Chlamydia trachomatis Whole-Proteome Microarray Analysis of The Netherlands Chlamydia Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
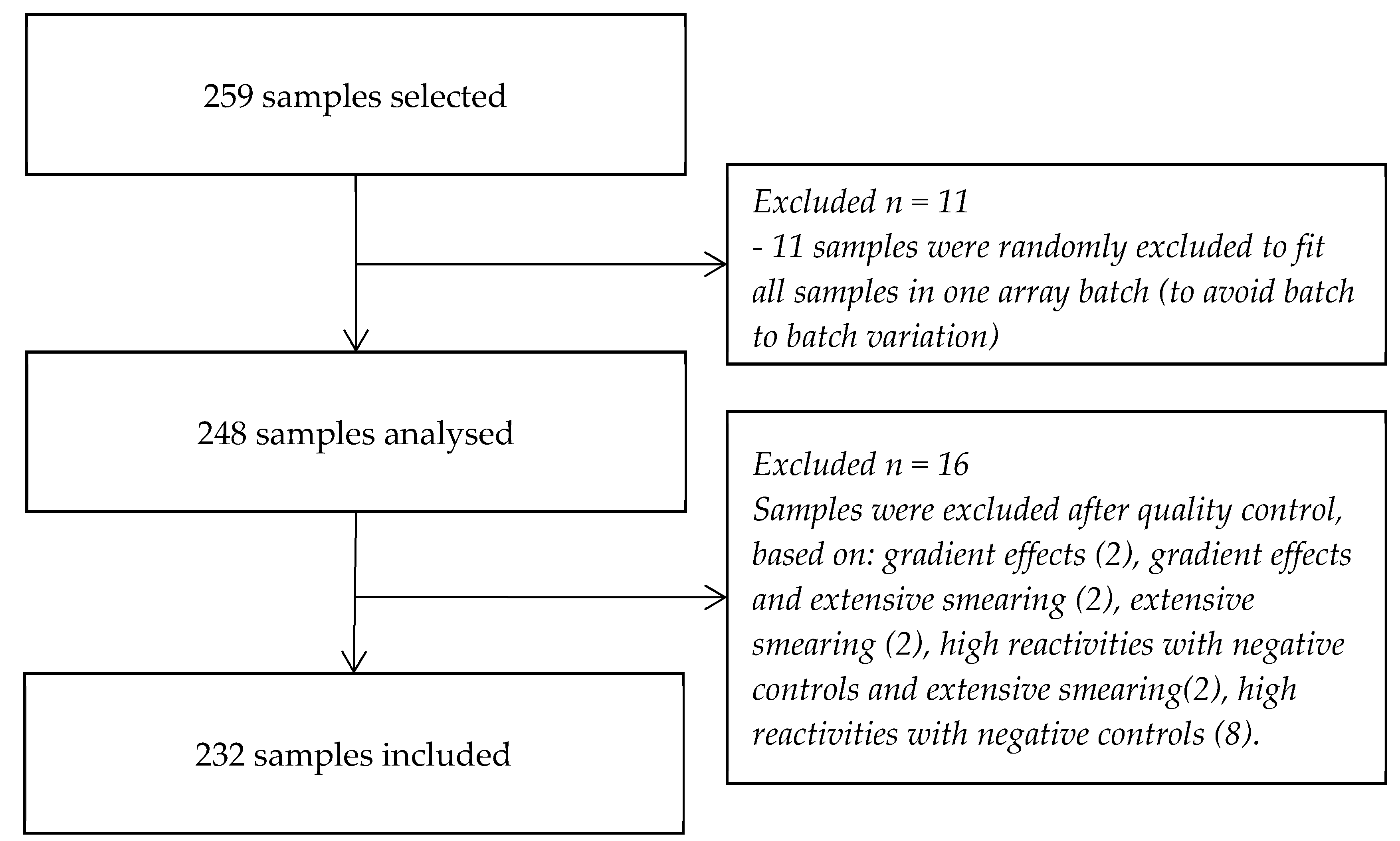
2.2. Sample Selection
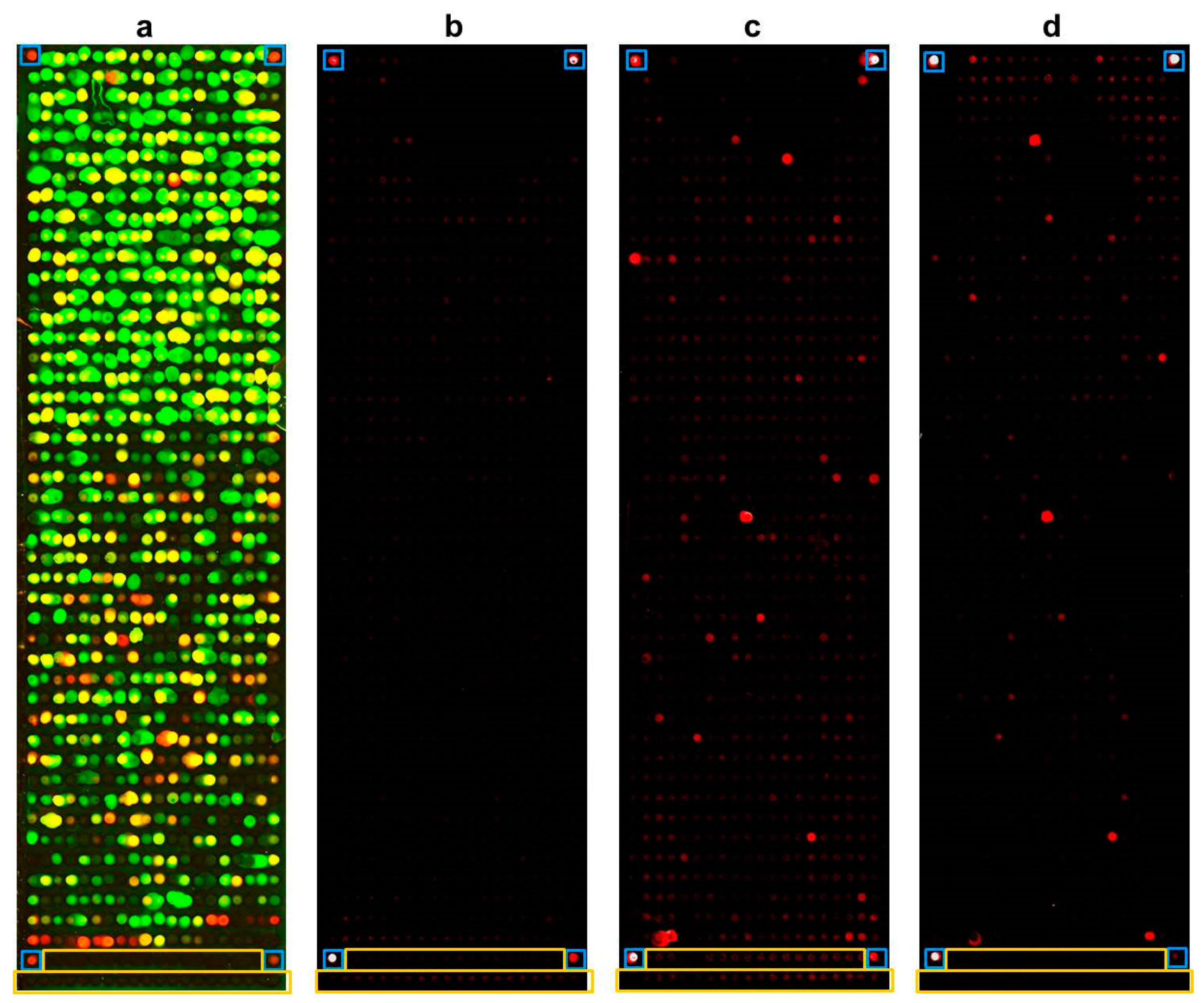
2.3. Whole-Proteome Microarray
- (i)
- gradient effects (e.g., loss of reactivity from top to bottom of a slide);
- (ii)
- smearing of spots (due to e.g., insufficient washing);
- (iii)
- disproportional high readouts for all negative controls (leading to the detection of false negative readouts of antigens).
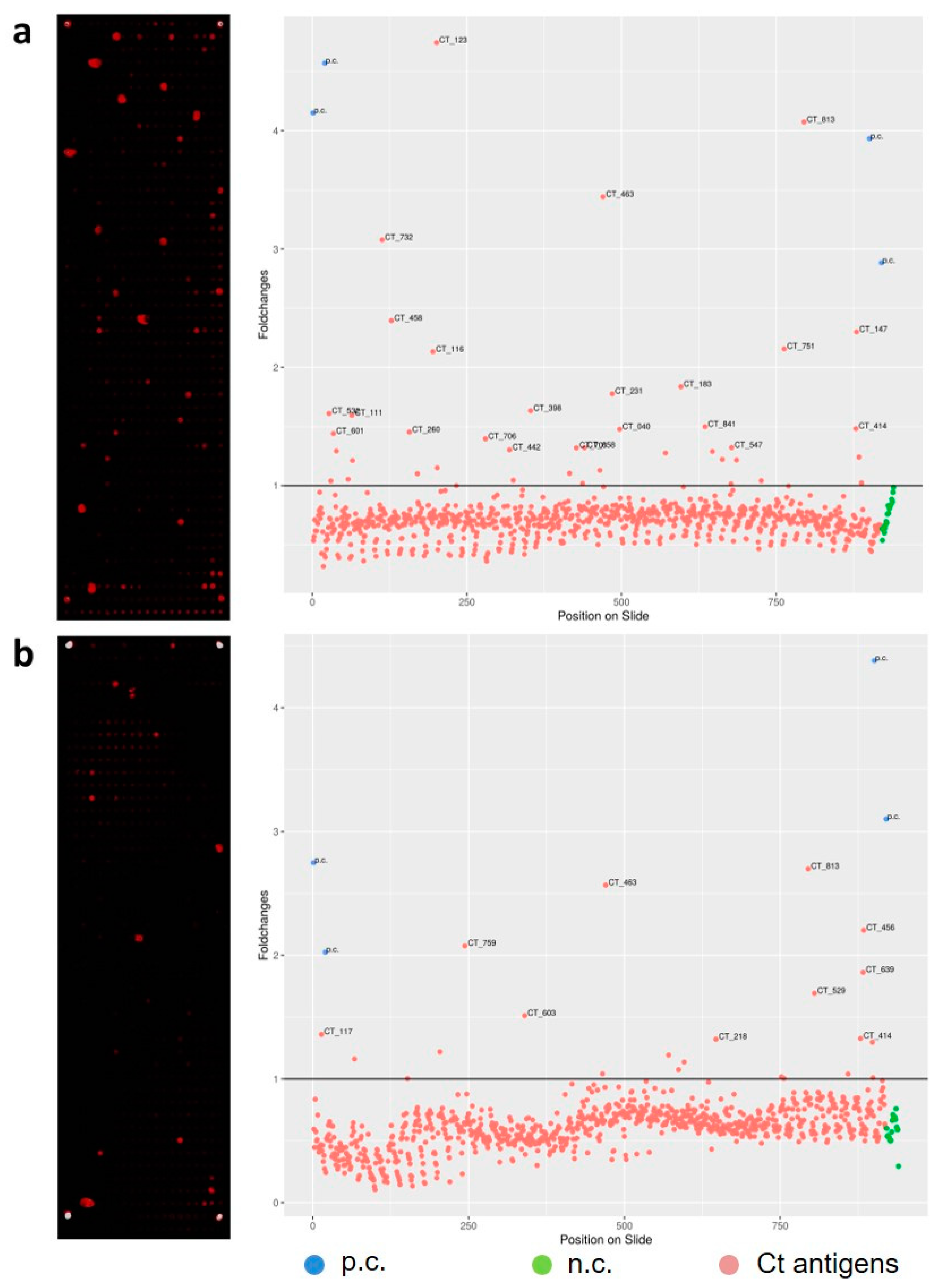
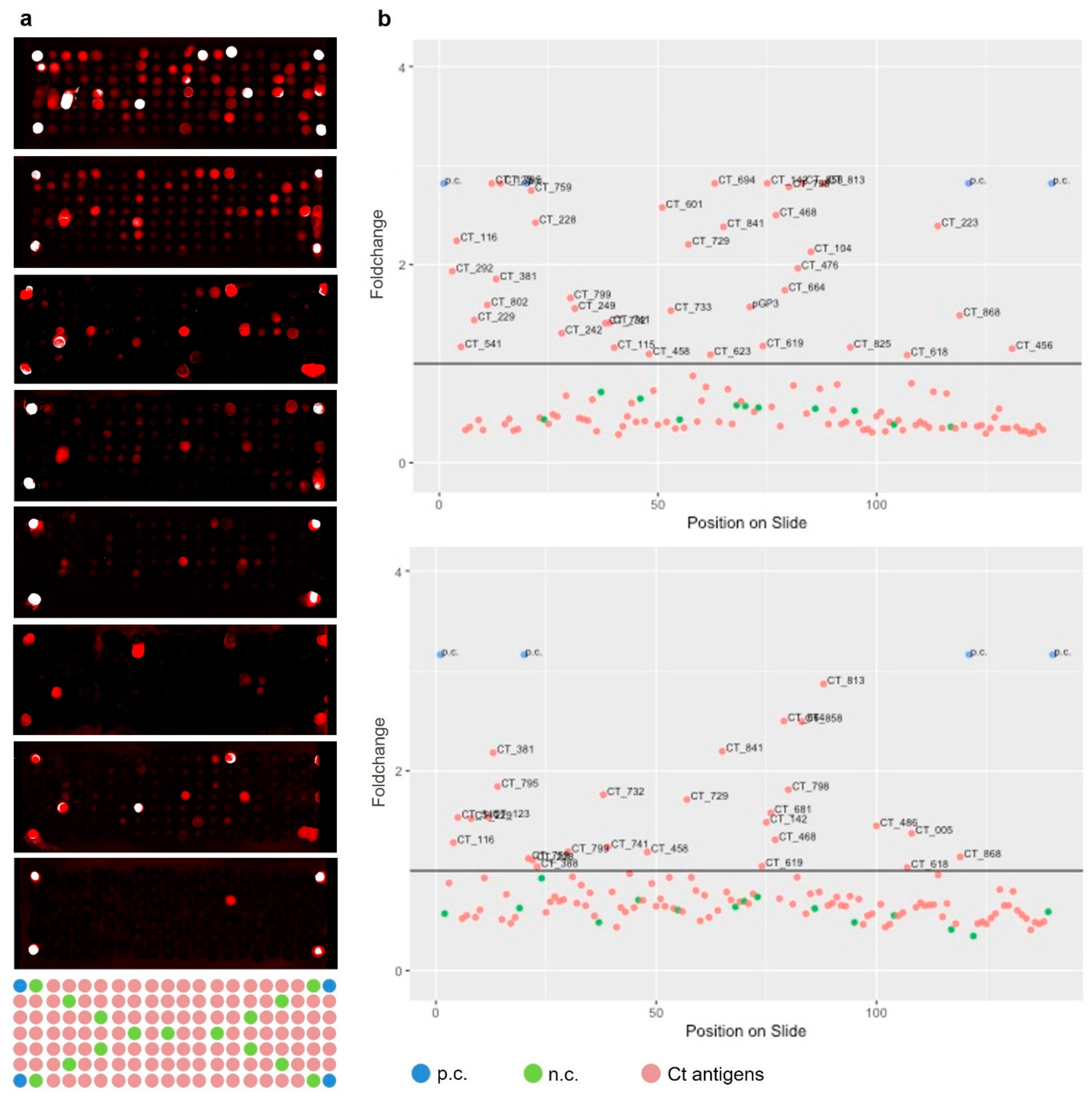
2.4. Selection of Informative Antigens
MED = median, MAD = median absolute deviation
2.5. Statistical Analyses
2.6. Ethics Approval
3. Results
3.1. Antigen Selection
3.2. Single Sera Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.L.; Gottlieb, S.L.; Taylor, B.D.; Low, N.; Xu, F.; Ness, R.B. Risk of sequelae after Chlamydia trachomatis genital infection in women. J. Infect. Dis 2010, 201, S134–S155. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm. Rep. 2014, 63, 1–19. [Google Scholar]
- Nwokolo, N.C.; Dragovic, B.; Patel, S.; Tong, C.Y.; Barker, G.; Radcliffe, K. 2015 UK national guideline for the management of infection with Chlamydia trachomatis. Int. J. STD AIDS 2016, 27, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Hokynar, K.; Korhonen, S.; Norja, P.; Paavonen, J.; Puolakkainen, M. Antibody to Chlamydia trachomatis proteins, TroA and HtrA, as a biomarker for Chlamydia trachomatis infection. Eur J. Clin. Microbiol Infect. Dis 2017, 36, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Grayston, J.T. Immunologic relationship between genital TRIC, Lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am. J. Ophthalmol. 1970, 70, 367–374. [Google Scholar] [CrossRef]
- Morre, S.A.; Munk, C.; Persson, K.; Kruger-Kjaer, S.; van Dijk, R.; Meijer, C.J.; van Den Brule, A.J. Comparison of three commercially available peptide-based immunoglobulin G (IgG) and IgA assays to microimmunofluorescence assay for detection of Chlamydia trachomatis antibodies. J. Clin. Microbiol. 2002, 40, 584–587. [Google Scholar] [CrossRef]
- Labsystems Diagnostics. Chlamydia Trachomatis EIA Tests. Available online: https://www.labsystemsdx.com /specialty/enzymatic-immuno-assays-eia/chlamydia-trachomatis-eia-tests (accessed on 11 November 2019).
- Savyon Diagnostics. SeroCTTM RT. Available online: https://www.savyondiagnostics.com/elisa/ (accessed on 11 November 2019).
- Wills, G.S.; Horner, P.J.; Reynolds, R.; Johnson, A.M.; Muir, D.A.; Brown, D.W.; Winston, A.; Broadbent, A.J.; Parker, D.; McClure, M.O. Pgp3 antibody enzyme-linked immunosorbent assay, a sensitive and specific assay for seroepidemiological analysis of Chlamydia trachomatis infection. Clin. Vaccine Immunol. 2009, 16, 835–843. [Google Scholar] [CrossRef]
- Horner, P.J.; Wills, G.S.; Righarts, A.; Vieira, S.; Kounali, D.; Samuel, D.; Winston, A.; Muir, D.; Dickson, N.P.; McClure, M.O. Chlamydia trachomatis Pgp3 antibody persists and correlates with self-reported infection and behavioural risks in a blinded cohort study. PLoS ONE 2016, 11, e0151497. [Google Scholar] [CrossRef]
- Winstanley, C.E.; Ramsey, K.H.; Marsh, P.; Clarke, I.N. Development and evaluation of an enzyme-linked immunosorbent assay for the detection of antibodies to a common urogenital derivative of Chlamydia trachomatis plasmid-encoded PGP3. J. Immunol. Methods 2017, 445, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Migchelsen, S.J.; Martin, D.L.; Southisombath, K.; Turyaguma, P.; Heggen, A.; Rubangakene, P.P.; Joof, H.; Makalo, P.; Cooley, G.; Gwyn, S.; et al. Defining seropositivity thresholds for use in trachoma elimination studies. PLoS Negl. Trop. Dis. 2017, 11, e0005230. [Google Scholar] [CrossRef] [PubMed]
- Waterboer, T.; Sehr, P.; Michael, K.M.; Franceschi, S.; Nieland, J.D.; Joos, T.O.; Templin, M.F.; Pawlita, M. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin. Chem. 2005, 51, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Waterboer, T.; Idahl, A.; Brenner, N.; Brinton, L.A.; Butt, J.; Coburn, S.B.; Hartge, P.; Hufnagel, K.; Inturrisi, F.; et al. Antibodies against Chlamydia trachomatis and ovarian cancer risk in two independent populations. J. Natl. Cancer Inst. 2018. [Google Scholar] [CrossRef]
- Goodhew, E.B.; Priest, J.W.; Moss, D.M.; Zhong, G.; Munoz, B.; Mkocha, H.; Martin, D.L.; West, S.K.; Gaydos, C.; Lammie, P.J. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl. Trop. Dis. 2012, 6, e1873. [Google Scholar] [CrossRef]
- Hufnagel, K.; Lueong, S.; Willhauck-Fleckenstein, M.; Hotz-Wagenblatt, A.; Miao, B.; Bauer, A.; Michel, A.; Butt, J.; Pawlita, M.; Hoheisel, J.D.; et al. Immunoprofiling of Chlamydia trachomatis using whole-proteome microarrays generated by on-chip in situ expression. Sci. Rep. 2018, 8, 7503. [Google Scholar] [CrossRef]
- Hoenderboom, B.M.; van Oeffelen, A.A.; van Benthem, B.H.; van Bergen, J.E.; Dukers-Muijrers, N.H.; Gotz, H.M.; Hoebe, C.J.; Hogewoning, A.A.; van der Klis, F.R.; van Baarle, D.; et al. The Netherlands Chlamydia cohort study (NECCST) protocol to assess the risk of late complications following Chlamydia trachomatis infection in women. BMC Infect. Dis. 2017, 17, 264. [Google Scholar] [CrossRef]
- van den Broek, I.V.; van Bergen, J.E.; Brouwers, E.E.; Fennema, J.S.; Gotz, H.M.; Hoebe, C.J.; Koekenbier, R.H.; Kretzschmar, M.; Over, E.A.; Schmid, B.V.; et al. Effectiveness of yearly, register based screening for chlamydia in the Netherlands: Controlled trial with randomised stepped wedge implementation. BMJ 2012, 345, e4316. [Google Scholar] [CrossRef]
- Angenendt, P.; Kreutzberger, J.; Glokler, J.; Hoheisel, J.D. Generation of high density protein microarrays by cell-free in situ expression of unpurified PCR products. Mol. Cell Proteomics 2006, 5, 1658–1666. [Google Scholar] [CrossRef]
- Woodhall, S.C.; Gorwitz, R.J.; Migchelsen, S.J.; Gottlieb, S.L.; Horner, P.J.; Geisler, W.M.; Winstanley, C.; Hufnagel, K.; Waterboer, T.; Martin, D.L.; et al. Advancing the public health applications of Chlamydia trachomatis serology. Lancet Infect. Dis. 2018, 18, e399–e407. [Google Scholar] [CrossRef]
- Horner, P.J.; Wills, G.S.; Reynolds, R.; Johnson, A.M.; Muir, D.A.; Winston, A.; Broadbent, A.J.; Parker, D.; McClure, M.O. Effect of time since exposure to Chlamydia trachomatis on chlamydia antibody detection in women: A cross-sectional study. Sex. Transm. Infect. 2013, 89, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Hoenderboom, B.M.; van Willige, M.E.; Land, J.A.; Pleijster, J.; Gotz, H.M.; van Bergen, J.; Dukers-Muijrers, N.; Hoebe, C.; van Benthem, B.H.B.; Morre, S.A. Antibody testing in estimating past exposure to Chlamydia trachomatis in the Netherlands Chlamydia Cohort Study. Microorganisms 2019, 7, 442. [Google Scholar] [CrossRef] [PubMed]
- Ohman, H.; Rantsi, T.; Joki-Korpela, P.; Tiitinen, A.; Surcel, H.M. Prevalence and persistence of Chlamydia trachomatis-specific antibodies after occasional and recurrent infections. Sex. Transm. Infect. 2019. [Google Scholar] [CrossRef]
- Michel, A.; Waterboer, T.; Kist, M.; Pawlita, M. Helicobacter pylori multiplex serology. Helicobacter 2009, 14, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Fan, P.; Ji, H.; Dong, F.; Huang, Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J. Exp. Med. 2001, 193, 935–942. [Google Scholar] [CrossRef]
- Chen, A.L.; Johnson, K.A.; Lee, J.K.; Sutterlin, C.; Tan, M. CPAF: A Chlamydial protease in search of an authentic substrate. PLoS Pathog. 2012, 8, e1002842. [Google Scholar] [CrossRef]
- Korhonen, S.; Hokynar, K.; Mannonen, L.; Paavonen, J.; Hiltunen-Back, E.; Puolakkainen, M. Transcriptional expression of the ompA, cpaf, tarp, and tox genes of Chlamydia trachomatis clinical isolates at different stages of the developmental cycle. Microorganisms 2019, 7, 153. [Google Scholar] [CrossRef]
- Van Ess, E.F.; Eck-Hauer, A.; Land, J.A.; Morre, S.A.; Ouburg, S. Combining individual Chlamydia trachomatis IgG antibodies MOMP, TARP, CPAF, OMP2, and HSP60 for tubal factor infertility prediction. Am. J. Reprod. Immunol. 2019, 81, e13091. [Google Scholar] [CrossRef]
- van Ess, E.F.; Ouburg, S.; Spaargaren, J.; Land, J.A.; Morre, S.A. Performance of the multitarget Mikrogen Chlamydia trachomatis IgG ELISA in the prediction of tubal factor infertility (TFI) in subfertile women: Comparison with the Medac MOMP IgG ELISA plus. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Sharma, J.; Cheng, W.; Zhong, Y.; Liu, K.; Jensen, J.; Shain, R.; Arulanandam, B.; Zhong, G. The hypothetical protein CT813 is localized in the Chlamydia trachomatis inclusion membrane and is immunogenic in women urogenitally infected with C. trachomatis. Infect. Immun. 2006, 74, 4826–4840. [Google Scholar] [CrossRef]


| Overall N = 259 | Ct Positive Without Complications N = 143 (55.2) | Ct Negative with Complications N = 80 (30.9) | Ct Positive with Complications N = 36 (13.9) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Age (years) | ||||
| <30 | 77 (29.7) | 41 (28.7) | 27 (33.8) | 9 (25.0) |
| 30–32 | 69 (26.6) | 31 (21.7) | 22 (27.5) | 16 (44.4) |
| 33–35 | 75 (29.0) | 45 (31.5) | 23 (28.8) | 7 (19.4) |
| ≥36 | 38 (14.7) | 26 (18.2) | 8 (10.0) | 4 (11.1) |
| CT history | ||||
| Negative | 80 (30.9) | 0 (0.0) | 80 (100.0) | 0 (0.0) |
| Positive by at least NAAT $ | 32 (12.4) | 25 (17.5) | 0 (0.0) | 7 (19.4) |
| Positive by at least MOMP ELISA o | 71 (27.4) | 56 (39.2) | 0 (0.0) | 15 (41.7) |
| Positive by only self-reported infections | 76 (29.3) | 62 (43.4) | 0 (0.0) | 14 (38.9) |
| Ct complications * | ||||
| None | 143 (56.9) | 143 (100.0) | 0 (0.0) | 0 (0.0) |
| PID | 52 (20.1) | 0 (0.0) | 31 (38.8) | 21 (58.3) |
| CPP | 54 (20.9) | 0 (0.0) | 39 (48.8) | 15 (41.7) |
| EP | 11 (5.5) | 0 (0.0) | 9 (11.3) | 2 (5.6) |
| TFI | 13 (5.0) | 0 (0.0) | 8 (10.0) | 5 (13.9) |
| Positive Serum Samples N (%, 95%CI) | No. of Positive Antigens Median (IQR) | Top Three Antigens with Highest Seroprevalence (≥40%) % | |
|---|---|---|---|
| Controls—Ct positive | 87 (65.9, 57.2–73.9) | 5 (2–15) | CT_858 (58.6), CT_813 (58.6), CT_142 (50.6), |
| Cases—Ct negative | 33 (48.5, 36.2–61.0) | 4 (2–7) | - |
| PID positive | 28 (57.1, 37.2–75.5) | 3.5 (2–6) | - |
| CPP positive | 13 (43.3, 25.5–62.6) | 5 (1–6) | - |
| EP positive | 2 (22.2, 2.8–60.0) | 10.5 (7–14) | NA |
| TFI positive | 4 (66.7, 22.3–95.7) | 7 (2–14) | CT_123 (50.0), CT_142 (50.0), CT_664 (50.0), CT_858 (50.0), CT_104 (50.0), CT_813 (50.0) |
| Cases—Ct positive | 25 (78.1, 60.0–90.7) | 3 (1–9) | CT_142 (56.0), CT_858 (52.0), CT_813 (44.0) |
| PID positive | 15 (83.3, 58.6–96.4) | 2 (1–9) | CT_858 (53.3), CT_142 (40.0), CT_813 (40.0), |
| CPP positive | 11 (78.6, 49.2–95.3) | 6 (1–11) | CT_142 (54.5), CT_841 (45.5), CT_858 (45.5), CT_813 (45.5) |
| EP positive | 1 (50.0, 1.25–98.7) | 1 | NA |
| TFI positive | 5 (100.0) | 6 (2–9) | CT_142 (100.0) *, CT_858 (60.0) * |
| Antigen | Name | No Reactive Sera | % | Antigen | Name | No Reactive Sera | % | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | CT_858 | protease-like activity factor CPAF | 74 | 51.0% | 26 | CT_228 | hypothetical protein | 17 | 11.7% |
| 2 | CT_813 | hypothetical protein | 72 | 49.7% | 27 | CT_664 | hypothetical protein | 16 | 11.0% |
| 3 | CT_142 | hypothetical protein | 64 | 44.1% | 28 | CT_798 | glycogen synthase | 16 | 11.0% |
| 4 | CT_841 | ATP-dependent zinc metalloprotease FtsH | 42 | 29.0% | 29 | CT_618 | hypothetical protein | 16 | 11.0% |
| 5 | CT_795 | hypothetical protein | 41 | 28.3% | 30 | CT_172 | hypothetical protein | 15 | 10.3% |
| 6 | CT_123 | acetyl-CoA carboxylase biotin carboxyl carrier protein | 39 | 26.9% | 31 | CT_687 | cysteine desulfurase | 14 | 9.7% |
| 7 | pGP3 | virulence plasmid protein | 37 | 25.5% | 32 | CT_456 | translocated actin-recruting phosphoprotein TARP | 14 | 9.7% |
| 8 | CT_104 | enoyl-(acyl carrier protein) reductase | 35 | 24.1% | 33 | CT_579 | hypothetical protein | 13 | 9.0% |
| 9 | CT_381 | arginine ABC transporter substrate-binding protein ArtJ | 35 | 24.1% | 34 | CT_117 | inclusion membrane protein F | 13 | 9.0% |
| 10 | CT_005 | hypothetical protein | 27 | 18.6% | 35 | CT_724 | hypothetical protein | 13 | 9.0% |
| 11 | CT_468 | 2-component regulatory system-ATPase | 26 | 17.9% | 36 | CT_143 | hypothetical protein | 12 | 8.3% |
| 12 | CT_694 | hypothetical protein | 25 | 17.2% | 37 | CT_476 | hypothetical protein | 12 | 8.3% |
| 13 | CT_249 | hypothetical protein | 24 | 16.6% | 38 | CT_336 | phosphoenolpyruvate-protein phosphotransferase | 12 | 8.3% |
| 14 | CT_729 | serine-tRNA ligase | 24 | 16.6% | 39 | CT_110 | HSP60/chepronin GroEL | 11 | 7.6% |
| 15 | CT_761 | peptidoglycan transferase | 23 | 15.9% | 40 | CT_732 | 7-dimethyl-8-ribityllumazine synthase | 11 | 7.6% |
| 16 | CT_223 | inclusion membrane protein | 21 | 14.5% | 41 | CT_332 | pyruvate kinase | 11 | 7.6% |
| 17 | CT_458 | acetyltransferase | 21 | 14.5% | 42 | CT_442 | cysteine-rich protein | 11 | 7.6% |
| 18 | CT_681 | major outer membrane protein | 20 | 13.8% | 43 | CT_529 | hypothetical protein | 10 | 6.9% |
| 19 | CT_802 | S18 ribosomal protein | 18 | 12.4% | 44 | CT_398 | hypothetical protein | 10 | 6.9% |
| 20 | CT_541 | peptidyl-prolyl cis-trans isomerase | 18 | 12.4% | 45 | CT_118 | inclusion membrane protein G | 10 | 6.9% |
| 21 | CT_229 | hypothetical protein | 18 | 12.4% | 46 | CT_868 | deubiquitinase and deneddylase Dub1 | 9 | 6.2% |
| 22 | CT_446 | hypothetical protein | 18 | 12.4% | 47 | CT_799 | 50S ribosomal protein | 9 | 6.2% |
| 23 | CT_116 | inclusion membrane protein E | 17 | 11.7% | 48 | CT_388 | hypothetical protein | 9 | 6.2% |
| 24 | CT_242 | OmpH-like outer membrane protein | 17 | 11.7% | 49 | CT_578 | hypothetical protein | 9 | 6.2% |
| 25 | CT_759 | muramidase | 17 | 11.7% | 50 | CT_226 | hypothetical protein | 9 | 6.2% |
| Control n (%) | Case n (%) | p-value | Control n (%) | Case n (%) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| PID | CPP | ||||||||
| Antigen | PID negative | PID positive | Antigen | CPP negative | CPP positive | ||||
| CT_813 | 51 (58.6) | 9 (29.0) | 0.005 | CT_858 | 51 (58.6) | 8 (33.3) | 0.028 | ||
| CT_142 | 44 (50.6) | 8 (25.8) | 0.017 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hufnagel, K.; Hoenderboom, B.; Harmel, C.; Rohland, J.K.; van Benthem, B.H.B.; Morré, S.A.; Waterboer, T. Chlamydia trachomatis Whole-Proteome Microarray Analysis of The Netherlands Chlamydia Cohort Study. Microorganisms 2019, 7, 703. https://doi.org/10.3390/microorganisms7120703
Hufnagel K, Hoenderboom B, Harmel C, Rohland JK, van Benthem BHB, Morré SA, Waterboer T. Chlamydia trachomatis Whole-Proteome Microarray Analysis of The Netherlands Chlamydia Cohort Study. Microorganisms. 2019; 7(12):703. https://doi.org/10.3390/microorganisms7120703
Chicago/Turabian StyleHufnagel, Katrin, Bernice Hoenderboom, Christoph Harmel, Juliane K. Rohland, Birgit H.B. van Benthem, Servaas A. Morré, and Tim Waterboer. 2019. "Chlamydia trachomatis Whole-Proteome Microarray Analysis of The Netherlands Chlamydia Cohort Study" Microorganisms 7, no. 12: 703. https://doi.org/10.3390/microorganisms7120703
APA StyleHufnagel, K., Hoenderboom, B., Harmel, C., Rohland, J. K., van Benthem, B. H. B., Morré, S. A., & Waterboer, T. (2019). Chlamydia trachomatis Whole-Proteome Microarray Analysis of The Netherlands Chlamydia Cohort Study. Microorganisms, 7(12), 703. https://doi.org/10.3390/microorganisms7120703






