteriol. 1990, 172, 3278–3283. [Google Scholar] [CrossRef]
- Soyer-Gobillard, M.O.; Geraud, M.L.; Coulaud, D.; Barray, M.; Theveny, B.; Revet, B.; Delain, E. Location of B- and Z-DNA in the chromosomes of a primitive eukaryote dinoflagellate. J. Cell Biol. 1990, 111, 293–304. [Google Scholar] [CrossRef]
- Ha, S.C.; Lowenhaupt, K.; Rich, A.; Kim, Y.-G.; Kim, K.K. Crystal structure of a junction between B-DNA and Z-DNA reveals two extruded bases. Nature 2005, 437, 1183. [Google Scholar] [CrossRef]
- de Rosa, M.; de Sanctis, D.; Rosario, A.L.; Archer, M.; Rich, A.; Athanasiadis, A.; Carrondo, M.A. Crystal structure of a junction between two Z-DNA helices Proc. Natl. Acad. Sci. USA 2010, 107, 9088–9092. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, J.T.Y. Architectural Organization of Dinoflagellate Liquid Crystalline Chromosomes. Microorganisms 2019, 7, 27. https://doi.org/10.3390/microorganisms7020027
Wong JTY. Architectural Organization of Dinoflagellate Liquid Crystalline Chromosomes. Microorganisms. 2019; 7(2):27. https://doi.org/10.3390/microorganisms7020027
Chicago/Turabian StyleWong, Joseph Tin Yum. 2019. "Architectural Organization of Dinoflagellate Liquid Crystalline Chromosomes" Microorganisms 7, no. 2: 27. https://doi.org/10.3390/microorganisms7020027
APA StyleWong, J. T. Y. (2019). Architectural Organization of Dinoflagellate Liquid Crystalline Chromosomes. Microorganisms, 7(2), 27. https://doi.org/10.3390/microorganisms7020027