Development and Evaluation of a Duo Zaire ebolavirus Real-Time RT-PCR Assay Targeting Two Regions within the Genome
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Analysis
2.2. Mekambo EBOV RNA
2.3. EBOV RNA Transcript for LoD Calculation
2.4. RT-qPCR Assays
2.5. Specificity
2.6. Cepheid GeneXpert Open Cartridge Development
3. Results
3.1. RT-qPCR Primers and Probe Matched against EBOV Multiple Sequence Alignment: In Silico Analysis
3.2. Study Using the Mekambo EBOV RNA
3.3. LoD Calculation Using the EBOV Synthetic RNA
3.4. Specificity of the Duo Gibb + Huang Assay
3.5. Transfer of the Duo Gibb + Huang Assay onto the Flex-03 Cartridge and Validation on the GeneXpert (Cepheid)
4. Discussion
4.1. In Silico Analysis
4.2. Sensitivity of the Duo Gibb + Huang Assay
4.3. Transfer of the Duo Gibb + Huang Assay onto the Flex-03 Cartridge and Validation on the GeneXpert (Cepheid)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bukreyev, A.A.; Chandran, K.; Dolnik, O.; Dye, J.M.; Ebihara, H.; Leroy, E.M.; Mühlberger, E.; Netesov, S.V.; Patterson, J.L.; Paweska, J.T.; et al. Discussions and Decisions of the 2012–2014 International Committee on Taxonomy of Viruses (ICTV) Filoviridae Study Group, January 2012–June 2013. Arch. Virol. 2014, 159, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Becker, S.; Ebihara, H.; Geisbert, T.W.; Johnson, K.M.; Kawaoka, Y.; Lipkin, W.I.; Negredo, A.I.; Netesov, S.V.; Nichol, S.T.; et al. Proposal for a Revised Taxonomy of the Family Filoviridae: Classification, Names of Taxa and Viruses, and Virus Abbreviations. Arch. Virol. 2010, 155, 2083–2103. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.E.; White, M.E.; Dayrit, M.M.; Hayes, C.G.; Ksiazek, T.G.; Burans, J.P. Seroepidemiological Study of Filovirus Related to Ebola in the Philippines. Lancet 1991, 337, 425–426. [Google Scholar] [CrossRef]
- 2014–2016 Ebola Outbreak Distribution in West Africa. Available online: https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/distribution-map.html (accessed on 30 September 2019).
- Cherpillod, P.; Schibler, M.; Vieille, G.; Cordey, S.; Mamin, A.; Vetter, P.; Kaiser, L. Ebola Virus Disease Diagnosis by Real-Time RT-PCR: A Comparative Study of 11 Different Procedures. J. Clin. Virol. 2016, 77, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Thirion, L.; Pezzi, L.; Corcostegui, I.; Dubot-Pérès, A.; Falchi, A.; de Lamballerie, X.; Charrel, R.N. Development and Evaluation of a Duo Chikungunya Virus Real-Time RT-PCR Assay Targeting Two Regions within the Genome. Viruses 2019, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acid. Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Gibb, T.R.; Norwood, D.A.; Woollen, N.; Henchal, E.A. Development and Evaluation of a Fluorogenic 5′ Nuclease Assay to Detect and Differentiate between Ebola Virus Subtypes Zaire and Sudan. J. Clin. Microbiol. 2001, 39, 4125–4130. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, H.; Wang, Y.; Shi, Z.; Raoul, H.; Yuan, Z. Rapid Detection of Filoviruses by Real-Time TaqMan Polymerase Chain Reaction Assays. Virol. Sin. 2012, 27, 273–277. [Google Scholar] [CrossRef]
- Panning, M.; Laue, T.; Olschlager, S.; Eickmann, M.; Becker, S.; Raith, S.; Courbot, M.-C.G.; Nilsson, M.; Gopal, R.; Lundkvist, A.; et al. Diagnostic Reverse-Transcription Polymerase Chain Reaction Kit for Filoviruses Based on the Strain Collections of All European Biosafety Level 4 Laboratories. J. Infect. Dis. 2007, 196, S199–S204. [Google Scholar] [CrossRef]
- Biava, M.; Colavita, F.; Marzorati, A.; Russo, D.; Pirola, D.; Cocci, A.; Petrocelli, A.; Delli Guanti, M.; Cataldi, G.; Kamara, T.A.; et al. Evaluation of a Rapid and Sensitive RT-QPCR Assay for the Detection of Ebola Virus. J. Virol. Methods 2018, 252, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Dedkov, V.G.; Magassouba, N.; Safonova, M.V.; Bodnev, S.A.; Pyankov, O.V.; Camara, J.; Sylla, B.; Agafonov, A.P.; Maleev, V.V.; Shipulin, G.A. Sensitive Multiplex Real-Time RT-QPCR Assay for the Detection of Filoviruses. Health Secur. 2018, 16, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ro, Y.-T.; Ticer, A.; Carrion, R.; Patterson, J.L. Rapid Detection and Quantification of Ebola Zaire Virus by One-Step Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction. Microbiol. Immunol. 2017, 61, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Rieger, T.; Kerber, R.; El Halas, H.; Pallasch, E.; Duraffour, S.; Günther, S.; Ölschläger, S. Evaluation of RealStar Reverse Transcription-Polymerase Chain Reaction Kits for Filovirus Detection in the Laboratory and Field. J. Infect. Dis. 2016, 214, S243–S249. [Google Scholar] [CrossRef]
- Liu, L.; Sun, Y.; Kargbo, B.; Zhang, C.; Feng, H.; Lu, H.; Liu, W.; Wang, C.; Hu, Y.; Deng, Y.; et al. Detection of Zaire Ebola Virus by Real-Time Reverse Transcription-Polymerase Chain Reaction, Sierra Leone, 2014. J. Virol. Methods 2015, 222, 62–65. [Google Scholar] [CrossRef]
- Jääskeläinen, A.J.; Moilanen, K.; Aaltonen, K.; Putkuri, N.; Sironen, T.; Kallio-Kokko, H.; Vapalahti, O. Development and Evaluation of a Real-Time EBOV-L-RT-QPCR for Detection of Zaire Ebolavirus. J. Clin. Virol. 2015, 67, 56–58. [Google Scholar] [CrossRef]
- Trombley, A.R.; Wachter, L.; Garrison, J.; Buckley-Beason, V.A.; Jahrling, J.; Hensley, L.E.; Schoepp, R.J.; Norwood, D.A.; Goba, A.; Fair, J.N.; et al. Comprehensive Panel of Real-Time TaqMan Polymerase Chain Reaction Assays for Detection and Absolute Quantification of Filoviruses, Arenaviruses, and New World Hantaviruses. Am. J. Trop. Med. Hyg. 2010, 82, 954–960. [Google Scholar] [CrossRef]
- Drosten, C.; Göttig, S.; Schilling, S.; Asper, M.; Panning, M.; Schmitz, H.; Günther, S. Rapid Detection and Quantification of RNA of Ebola and Marburg Viruses, Lassa Virus, Crimean-Congo Hemorrhagic Fever Virus, Rift Valley Fever Virus, Dengue Virus, and Yellow Fever Virus by Real-Time Reverse Transcription-PCR. J. Clin. Microbiol. 2002, 40, 2323–2330. [Google Scholar] [CrossRef]
- Dedkov, V.G.; Magassouba, N.F.; Safonova, M.V.; Deviatkin, A.A.; Dolgova, A.S.; Pyankov, O.V.; Sergeev, A.A.; Utkin, D.V.; Odinokov, G.N.; Safronov, V.A.; et al. Development and Evaluation of a Real-Time RT-PCR Assay for the Detection of Ebola Virus (Zaire) during an Ebola Outbreak in Guinea in 2014-2015. J. Virol. Methods 2016, 228, 26–30. [Google Scholar] [CrossRef]
- Templer, S.P.; Seiverth, B.; Baum, P.; Stevens, W.; Seguin-Devaux, C.; Carmona, S. Improved Sensitivity of a Dual-Target HIV-1 Qualitative Test for Plasma and Dried Blood Spots. J. Clin. Microbiol. 2016, 54, 1877–1882. [Google Scholar] [CrossRef]
- Sizmann, D.; Glaubitz, J.; Simon, C.O.; Goedel, S.; Buergisser, P.; Drogan, D.; Hesse, M.; Kröh, M.; Simmler, P.; Dewald, M.; et al. Improved HIV-1 RNA Quantitation by COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0 Using a Novel Dual-Target Approach. J. Clin. Virol. 2010, 49, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Damond, F.; Avettand-Fenoel, V.; Collin, G.; Roquebert, B.; Plantier, J.C.; Ganon, A.; Sizmann, D.; Babiel, R.; Glaubitz, J.; Chaix, M.L.; et al. Evaluation of an Upgraded Version of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 Test for HIV-1 Load Quantification. J. Clin. Microbiol. 2010, 48, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, B.A.; Sahoo, M.K.; Sandlund, J.; Kleman, M.; Kulkarni, M.; Grufman, P.; Nygren, M.; Kwiatkowski, R.; Baron, E.J.; Tenover, F.; et al. Analytical Performance Characteristics of the Cepheid GeneXpert Ebola Assay for the Detection of Ebola Virus. PLoS ONE 2015, 10, e0142216. [Google Scholar] [CrossRef] [PubMed]
- Jansen van Vuren, P.; Grobbelaar, A.; Storm, N.; Conteh, O.; Konneh, K.; Kamara, A.; Sanne, I.; Paweska, J.T. Comparative Evaluation of the Diagnostic Performance of the Prototype Cepheid GeneXpert Ebola Assay. J. Clin. Microbiol. 2016, 54, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Semper, A.E.; Broadhurst, M.J.; Richards, J.; Foster, G.M.; Simpson, A.J.; Logue, C.H.; Kelly, J.D.; Miller, A.; Brooks, T.J.; Murray, M.; et al. Performance of the GeneXpert Ebola Assay for Diagnosis of Ebola Virus Disease in Sierra Leone: A Field Evaluation Study. PLoS Med. 2016, 13, e1001980. [Google Scholar] [CrossRef]
- Raftery, P.; Condell, O.; Wasunna, C.; Kpaka, J.; Zwizwai, R.; Nuha, M.; Fallah, M.; Freeman, M.; Harris, V.; Miller, M.; et al. Establishing Ebola Virus Disease (EVD) diagnostics using GeneXpert technology at a mobile laboratory in Liberia: Impact on outbreak response, case management and laboratory systems strengthening. PLoS Negl. Trop. Dis. 2018, 12, e0006135. [Google Scholar] [CrossRef]
- Mbala-Kingebeni, P.; Villabona-Arenas, C.J.; Vidal, N.; Likofata, J.; Nsio-Mbeta, J.; Makiala-Mandanda, S.; Mukadi, D.; Mukadi, P.; Kumakamba, C.; Djokolo, B.; et al. Rapid Confirmation of the Zaire Ebola Virus in the Outbreak of the Equateur Province in the Democratic Republic of Congo: Implications for Public Health Interventions. Clin. Infect. Dis. 2019, 68, 330–333. [Google Scholar] [CrossRef]
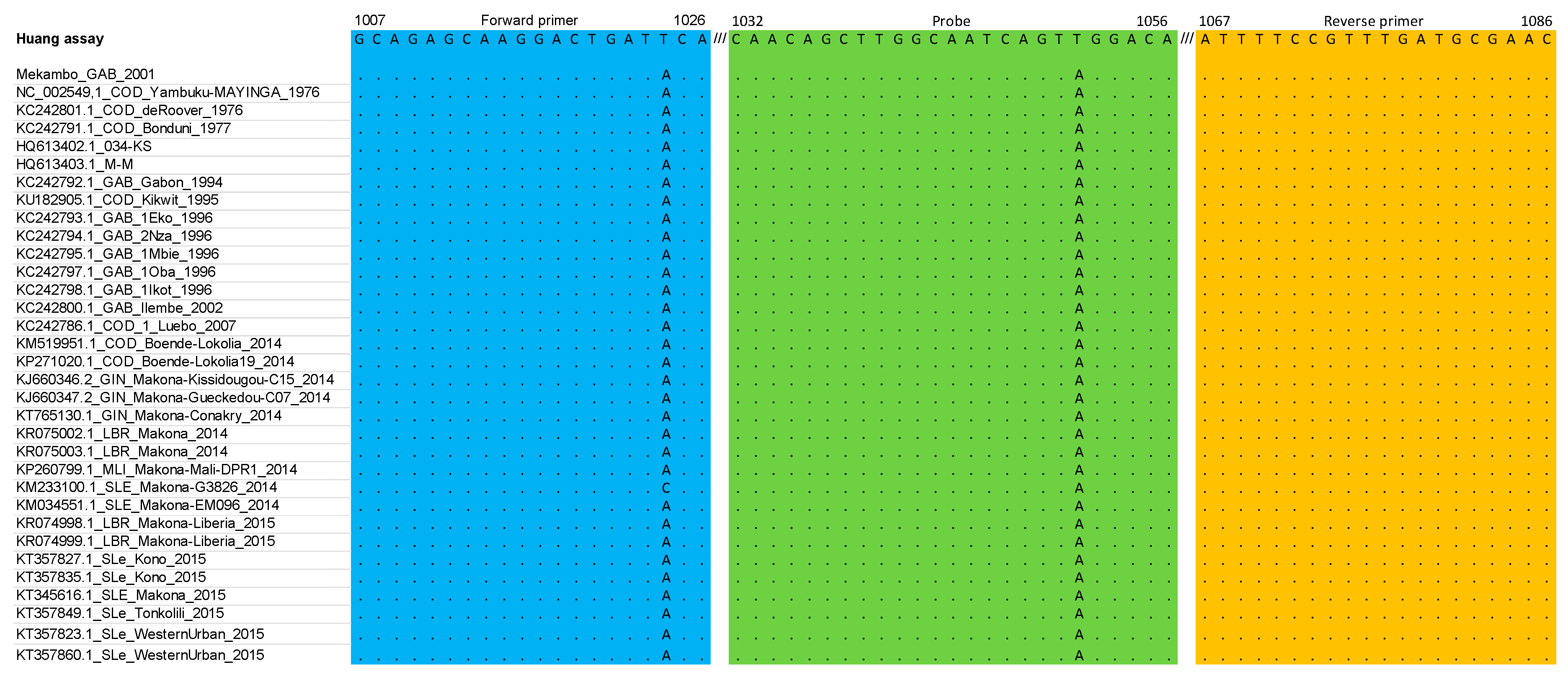



| Assay | Target | Amplicon | Primers/Probe | Sequence (5′-3′) |
|---|---|---|---|---|
| Gibb | GP | 112 bp | EBOGP1D-fwd | TGGGCTGAAAAYTGCTACAATC |
| EBOGP1D-rev | GTGCCGSTATGTKCACAAAG | |||
| EBOGP1DZ-Prb | FAM-CTACCAGCAGCGCCAGACGG-TAMRA | |||
| Huang | NP | 80 bp | enp-F | GCAGAGCAAGGACTGATTCA |
| Enp-F2 | GCAGAGCAAGGACTGATACA | |||
| enp-R | ATTTTCCGTTTGATGCGAAC | |||
| enp-Probe | FAMa-CAACAGCTTGGCAATCAGTTGGACA-TAMRA | |||
| Panning | L | 293 bp | FiloA2.2 | AAGCCTTTCCTAGCAACATGATGGT |
| FiloA2.3 | AAGCATTCCCTAGCAACATGATGGT | |||
| FiloA2.4 | AAGCATTTCCTAGCAATATGATGGT | |||
| FiloB | CATGTCAGTGATTATTATAACCCACCACAT | |||
| Filo B-Prime | CATGTCAGTGATTATTATAAYCCACCRCAT | |||
| Filo B-Ra | CATGTCAGTGACTTTTATAGCCCTCCTCAC | |||
| FAMEBOSu | FAMb-TGGCACCAIACIAGTGATGATTTCGG-BHQ1 | |||
| FAMEBOg | FAMb-TGGCACCACACIAGTGATGATTTTGG-BHQ1 |
| Gibb et al. [9] | Huang et al. [10] | Panning et al. [11] | Standard Protocol Mekambo EBOV RNA | Standard Protocol EBOV Synthetic RNA | RealStar® Filovirus Altona Diagnostics | |
|---|---|---|---|---|---|---|
| Reverse Transcription | 55 °C/45 min | 50 °C/30 min | 50 °C/30 min | 50 °C/15 min | 50 °C/15 min | 55 °C/20 min |
| Denaturation | 94 °C/1 min | 94 °C/5 min | 95 °C/1.5 min | 95 °C/2 min | 95 °C/2 min | 95 °C/2 min |
| Cycling | 40 | 45 | 45 | 40 | 40 | 45 |
| Denaturation | 94 °C/15 s | 94 °C/15 s | 95 °C/15 s | 95 °C/15 s | 95 °C/15 s | 95 °C/15 s |
| Amplification | 60 °C/30 s | 60 °C/1 min | 72 °C/20 s | 60 °C/1 min | 60 °C/1 min | 72 °C/15 s |
| RNA volume | 5 µL | 1–3 µL | 3 µL | 5 µL | 10 µL | 10 µL |
| Total volume | 50 µL | 23–25 µL | 25 µL | 25 µL | 30 µL | 30 µL |
| Primers concentration | 0.5 µM | 0.4 µM | 0.2 µM Forward 0.3 µM Reverse | 0.4 µM Gibb 0.4 µM Huang 0.2 µM Panning | 0.625 µM Gibb 0.5 µM Huang | - |
| Probes concentration | 0.2 µM | 0.1 µM | 0.0667 µM | 0.16 µM Gibb and Huang | 0.25 µM Gibb | - |
| 0.2 µM each Panning | 0.125 µM Huang |
| Genus | Virus | Acronyms | Strain (Viral Load TCID50/mL) | Reference (a) |
|---|---|---|---|---|
| Phlebovirus | Toscana virus | TOSV | UVE/TOSV/2014/FR/5904 (10 8,22) | 001v-02452 |
| Sandfly Fever Sicilian virus | SFSV | UVE/SFSV/1943/IT/Sabin (10 6,82) | 001v-EVA77 | |
| Flavivirus | Japanese encephalitis virus | JEV | UVE/JEV/2009/LA/CNS769 (10 5,57) | 001v-02217 |
| Saint-Louis encephalitis virus | SLEV | UVE/SLEV/UNK/US/MSI-7 (10 4,82) | 001v-EVA128 | |
| Tick-borne encephalitis virus | TBEV | UVE/TBEV/2013/FR/32.11 WT-PCR (10 8,82) | 001v-02352 | |
| West-Nile virus | WNV | UVE/WNV/2008/US/R94224 (10 7,32) | 001v-02224 | |
| Yellow Fever virus | YFV | UVE/YFV/UNK/XX/French neurotropic R94224 (10 7,32) | 001v-02226 | |
| Usutu virus | USUV | UVE/USUV/1959/ZA/SAAR-1776 (10 5,32) | 001v-EVA138 | |
| Murray Valley virus | MVEV | UVE/MVEV/UNK/AU/3329 (10 4,32) | 001v-EVA145 | |
| Zika virus | ZIKV | UVE/ZIKV/1947/UG/MR766 (10 4,32) | 001v-EVA143 | |
| Dengue virus | DENV-1 | UVE/DENV-1/2013/NC/CNR_17132 (10 7,57) | 001v-03151 | |
| Alphavirus | Venezuelan equine encephalitis virus | VEEV | UVE/VEEV/UNK/XX/TC83 vaccine (10 9,42) | 001v-EVA1459 |
| Western equine encephalitis virus | WEEV | UVE/WEEV/UNK/XX/47a (10 8,32) | 001v-EVA1479 | |
| Eastern equine encephalitis virus | EEEV | UVE/EEEV/1999/XX/H178_99 (10 7,82) | 001v-EVA1480 | |
| O’nyong-nyong virus | ONNV | UVE/ONNV/UNK/SN/Dakar 234 (10 4,22) | 001v-EVA1044 | |
| Chikungunya virus | CHIKV | UVE/CHIKV/2017/FR/45625-26 (10 6,16) | 001v-03433 | |
| Semliki Forest virus | SFV | UVE/SFV/UNK/XX/1745 (10 4,42) | 001v-02468 | |
| Sindbis virus | SINV | UVE/SINV/UNK/EG/Egypt 339 (10 4,32) | 001v-02469 | |
| Filovirus | Marburg virus | MBGV | Popp | n/a |
| Marburg virus | MBGV | Musoke | n/a | |
| Nairovirus | Crimean-Congo hemorrhagic fever virus | CCHF | Unk | n/a |
| Replicate | RNA Copies/Reaction | ||||
|---|---|---|---|---|---|
| 16.8 | 12.6 | 8.4 | 4.2 | 1.7 | |
| Flex-C#1 | 37.1 | 38.7 | 39.6 | negative | negative |
| Flex-C#2 | 36.3 | 36.0 | 38.9 | 38.0 | negative |
| Flex-C#3 | 36.4 | 36.9 | 39.2 | negative | negative |
| Flex-C#4 | 38.4 | 37.6 | 37.9 | negative | negative |
| Flex-C#5 | 36.2 | 38.2 | negative | 36.7 | negative |
| Flex-C#6 | 36.5 | 39.3 | negative | 38.0 | negative |
| Flex-C#7 | 39.7 | 37.8 | negative | negative | negative |
| Flex-C#8 | 36.1 | 36.2 | negative | negative | negative |
| Mean | 37.1 (1.3) | 37.6 (1.2) | 38.9 (0.6) | 37.6 (0.6) | - |
| GIBB et al. [9] | Replicate | RNA Copies/µL | ||||||
| 1 | 0.75 | 0.5 | 0.2 | 0.1 | 0.075 | 0.05 | ||
| 1 | 36.57 | 36.66 | 37.28 | 38.76 | >40 | >40 | >40 | |
| 2 | 36.59 | 35.99 | 37.25 | 38.43 | >40 | >40 | >40 | |
| 3 | 37.02 | 36.62 | 36.98 | >40 | 38.36 | >40 | >40 | |
| 4 | 36.47 | 37.07 | >40 | 38.27 | >40 | >40 | >40 | |
| 5 | 38.47 | 36.17 | >40 | 38.27 | >40 | >40 | >40 | |
| 6 | 37.49 | >40 | 38.54 | >40 | 38.54 | >40 | >40 | |
| 7 | >40 | 38.53 | 37.59 | >40 | 38.52 | 39.85 | >40 | |
| Mean Ct (SD) | 37.10 (0.7) | 36.84 (0.8) | 37.53 (0.5) | 38.43 (0.2) | 38.47 (0.1) | 39.85 | >40 | |
| HUANG et al. [10] | Replicate | RNA Copies/µL | ||||||
| 1 | 0.75 | 0.5 | 0.2 | 0.1 | 0.075 | 0.05 | ||
| 1 | 37.59 | >40 | 38.14 | >40 | 38.60 | >40 | >40 | |
| 2 | 37.92 | 38.02 | >40 | 40 | >40 | >40 | >40 | |
| 3 | 37.24 | 38.42 | 39.62 | >40 | 39.09 | >40 | >40 | |
| 4 | 37.30 | 38.48 | >40 | >40 | >40 | 37.95 | >40 | |
| 5 | 38.35 | 37.39 | >40 | >40 | >40 | 39.98 | >40 | |
| 6 | 37.89 | >40 | 38.07 | >40 | >40 | 39.98 | >40 | |
| 7 | 37.48 | 37.48 | 39.13 | 39.31 | 39.92 | >40 | >40 | |
| Mean Ct (SD) | 37.68 (0.4) | 37.96 (0.5) | 38.74 (0.7) | 39.66 | 39.20 (0.5) | 39.30 (1.0) | >40 | |
| DUO GIBB + HUANG (this study) | Replicate | RNA Copies/µL | ||||||
| 1 | 0.75 | 0.5 | 0.2 | 0.1 | 0.075 | 0.05 | ||
| 1 | NT | 32.14 | 33.04 | 34.89 | 36.40 | 32.98 | >40 | |
| 2 | NT | 33.01 | 32.78 | 34.93 | 36.24 | 33.17 | >40 | |
| 3 | NT | 32.87 | 33.10 | 34.03 | >40 | 33.80 | 38.27 | |
| 4 | NT | 32.83 | 33.20 | 34.88 | >40 | 33.48 | >40 | |
| 5 | NT | 32.77 | 36.61 | 34.67 | 37.45 | > 40 | >40 | |
| 6 | NT | 32.28 | 33.53 | 35.63 | 35.34 | 32.95 | >40 | |
| 7 | NT | 32.71 | 32.74 | >40 | 35.43 | >40 | >40 | |
| 8 | NT | 32.59 | 32.84 | 34.64 | 35.18 | 33.41 | >40 | |
| 9 | NT | 31.98 | 33.40 | 34.04 | 35.39 | 33.97 | >40 | |
| 10 | NT | 32.44 | 33.12 | 35.12 | >40 | 33.01 | >40 | |
| 11 | NT | 33.11 | 33.33 | 34.67 | 35.88 | 33.83 | >40 | |
| 12 | NT | 32.27 | 32.73 | 35.36 | 35.36 | 33.56 | >40 | |
| 13 | NT | 32.19 | 33.45 | >40 | 38.37 | 36.71 | >40 | |
| 14 | NT | 32.41 | 33.04 | 34.29 | >40 | 34.74 | >40 | |
| 15 | NT | 33.06 | 33.29 | 34.48 | 36.04 | 34.18 | >40 | |
| 16 | NT | 32.47 | 32.41 | 35.69 | >40 | >40 | 34.65 | |
| 17 | NT | 32.07 | 32.93 | 34.70 | >40 | 33.18 | >40 | |
| 18 | NT | 32.22 | 33.15 | >40 | 35.86 | 32.29 | >40 | |
| 19 | NT | 34.00 | 33.46 | 36.39 | 35.78 | 36.10 | >40 | |
| 20 | NT | 32.38 | 34.32 | >40 | 33.92 | 33.17 | >40 | |
| 21 | NT | 33.12 | 33.05 | 34.57 | 36.72 | >40 | > 40 | |
| 22 | NT | 32.44 | 32.55 | 34.31 | >40 | >40 | >40 | |
| 23 | NT | 33.08 | 32.23 | >40 | > 40 | >40 | >40 | |
| Mean Ct (SD) | 32.63 (0.5) | 33.23 (0.8) | 34.85 (0.6) | 35.96 (1.0) | 33.80 (1.1) | 36.46 (1.8) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirion, L.; Charrel, R.N.; Boehmann, Y.; Corcostegui, I.; Raoul, H.; de Lamballerie, X. Development and Evaluation of a Duo Zaire ebolavirus Real-Time RT-PCR Assay Targeting Two Regions within the Genome. Microorganisms 2019, 7, 652. https://doi.org/10.3390/microorganisms7120652
Thirion L, Charrel RN, Boehmann Y, Corcostegui I, Raoul H, de Lamballerie X. Development and Evaluation of a Duo Zaire ebolavirus Real-Time RT-PCR Assay Targeting Two Regions within the Genome. Microorganisms. 2019; 7(12):652. https://doi.org/10.3390/microorganisms7120652
Chicago/Turabian StyleThirion, Laurence, Remi N. Charrel, Yannik Boehmann, Iban Corcostegui, Hervé Raoul, and Xavier de Lamballerie. 2019. "Development and Evaluation of a Duo Zaire ebolavirus Real-Time RT-PCR Assay Targeting Two Regions within the Genome" Microorganisms 7, no. 12: 652. https://doi.org/10.3390/microorganisms7120652
APA StyleThirion, L., Charrel, R. N., Boehmann, Y., Corcostegui, I., Raoul, H., & de Lamballerie, X. (2019). Development and Evaluation of a Duo Zaire ebolavirus Real-Time RT-PCR Assay Targeting Two Regions within the Genome. Microorganisms, 7(12), 652. https://doi.org/10.3390/microorganisms7120652






