Diverse Stress-Inducing Treatments cause Distinct Aberrant Body Morphologies in the Chlamydia-Related Bacterium, Waddlia chondrophila
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies, Reagents, and Drugs
2.2. Culture of Amoebae and W. chondrophila
2.3. Cell Culture and Infection Procedure
2.4. Cell Viability Monitoring Using Resazurin
2.5. Propidium Iodide-Based Assay
2.6. Immunofluorescence
2.7. Quantification of Recoverable Infectious Progeny
2.8. Quantitative PCR
2.9. RNA Extraction, cDNA Synthesis, and qPCR
3. Results
3.1. Stress Stimuli Induce Different Morphological Subtypes of Aberrant Bodies
3.2. ABs Length and Area Vary According to the Stress Stimuli Applied to Infected Cells
3.3. Number of ABs Per Infected Cell and Inclusion Composition Vary with the Different Stress Stimuli
3.4. Treatment with the Stress Stimuli Reduces the Production of Infectious EBs
3.5. DNA Replication is Differentially Affected in Presence of Stress Stimuli
3.6. Number of Genome Copies per AB with Phosphomycin and Mecillinam
3.7. Stress Stimuli Differently Modulate the Expression of mreB and rodZ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greub, G. The medical importance of Chlamydiae. Clin. Microbiol. Infect. 2009, 15, 2–3. [Google Scholar] [CrossRef][Green Version]
- Rurangirwa, F.R.; Dilbeck, P.M.; Crawford, T.B.; McGuire, T.C.; McElwain, T.F. Analysis of the 16S rRNA gene of micro-organism WSU 86-1044 from an aborted bovine foetus reveals that it is a member of the order Chlamydiales: Proposal of Waddliaceae fam. nov., Waddlia chondrophila gen. nov., sp. nov. Int. J. Syst. Bacteriol. 1999, 49 Pt 2, 577–581. [Google Scholar] [CrossRef]
- Dilbeck-Robertson, P.; McAllister, M.M.; Bradway, D.; Evermann, J.F. Results of a new serologic test suggest an association of Waddlia chondrophila with bovine abortion. J. Vet. Diagn. Investig. 2003, 15, 568–569. [Google Scholar] [CrossRef]
- Barkallah, M.; Gharbi, Y.; Slima, A.B.; Elleuch, F.; Mallek, Z.; Saad, R.B.; Gautier, M.; Gdoura, R.; Fendri, I. Simultaneous detection of Waddlia chondrophila and Listeria monocytogenes in aborted ruminant samples by real-time quantitative PCR. J. Microbiol. Methods 2016, 125, 64–69. [Google Scholar] [CrossRef]
- Baud, D.; Thomas, V.; Arafa, A.; Regan, L.; Greub, G. Waddlia chondrophila, a potential agent of human fetal death. Emerg. Infect. Dis. 2007, 13, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Goy, G.; Osterheld, M.C.; Borel, N.; Vial, Y.; Pospischil, A.; Greub, G. Waddlia chondrophila: From bovine abortion to human miscarriage. Clin. Infect. Dis. 2011, 52, 1469–1471. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Goy, G.; Osterheld, M.C.; Croxatto, A.; Borel, N.; Vial, Y.; Pospischil, A.; Greub, G. Role of Waddlia chondrophila placental infection in miscarriage. Emerg. Infect. Dis. 2014, 20, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Hornung, S.; Thuong, B.C.; Gyger, J.; Kebbi-Beghdadi, C.; Vasilevsky, S.; Greub, G.; Baud, D. Role of Chlamydia trachomatis and emerging Chlamydia-related bacteria in ectopic pregnancy in Vietnam. Epidemiol. Infect. 2015, 143, 2635–2638. [Google Scholar] [CrossRef]
- Verweij, S.P.; Kebbi-Beghdadi, C.; Land, J.A.; Ouburg, S.; Morre, S.A.; Greub, G. Waddlia chondrophila and Chlamydia trachomatis antibodies in screening infertile women for tubal pathology. Microbes Infect. 2015, 17, 745–748. [Google Scholar] [CrossRef]
- Goy, G.; Croxatto, A.; Posfay-Barbe, K.M.; Gervaix, A.; Greub, G. Development of a real-time PCR for the specific detection of Waddlia chondrophila in clinical samples. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1483–1486. [Google Scholar] [CrossRef]
- Haider, S.; Collingro, A.; Walochnik, J.; Wagner, M.; Horn, M. Chlamydia-like bacteria in respiratory samples of community-acquired pneumonia patients. FEMS Microbiol. Lett. 2008, 281, 198–202. [Google Scholar] [CrossRef]
- Matsumoto, A. Fine structures of cell envelopes of Chlamydia organisms as revealed by freeze-etching and negative staining techniques. J. Bacteriol. 1973, 116, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Greub, G.; Raoult, D. Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: An electron micrograph study. Appl. Environ. Microbiol. 2002, 68, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Friis, R.R. Interaction of L cells and Chlamydia psittaci: Entry of the parasite and host responses to its development. J. Bacteriol. 1972, 110, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Y.M.; Belland, R.J. The chlamydial developmental cycle. FEMS Microbiol. Rev. 2005, 29, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Wyrick, P.B. Chlamydia trachomatis persistence in vitro: An overview. J. Infect. Dis. 2010, 201 (Suppl. S2), S88–S95. [Google Scholar] [CrossRef]
- Kintner, J.; Lajoie, D.; Hall, J.; Whittimore, J.; Schoborg, R.V. Commonly prescribed beta-lactam antibiotics induce C. trachomatis persistence/stress in culture at physiologically relevant concentrations. Front. Cell. Infect. Microbiol. 2014, 4, 44. [Google Scholar] [CrossRef]
- Jacquier, N.; Frandi, A.; Pillonel, T.; Viollier, P.H.; Greub, G. Cell wall precursors are required to organize the chlamydial division septum. Nat. Commun. 2014, 5, 3578. [Google Scholar] [CrossRef]
- Raulston, J.E. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect. Immun. 1997, 65, 4539–4547. [Google Scholar] [CrossRef]
- Coles, A.M.; Reynolds, D.J.; Harper, A.; Devitt, A.; Pearce, J.H. Low-nutrient induction of abnormal chlamydial development: A novel component of chlamydial pathogenesis? FEMS Microbiol. Lett. 1993, 106, 193–200. [Google Scholar] [CrossRef]
- Beatty, W.L.; Byrne, G.I.; Morrison, R.P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 1993, 90, 3998–4002. [Google Scholar] [CrossRef] [PubMed]
- Deka, S.; Vanover, J.; Dessus-Babus, S.; Whittimore, J.; Howett, M.K.; Wyrick, P.B.; Schoborg, R.V. Chlamydia trachomatis enters a viable but non-cultivable (persistent) state within herpes simplex virus type 2 (HSV-2) co-infected host cells. Cell. Microbiol. 2006, 8, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Lambden, P.R.; Pickett, M.A.; Clarke, I.N. The effect of penicillin on Chlamydia trachomatis DNA replication. Microbiology 2006, 152, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Kebbi-Beghdadi, C.; Cisse, O.; Greub, G. Permissivity of Vero cells, human pneumocytes and human endometrial cells to Waddlia chondrophila. Microbes Infect. 2011, 13, 566–574. [Google Scholar] [CrossRef]
- de Barsy, M.; Bottinelli, L.; Greub, G. Antibiotic susceptibility of Estrella lausannensis, a potential emerging pathogen. Microbes Infect. 2014, 16, 746–754. [Google Scholar] [CrossRef]
- Vouga, M.; Baud, D.; Greub, G. Simkania negevensis, an example of the diversity of the antimicrobial susceptibility pattern among Chlamydiales. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Pospischil, A.; Borel, N.; Chowdhury, E.H.; Guscetti, F. Aberrant chlamydial developmental forms in the gastrointestinal tract of pigs spontaneously and experimentally infected with Chlamydia suis. Vet. Microbiol. 2009, 135, 147–156. [Google Scholar] [CrossRef]
- Lewis, M.E.; Belland, R.J.; AbdelRahman, Y.M.; Beatty, W.L.; Aiyar, A.A.; Zea, A.H.; Greene, S.J.; Marrero, L.; Buckner, L.R.; Tate, D.J.; et al. Morphologic and molecular evaluation of Chlamydia trachomatis growth in human endocervix reveals distinct growth patterns. Front. Cell. Infect. Microbiol. 2014, 4, 71. [Google Scholar] [CrossRef]
- Fox, A.; Rogers, J.C.; Gilbart, J.; Morgan, S.; Davis, C.H.; Knight, S.; Wyrick, P.B. Muramic acid is not detectable in Chlamydia psittaci or Chlamydia trachomatis by gas chromatography-mass spectrometry. Infect. Immun. 1990, 58, 835–837. [Google Scholar] [CrossRef]
- Matsumoto, A.; Manire, G.P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J. Bacteriol. 1970, 101, 278–285. [Google Scholar] [CrossRef]
- Barbour, A.G.; Amano, K.; Hackstadt, T.; Perry, L.; Caldwell, H.D. Chlamydia trachomatis has penicillin-binding proteins but not detectable muramic acid. J. Bacteriol. 1982, 151, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.W. Why is Chlamydia sensitive to penicillin in the absence of peptidoglycan? Infect. Agents Dis. 1993, 2, 87–99. [Google Scholar]
- Pilhofer, M.; Aistleitner, K.; Biboy, J.; Gray, J.; Kuru, E.; Hall, E.; Brun, Y.V.; VanNieuwenhze, M.S.; Vollmer, W.; Horn, M.; et al. Discovery of chlamydial peptidoglycan reveals bacteria with murein sacculi but without FtsZ. Nat. Commun. 2013, 4, 2856. [Google Scholar] [CrossRef] [PubMed]
- Liechti, G.W.; Kuru, E.; Hall, E.; Kalinda, A.; Brun, Y.V.; VanNieuwenhze, M.; Maurelli, A.T. A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis. Nature 2014, 506, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Liechti, G.; Kuru, E.; Packiam, M.; Hsu, Y.P.; Tekkam, S.; Hall, E.; Rittichier, J.T.; VanNieuwenhze, M.; Brun, Y.V.; Maurelli, A.T. Pathogenic Chlamydia lack a classical sacculus but synthesize a narrow, mid-cell peptidoglycan ring, regulated by MreB, for cell division. PLoS Pathog. 2016, 12, e1005590. [Google Scholar] [CrossRef] [PubMed]
- Goy, G.; Greub, G. Antibiotic susceptibility of Waddlia chondrophila in Acanthamoeba castellanii amoebae. Antimicrob. Agents Chemother. 2009, 53, 2663–2666. [Google Scholar] [CrossRef]
- Bi, E.F.; Lutkenhaus, J. FtsZ ring structure associated with division in Escherichia coli. Nature 1991, 354, 161–164. [Google Scholar] [CrossRef]
- Stephens, R.S.; Kalman, S.; Lammel, C.; Fan, J.; Marathe, R.; Aravind, L.; Mitchell, W.; Olinger, L.; Tatusov, R.L.; Zhao, Q.; et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 1998, 282, 754–759. [Google Scholar] [CrossRef]
- Bertelli, C.; Collyn, F.; Croxatto, A.; Ruckert, C.; Polkinghorne, A.; Kebbi-Beghdadi, C.; Goesmann, A.; Vaughan, L.; Greub, G. The Waddlia genome: A window into chlamydial biology. PLoS ONE 2010, 5, e10890. [Google Scholar] [CrossRef]
- Brown, W.J.; Rockey, D.D. Identification of an antigen localized to an apparent septum within dividing chlamydiae. Infect. Immun. 2000, 68, 708–715. [Google Scholar] [CrossRef][Green Version]
- Dominguez-Escobar, J.; Chastanet, A.; Crevenna, A.H.; Fromion, V.; Wedlich-Soldner, R.; Carballido-Lopez, R. Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science 2011, 333, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Garner, E.C.; Bernard, R.; Wang, W.; Zhuang, X.; Rudner, D.Z.; Mitchison, T. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science 2011, 333, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, S.P.; Karimova, G.; Subtil, A.; Ladant, D. Chlamydia co-opts the rod shape-determining proteins MreB and Pbp2 for cell division. Mol. Microbiol. 2012, 85, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Croxatto, A.; Greub, G. Early intracellular trafficking of Waddlia chondrophila in human macrophages. Microbiology 2010, 156, 340–355. [Google Scholar] [CrossRef]
- Jacquier, N.; Viollier, P.H.; Greub, G. The role of peptidoglycan in chlamydial cell division: Towards resolving the chlamydial anomaly. FEMS Microbiol. Rev. 2015, 39, 262–275. [Google Scholar] [CrossRef]
- Drawz, S.M.; Bonomo, R.A. Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef]
- Eberhardt, C.; Kuerschner, L.; Weiss, D.S. Probing the catalytic activity of a cell division-specific transpeptidase in vivo with beta-lactams. J. Bacteriol. 2003, 185, 3726–3734. [Google Scholar] [CrossRef]
- Spratt, B.G. Comparison of the binding properties of two 6 beta-amidinopenicillanic acid derivatives that differ in their physiological effects on Escherichia coli. Antimicrob. Agents Chemother. 1977, 11, 161–166. [Google Scholar] [CrossRef][Green Version]
- Reynolds, P.E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 943–950. [Google Scholar] [CrossRef]
- Thompson, C.C.; Carabeo, R.A. An optimal method of iron starvation of the obligate intracellular pathogen, Chlamydia trachomatis. Front. Microbiol. 2011, 2, 20. [Google Scholar] [CrossRef]
- Gellert, M.; O’Dea, M.H.; Itoh, T.; Tomizawa, J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc. Natl. Acad. Sci. USA 1976, 73, 4474–4478. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.; Yadav, A.K.; Pillonel, T.; Viollier, P.H.; Cava, F.; Greub, G. A SpoIID homolog cleaves glycan strands at the chlamydial division septum. MBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- de Barsy, M.; Herrgott, L.; Martin, V.; Pillonel, T.; Viollier, P.H.; Greub, G. Identification of new DNA-associated proteins from Waddlia chondrophila. Sci. Rep. 2019, 9, 4885. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, L.G.; Miller, R.D.; Ramirez, J.A.; Molestina, R.E.; Summersgill, J.T. Characterization of Chlamydia pneumoniae persistence in HEp-2 cells treated with gamma interferon. Infect. Immun. 2001, 69, 7927–7932. [Google Scholar] [CrossRef] [PubMed]
- Spratt, B.G.; Jobanputra, V.; Zimmermann, W. Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob. Agents Chemother. 1977, 12, 406–409. [Google Scholar] [CrossRef][Green Version]
- McCoy, A.J.; Sandlin, R.C.; Maurelli, A.T. In vitro and in vivo functional activity of Chlamydia MurA, a UDP-N-acetylglucosamine enolpyruvyl transferase involved in peptidoglycan synthesis and fosfomycin resistance. J. Bacteriol. 2003, 185, 1218–1228. [Google Scholar] [CrossRef]
- Sougakoff, W.; Jarlier, V. Comparative potency of mecillinam and other beta-lactam antibiotics against Escherichia coli strains producing different beta-lactamases. J. Antimicrob. Chemother. 2000, 46 (Suppl. S1), 9–14. [Google Scholar] [CrossRef]
- Mathews, S.; George, C.; Flegg, C.; Stenzel, D.; Timms, P. Differential expression of ompA, ompB, pyk, nlpD and Cpn0585 genes between normal and interferon-gamma treated cultures of Chlamydia pneumoniae. Microb. Pathog. 2001, 30, 337–345. [Google Scholar] [CrossRef]
- Belland, R.J.; Nelson, D.E.; Virok, D.; Crane, D.D.; Hogan, D.; Sturdevant, D.; Beatty, W.L.; Caldwell, H.D. Transcriptome analysis of chlamydial growth during IFN-gamma-mediated persistence and reactivation. Proc. Natl. Acad. Sci. USA 2003, 100, 15971–15976. [Google Scholar] [CrossRef]
- Kokab, A.; Jennings, R.; Eley, A.; Pacey, A.A.; Cross, N.A. Analysis of modulated gene expression in a model of Interferon-gamma-induced persistence of Chlamydia trachomatis in HEp-2 cells. Microb. Pathog. 2010, 49, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Pokorzynski, N.D.; Thompson, C.C.; Carabeo, R.A. Ironing out the unconventional mechanisms of iron acquisition and gene regulation in Chlamydia. Front. Cell. Infect. Microbiol. 2017, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Galasso, G.J.; Manire, G.P. Effect of antiserum and antibiotics on persistent infection of HeLa cells with meningopneumonitis virus. J. Immunol. 1961, 86, 382–385. [Google Scholar] [PubMed]
- Muheim, C.; Gotzke, H.; Eriksson, A.U.; Lindberg, S.; Lauritsen, I.; Norholm, M.H.H.; Daley, D.O. Increasing the permeability of Escherichia coli using MAC13243. Sci. Rep. 2017, 7, 17629. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.; Lee, C.R. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 2019, 10, 953. [Google Scholar] [CrossRef]
- Pickett, M.A.; Everson, J.S.; Pead, P.J.; Clarke, I.N. The plasmids of Chlamydia trachomatis and Chlamydophila pneumoniae (N16): Accurate determination of copy number and the paradoxical effect of plasmid-curing agents. Microbiology 2005, 151, 893–903. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.M.; Nicks, K.M. A plasmid-cured Chlamydia muridarum strain displays altered plaque morphology and reduced infectivity in cell culture. Microbiology 2006, 152, 1601–1607. [Google Scholar] [CrossRef]
- Al-Younes, H.M.; Rudel, T.; Brinkmann, V.; Szczepek, A.J.; Meyer, T.F. Low iron availability modulates the course of Chlamydia pneumoniae infection. Cell. Microbiol. 2001, 3, 427–437. [Google Scholar] [CrossRef]

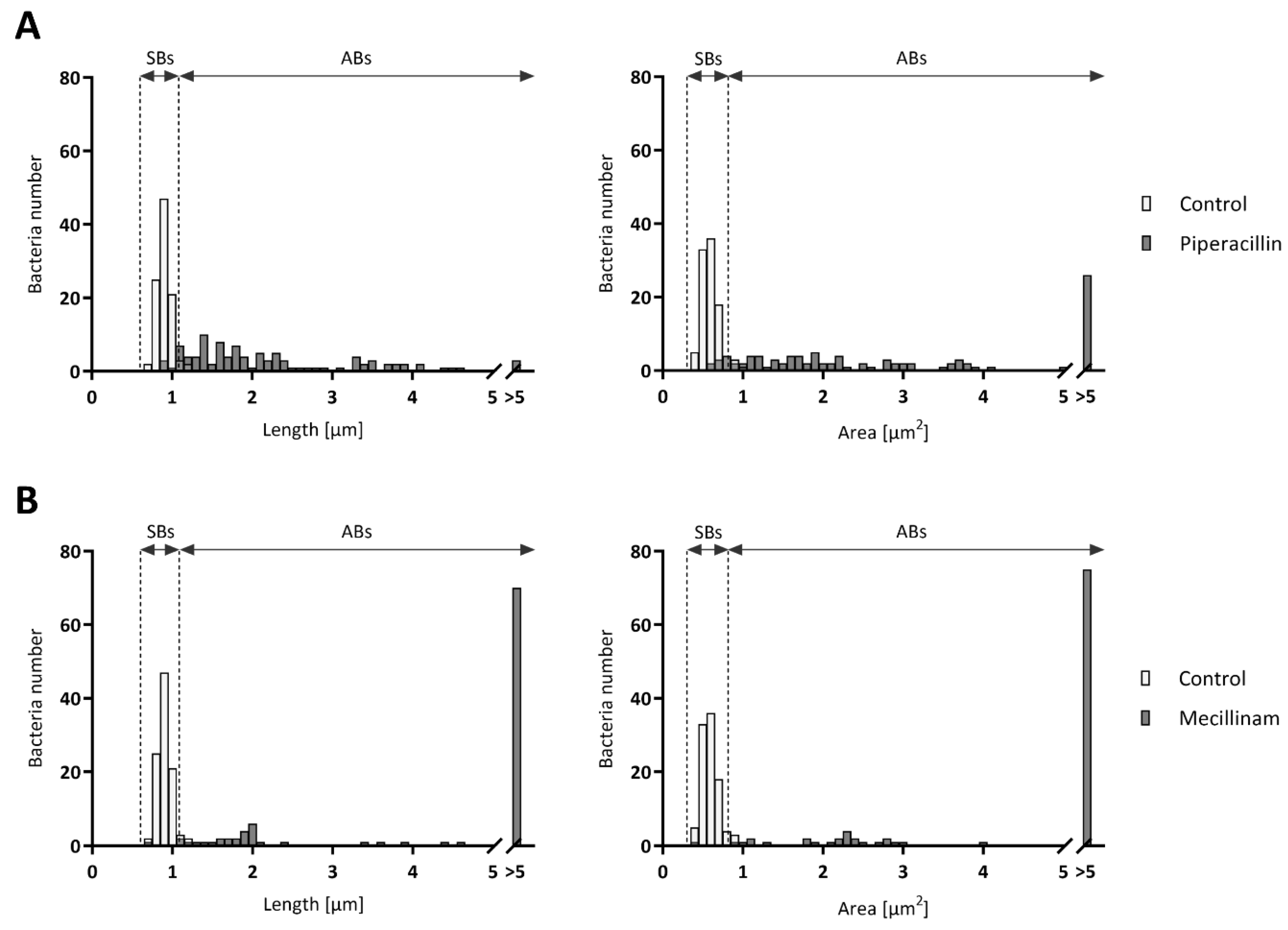
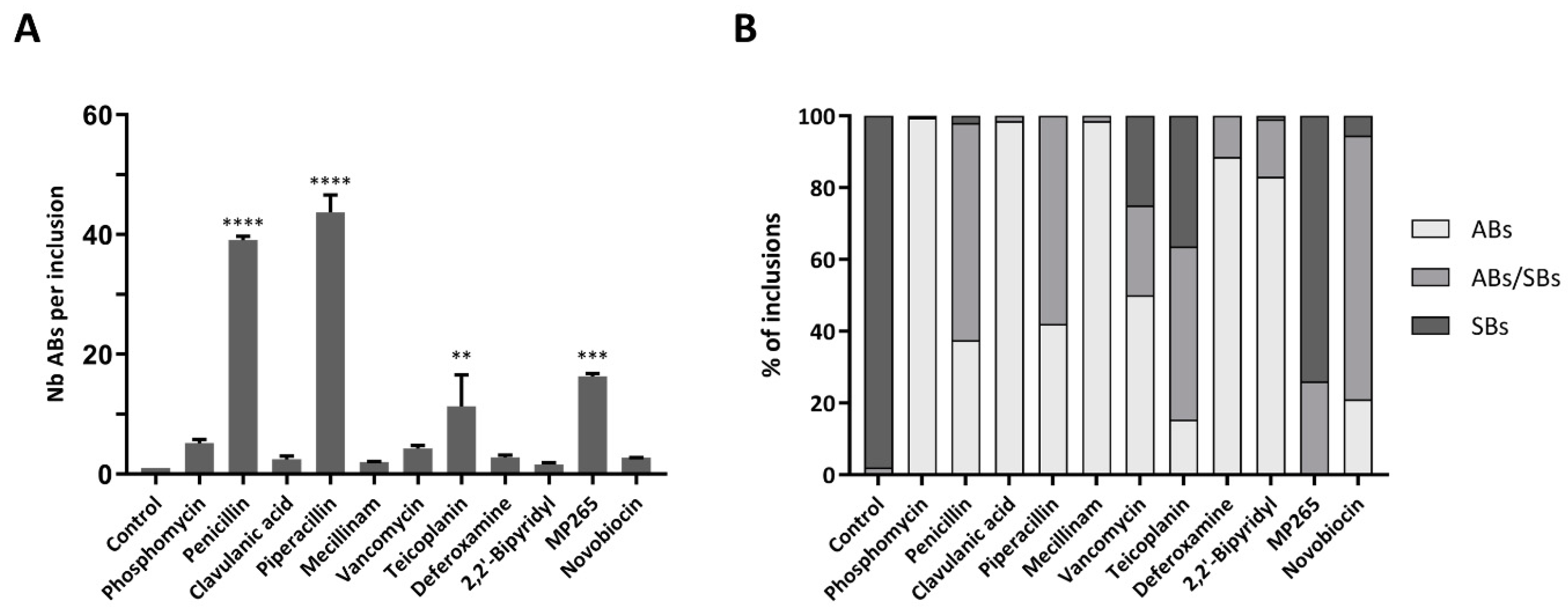

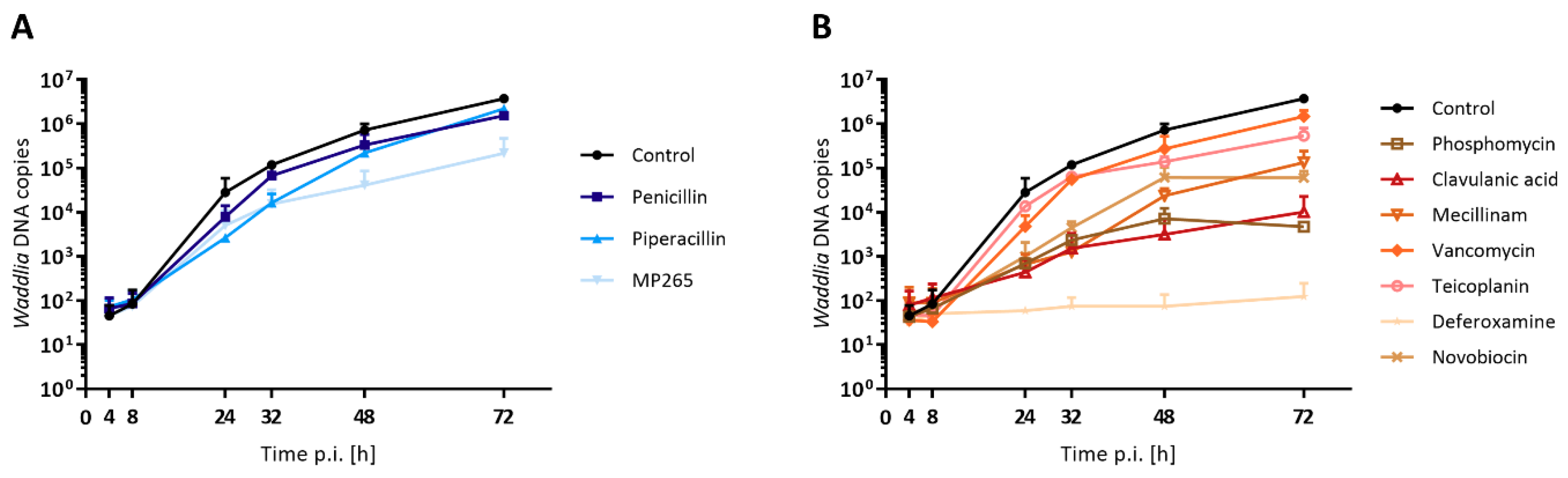
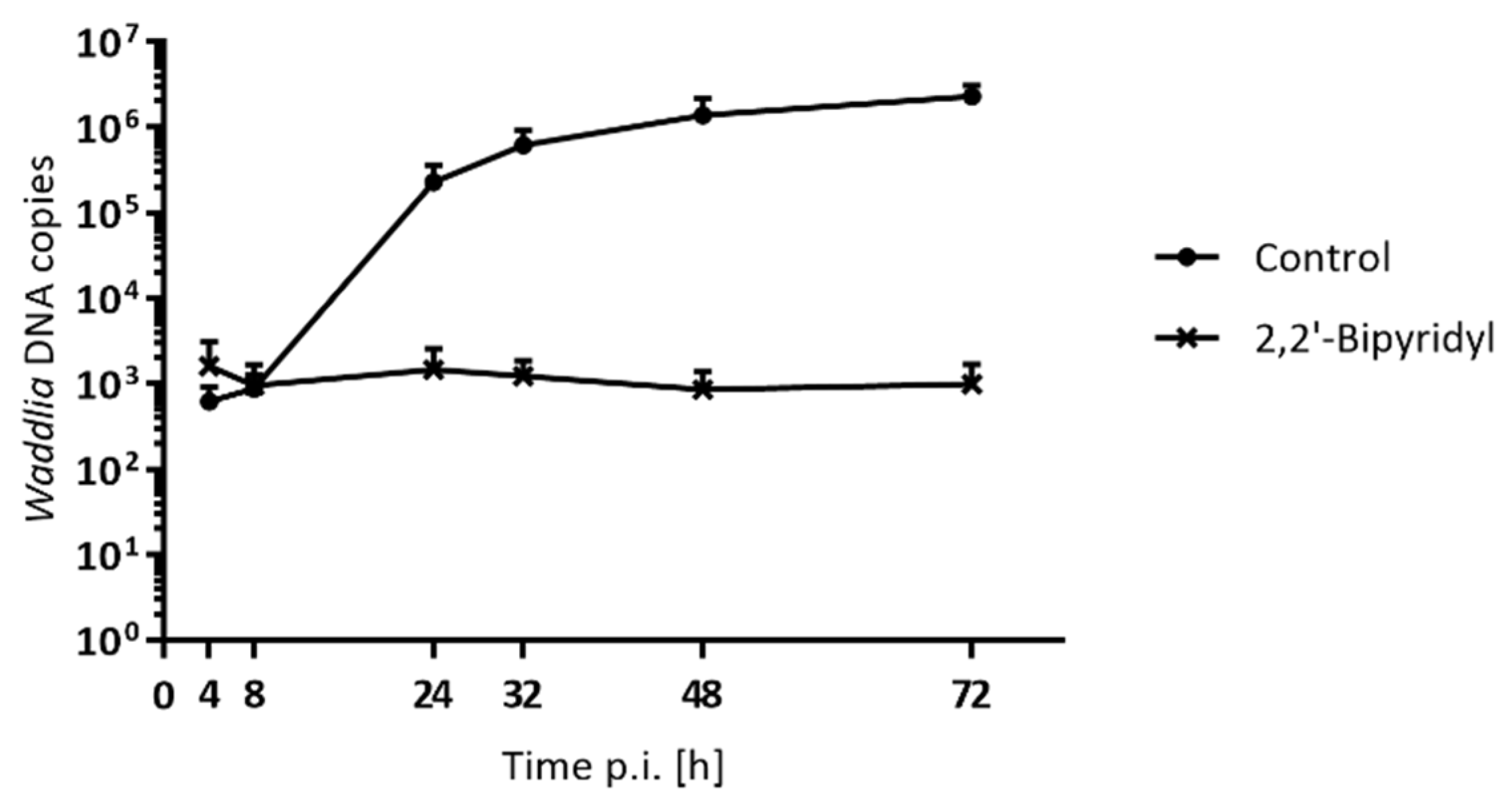
| Drug | Class | Target | Concentration | Infected Cells [%]a | Length [µm]b* | Area [µm2 ]b* | ABs [%]c | ABs Length [µm]d | ABs Area [µm2]d |
|---|---|---|---|---|---|---|---|---|---|
| Control | / | / | / | 13 | 0.90 | 0.57 | 2 | 1.17 | 0.89 |
| Phosphomycin | Other | MurA [18] | 500 µg/mL | 19 | 4.24 | 10.73 | 100 | 4.24 | 10.73 |
| Penicillin | Penicillin | Pbp2/Pbp3/FtsI/AmiA [45] | 1000 µg/mL | 10 | 2.11 | 2.64 | 98 | 2.11 | 2.78 |
| Clavulanic acid | β-lactamase inhibitor | β-lactamase [46] | 900 µg/mL | 19 | 3.10 | 6.00 | 99 | 3.10 | 6.15 |
| Piperacillin | Penicillin | Pbp3/(FtsI) [47] | 500 µg/mL | 13 | 1.84 | 2.24 | 93 | 1.93 | 2.57 |
| Mecillinam | Broad-spectrum penicillin | Pbp2 [48] | 200 µg/mL | 13 | 6.65 | 26.48 | 99 | 6.69 | 26.54 |
| Vancomycin | Glycopeptide | D-Ala-D-Ala moiety of NAM/NAG [49] | 500 µg/mL | 48 | 1.47 | 1.26 | 64 | 2.63 | 4.74 |
| Teicoplanin | Glycopeptide | D-Ala-D-Ala moiety of NAM/NAG [49] | 250 µg/mL | 13 | 1.30 | 1.18 | 76 | 1.49 | 1.44 |
| Deferoxamine | Iron chelator | Binds Fe3+ [50] | 400 µM | 17 | 1.78 | 1.80 | 92 | 1.80 | 1.90 |
| 2,2’-Bipyridyl | Iron chelator | Binds Fe2+ and Fe3+ [50] | 100 µM | 25 | 2.26 | 3.10 | 93 | 2.29 | 3.24 |
| MP265 | Other | Inhibitor of MreB [43] | 100 µM | 19 | 1.01 | 0.73 | 29 | 1.26 | 1.05 |
| Novobiocin | Aminocoumarin | DNA gyrase subunit B (GyrB) [51] | 450 µM | 13.5 | 1.21 | 0.98 | 59 | 1.38 | 1.26 |
| mreB | rodZ | |||||||
|---|---|---|---|---|---|---|---|---|
| 12 hpi | 24 hpi | 36 hpi | 48 hpi | 12 hpi | 24 hpi | 36 hpi | 48 hpi | |
| Phosphomycin | 0.47 ± 0.17 | 0.34 ± 0.03 | 0.37 ± 0.06 | 0.14 ± 0.01 | 0.72 ± 0.17 | 0.54 ± 0.01 | 0.88 ± 0.12 | 0.23 ± 0.02 |
| Penicillin | 0.43 ± 0.00 | 0.71 ± 0.51 | 0.86 ± 0.64 | 0.24 ± 0.19 | 0.95 ± 0.00 | 0.89 ± 0.43 | 1.04 ± 0.51 | 0.33 ± 0.23 |
| Clavulanic acid | 1.43 ± 0.40 | 1.32 ± 0.10 | 1.30 ± 0.30 | 1.12 ± 0.05 | 1.12 ± 0.17 | 1.95 ± 0.80 | 2.37 ± 0.41 | 0.53 ± 0.11 |
| Piperacillin | 0.47 ± 0.05 | 0.50 ± 0.03 | 0.59 ± 0.21 | 0.15 ± 0.01 | 0.39 ± 0.06 | 0.36 ± 0.08 | 0.47 ± 0.23 | 0.13 ± 0.02 |
| Mecillinam | 1.46 ± 0.10 | 0.76 ± 0.12 | 0.65 ± 0.03 | 1.78 ± 0.00 | 1.08 ± 0.26 | 1.06 ± 0.14 | 1.81 ± 0.11 | 0.88 ± 0.14 |
| Vancomycin | 0.43 ± 0.12 | 0.40 ± 0.08 | 0.41 ± 0.07 | 0.24 ± 0.04 | 3.20 ± 0.04 | 2.99 ± 0.29 | 2.97 ± 0.95 | 1.98 ± 0.37 |
| Teicoplanin | 0.95 ± 0.01 | 1.15 ± 0.02 | 0.93 ± 0.07 | 0.83 ± 0.07 | 1.11 ± 0.19 | 1.11 ± 0.16 | 0.96 ± 0.06 | 0.59 ± 0.07 |
| Deferoxamine | 0.42 ± 0.16 | 0.21 ± 0.05 | 0.16 ± 0.05 | 0.04 ± 0.00 | 0.64 ± 0.20 | 0.31 ± 0.08 | 0.49 ± 0.17 | 0.09 ± 0.12 |
| 2,2’-Bipyridyl | 0.64 ± 0.04 | 0.34 ± 0.14 | 0.42 ± 0.34 | 0.15 ± 0.05 | 0.20 ± 0.02 | 0.34 ± 0.14 | 0.54 ± 0.18 | 0.15 ± 0.05 |
| MP265 | 0.50 ± 0.17 | 0.62 ± 0.31 | 1.06 ± 0.06 | 0.14 ± 0.00 | 0.35 ± 0.10 | 0.52 ± 0.15 | 0.77 ± 0.09 | 0.10 ± 0.01 |
| Novobiocin | 0.06 ± 0.06 | 0.93 ± 0.39 | 1.20 ± 0.12 | 0.80 ± 0.00 | 0.04 ± 0.03 | 0.80 ± 0.12 | 1.56 ± 0.43 | 0.44 ± 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherler, A.; Jacquier, N.; Kebbi-Beghdadi, C.; Greub, G. Diverse Stress-Inducing Treatments cause Distinct Aberrant Body Morphologies in the Chlamydia-Related Bacterium, Waddlia chondrophila. Microorganisms 2020, 8, 89. https://doi.org/10.3390/microorganisms8010089
Scherler A, Jacquier N, Kebbi-Beghdadi C, Greub G. Diverse Stress-Inducing Treatments cause Distinct Aberrant Body Morphologies in the Chlamydia-Related Bacterium, Waddlia chondrophila. Microorganisms. 2020; 8(1):89. https://doi.org/10.3390/microorganisms8010089
Chicago/Turabian StyleScherler, Aurélie, Nicolas Jacquier, Carole Kebbi-Beghdadi, and Gilbert Greub. 2020. "Diverse Stress-Inducing Treatments cause Distinct Aberrant Body Morphologies in the Chlamydia-Related Bacterium, Waddlia chondrophila" Microorganisms 8, no. 1: 89. https://doi.org/10.3390/microorganisms8010089
APA StyleScherler, A., Jacquier, N., Kebbi-Beghdadi, C., & Greub, G. (2020). Diverse Stress-Inducing Treatments cause Distinct Aberrant Body Morphologies in the Chlamydia-Related Bacterium, Waddlia chondrophila. Microorganisms, 8(1), 89. https://doi.org/10.3390/microorganisms8010089






