Isolation and Molecular Typing of Leishmania infantum from Phlebotomus perfiliewi in a Re-Emerging Focus of Leishmaniasis, Northeastern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sand Fly Sampling and Identification
2.2. Leishmania Detection and Determination of Parasite Load by qPCR
2.3. Leishmania Isolation and Typing
2.4. Captivity of Field Collected Sand Flies
3. Results
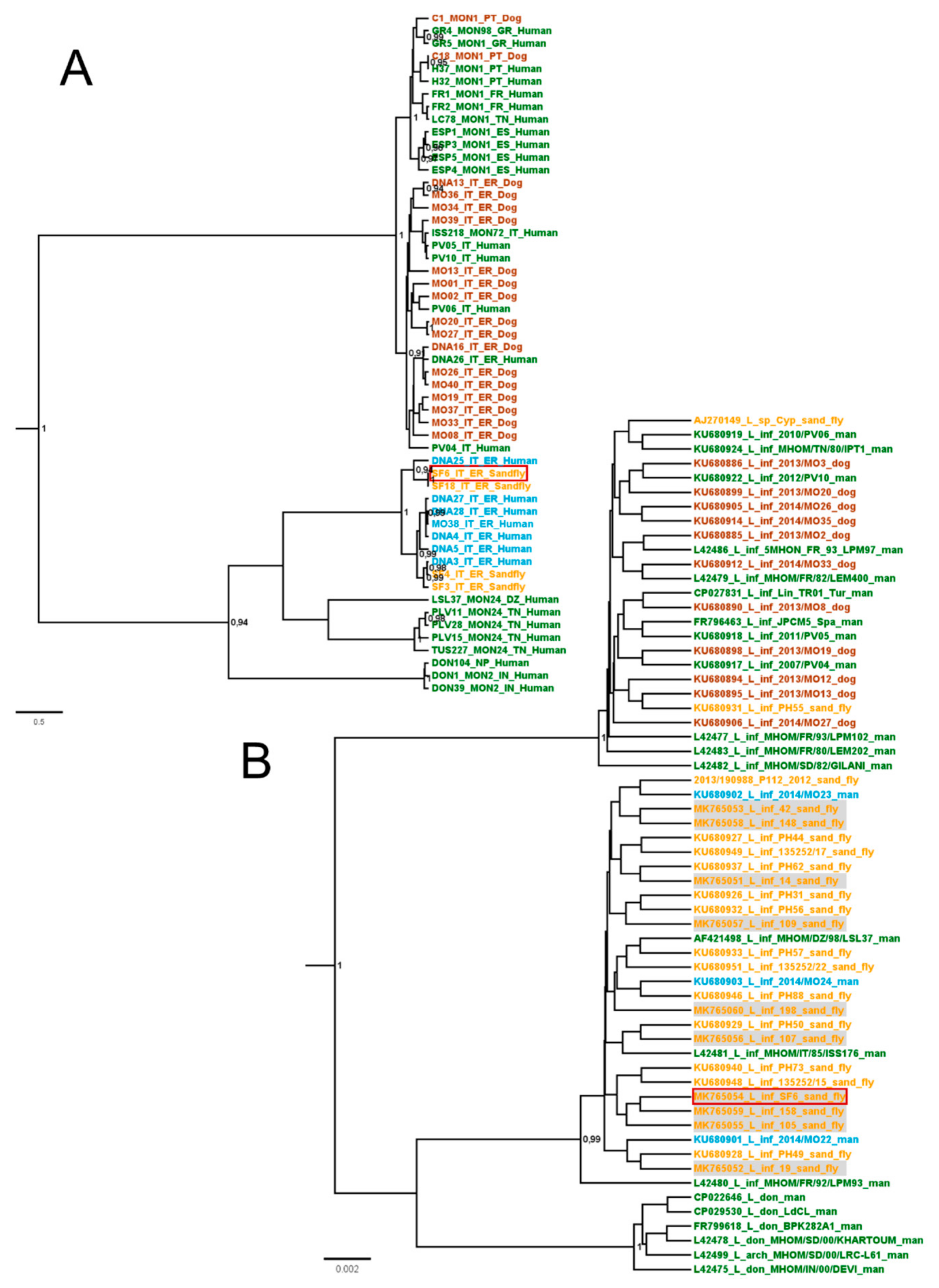
3.1. Leishmania Isolation from Sand Fly and Species Definition
3.2. Lein-pw Molecular Typing
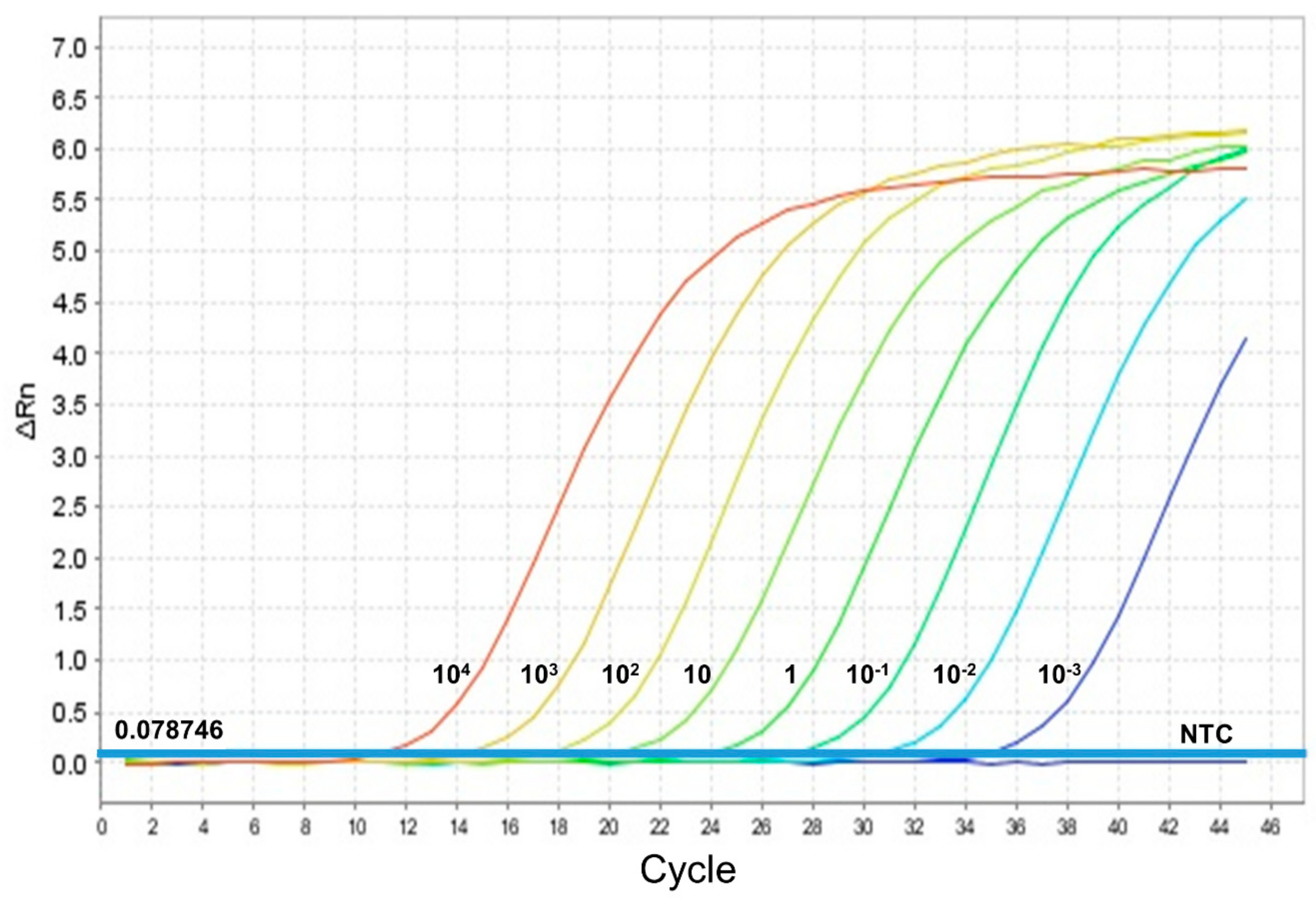
3.3. Infection Rate in Sand Flies and Parasite Load by qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moriconi, M.; Rugna, G.; Calzolari, M.; Bellini, R.; Albieri, A.; Angelini, P.; Cagarelli, R.; Landini, M.P.; Charrel, R.N.; Varani, S. Phlebotomine sand fly–borne pathogens in the Mediterranean Basin: Human leishmaniasis and phlebovirus infections. PLoS Negl. Trop. Dis. 2017, 11, e0005660. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Control of the Leishmaniases: Report of a Meeting of the WHO Expert Commitee on the Control of Leishmaniases, Geneva, 22–26 March 2010; WHO Technical Report series; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Gramiccia, M.; Scalone, A.; Di Muccio, T.; Orsini, S.; Orsini, E.; Gradoni, L. The burden of visceral leishmaniasis in Italy from 1982 to 2012: A retrospective analysis of the multi-annual epidemic that occurred from 1989 to 2009. Euro. Surveill. 2013, 18, 20535. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, A. Phlebotomus and leishmaniasis in North-Central Italy (Apennine Region). Scientific Report Istituto Superiore di Sanità 1962, 2, 103–109. [Google Scholar]
- Maroli, M.; Gramiccia, M.; Gradoni, L. Natural infection of the sand fly Phlebotomus perfiliewi Parrot, 1930 with Leishmania infantum Nicolle, 1908 in a cutaneous leishmaniasis focus of the Abruzzi region (Italy). Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 596–598. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef]
- Pampiglione, S.; La Placa, M.; Schlick, G. Studies on Mediterranean Leishmaniasis. I. An outbreak of visceral leishmaniasis in Northern Italy. Trans. R. Soc. Trop. Med. Hyg. 1974, 68, 349–359. [Google Scholar] [CrossRef]
- Varani, S.; Cagarelli, R.; Melchionda, F.; Attard, L.; Salvadori, C.; Finarelli, A.C.; Gentilomi, G.A.; Tigani, R.; Rangoni, R.; Todeschini, R.; et al. Ongoing outbreak of visceral leishmaniasis in Bologna Province, Italy, November 2012 to May 2013. Euro. Surveill. 2013, 18, 20530. [Google Scholar] [CrossRef]
- Calzolari, M.; Angelini, P.; Finarelli, A.C.; Cagarelli, R.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Tamba, M.; Dottori, M.; et al. Human and entomological surveillance of Toscana virus in the Emilia-Romagna region, Italy, 2010 to 2012. Euro. Surveill. 2014, 19, 20978. [Google Scholar] [CrossRef] [PubMed]
- Killick-Kendrick, R.; Ready, P.D.; Pampiglione, S. Notes on the prevalence and host preferences of Phlebotomus perfiliewi in Emilia Romagna, Italy. Ecologic des Leishmunioses. Colloq. Int. CNRS 1977, 239, 169–175. [Google Scholar]
- Coluzzi, A.; Costantini, R.; Nannarone, L.; Sacchetti, A.; Bergomi, S. Investigations on the Phlebotomus in the Province of Emilia-Romagna and anti-leishmaniosis prophylactic directions. G. Mal. Infett. Parassit. 1982, 34, 1481–1487. [Google Scholar]
- Nicolas, L.; Prina, E.; Lang, T.; Milon, G. Real-time PCR for detection and quantification of Leishmania in mouse tissues. J. Clin. Microbiol. 2002, 40, 1666–1669. [Google Scholar] [CrossRef]
- Mary, C.; Faraut, F.; Lascombe, L.; Dumon, H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J. Clin. Microbiol. 2004, 42, 5249–5255. [Google Scholar] [CrossRef] [PubMed]
- Galletti, E.; Bonilauri, P.; Bardasi, L.; Fontana, M.C.; Ramini, M.; Renzi, M.; Dosa, G.; Merialdi, G. Development of a minor groove binding probe based real-time PCR for the diagnosis and quantification of Leishmania infantum in dog specimens. Res. Vet. Sci. 2011, 91, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Myskova, J.; Votypka, J.; Volf, P. Leishmania in sand flies: Comparison of quantitative polymerase chain reaction with other techniques to determine the intensity of infection. J. Med. Entomol. 2008, 45, 133–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bezerra-Vasconcelos, D.R.; Melo, L.M.; Albuquerque, É.S.; Luciano, M.C.; Bevilaqua, C.M. Real-time PCR to assess the Leishmania load in Lutzomyia longipalpis sand flies: Screening of target genes and assessment of quantitative methods. Exp. Parasitol. 2011, 129, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.C.; Andreotti, R.; Cominetti, M.C.; Silva, E.A. Detection of Leishmania infantum in Lutzomyia longipalpis captured in Campo Grande, MS. Rev. Bras. Parasitol Vet. 2014, 23, 269–273. [Google Scholar] [CrossRef]
- González, E.; Álvarez, A.; Ruiz, S.; Molina, R.; Jiménez, M. Detection of high Leishmania infantum loads in Phlebotomus perniciosus captured in the leishmaniasis focus of southwestern Madrid region (Spain) by real time PCR. Acta Trop. 2017, 171, 68–73. [Google Scholar] [CrossRef]
- Van der Auwera, G.; Dujardin, J.C. Species typing in dermal leishmaniasis. Clin. Microbiol. Rev. 2015, 28, 265294. [Google Scholar] [CrossRef] [PubMed]
- Rugna, G.; Carra, E.; Corpus, F.; Calzolari, M.; Salvatore, D.; Bellini, R.; Di Francesco, A.; Franceschini, E.; Bruno, A.; Poglayen, G.; et al. Distinct Leishmania infantum Strains Circulate in Humans and Dogs in the Emilia-Romagna Region, Northeastern Italy. Vector Borne Zoonotic Dis. 2017, 17, 409–415. [Google Scholar] [CrossRef]
- Romi, R.; Khoury, C.; Bigliocchi, F.; Maroli, M. Fact Sheet on Mites and Insects of Medical Importance [Schede Guida su Acari e Insetti di Interesse Sanitario]; Istituto Superiore di Sanità: Rome, Italy, 1994. [Google Scholar]
- Dantas-Torres, F.; Tarallo, V.D.; Otranto, D. Morphological keys for the identification of Italian phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae). Parasites Vectors 2014, 7, 479. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Peacock, C.S.; Seeger, K.; Harris, D.; Murphy, L.; Ruiz, J.C.; Quail, M.A.; Peters, N.; Adlem, E.; Tivey, A.; Aslett, M.; et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat. Genet. 2007, 39, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Kuhls, K.; Mauricio, I.L.; Pratlong, F.; Presber, W.; Schonian, G. Analysis of ribosomal DNA internal transcribed spacer sequences of the Leishmania donovani complex. Microbes Infect. 2005, 7, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Rugna, G.; Carra, E.; Bergamini, F.; Calzolari, M.; Salvatore, D.; Corpus, F.; Gennari, W.; Baldelli, R.; Fabbi, M.; Natalini, S.; et al. Multilocus microsatellite typing (MLMT) reveals host-related population structure in Leishmania infantum from northeastern Italy. PLoS Negl. Trop. Dis. 2018, 12, e0006595. [Google Scholar] [CrossRef] [PubMed]
- Sainudiin, R.; Durrett, R.T.; Aquadro, C.F.; Nielsen, R. Microsatellite mutation models: Insights from a comparison of humans and chimpanzees. Genetics 2004, 168, 383–395. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef]
- Piarroux, R.; Fontes, M.; Perasso, R.; Gambarelli, F.; Joblet, C.; Dumon, H.; Quilici, M. Phylogenetic relationship between Old World Leishmania strains revealed by analysis of a repetitive DNA sequence. Mol. Biochem. Parasitol. 1995, 73, 249–252. [Google Scholar] [CrossRef]
- Harrat, Z.; Pratlong, F.; Belazzoug, S.; Dereure, J.; Deniau, M.; Rioux, J.A.; Belkaid, M.; Dedet, J.P. Leishmania infantum and L. major in Algeria. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 625–629. [Google Scholar] [CrossRef]
- Izri, M.A.; Belazzoug, S. Phlebotomus (Larroussius) perfiliewi naturally infected with dermotropic Leishmania infantum at Tenes, Algeria. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 399. [Google Scholar] [CrossRef]
- Kallel, K.; Haouas, N.; Pratlong, F.; Kaouech, E.; Belhadj, S.; Anane, S.; Dedet, J.P.; Babba, H.; Chaker, E. Cutaneous leishmaniasis caused by Leishmania infantum MON-24 in Tunisia: Extension of the focus to the center of the country. Bull. Soc. Pathol. Exot. 2008, 101, 29–31. [Google Scholar] [PubMed]
- Bennai, K.; Tahir, D.; Lafri, I.; Bendjaballah-Laliam, A.; Bitam, I.; Parola, P. Molecular detection of Leishmania infantum DNA and host blood meal identification in Phlebotomus in a hypoendemic focus of human leishmaniasis in northern Algeria. PLoS Negl. Trop. Dis. 2018, 12, e0006513. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Ferroglio, E.; Solano-Gallego, L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol. Res. 2014, 113, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, E.; Moreno, J.; Cruz, I. What is responsible for a large and unusual outbreak of leishmaniasis in Madrid? Trends Parasitol. 2013, 29, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Barriga, D.; Parreira, R.; Maia, C.; Afonso, M.O.; Blanco-Ciudad, J.; Serrano, F.J.; Pérez-Martín, J.E.; Gómez-Gordo, L.; Campino, L.; Reina, D.; et al. Detection of Leishmania DNA and blood meal sources in phlebotomine sand flies (Diptera: Psychodidae) in western of Spain: Update on distribution and risk factors associated. Acta Trop. 2016, 164, 414–424. [Google Scholar] [CrossRef]
- Jiménez, M.; González, E.; Iriso, A.; Marco, E.; Alegret, A.; Fúster, F.; Molina, R. Detection of Leishmania infantum and identification of blood meals in Phlebotomus perniciosus from a focus of human leishmaniosis in Madrid. Spain. Parasitol. Res. 2013, 112, 2453–2459. [Google Scholar] [CrossRef]
- Dvorak, V.; Shaw, J.; Volf, P. Parasite Biology: The Vectors. In The Leishmaniases: Old Neglected Tropical Diseases; Bruschi, F., Gradoni, L., Eds.; Springer International Publishing: Berlin, Germany, 2018; pp. 31–77. [Google Scholar]
- Sadlova, J.; Volf, P. Occurrence of Leishmania major in sand fly urine. Parasitology 1999, 118, 455–460. [Google Scholar] [CrossRef]


| Date | N | Tested in Pool | Individually-Tested | pw/pe | Leish-Pos Pools/Total Pools |
|---|---|---|---|---|---|
| 27-07-16 | 2728 | 1000 | 209 | 95/5 | 1/10 |
| 11-08-16 | 431 | 300 | 57 | 16/2 | 3/3 |
| 18-08-16 | 821 | 500 | 80 | 101/4 | 5/5 |
| 31-08-16 | 1084 | 500 | 176 | 100/3 | 5/5 |
| 14-09-16 | 1050 | 700 | 80 | 101/1 | 6/7 |
| 6114 | 4030 | 602 | 413 (96.5%)/15 (3.5%) | 20/30 |
| Date | Female | Male | ||||
|---|---|---|---|---|---|---|
| N | <10 (%) | ≥10 (%) | N | <10 (%) | ≥10 | |
| 27-07-16 | 51 | 6 (11.8) | - | 30 | - | - |
| 11-08-16 | 52 | 5 (9.6) | - | 5 | - | - |
| 18-08-16 | 50 | 20 (40.0) | 3 (6.0) | 30 | 3 (10.0) | - |
| 31-08-16 | 50 | 19 (38.0) | 4 (8.0) | 32 | 11 (34.4) | - |
| 14-09-16 | 50 | 6 (12.0) | 5 (10.0) | 30 | - | - |
| - | 253 | 56 (22.1) | 12 (4.7) | 127 | 14 (11.0) | - |
| Date | Sex | <0.01 | 0.01–0.1 | 0.1–1 | 1–10 | 10–102 | 102–103 | 103–104 | 104–105 | >105 |
|---|---|---|---|---|---|---|---|---|---|---|
| 27-07-16 | F | 5 | 1 | |||||||
| 11-08-16 | F | 3 | 2 | |||||||
| 18-08-16 | F | 18 | 1 | 1 | 2 | 1 | ||||
| 18-08-16 | M | 3 | ||||||||
| 31-08-16 | F | 5 | 10 | 4 | 2 | 1 | 1 | |||
| 31-08-16 | M | 3 | 5 | 3 | ||||||
| 14-09-16 | F | 3 | 2 | 1 | 2 | 3 | ||||
| Total | 34 | 25 | 9 | 2 | 2 | 1 | 2 | 4 | 3 |
| Faeces and Excreta | Females | Males | ||||
|---|---|---|---|---|---|---|
| Jar | Sampling Date | QPN | N/N Pools | Positive Pools (QPN) | N/N Pools | Positive Pools (QPN) |
| 1 | 17-08-17 | 0.3 | 142/6 | 2 (945, 6000) | 62/3 | 1 (0.5) |
| 2 | 29-08-17 | 7 | 71/3 | 2 (2, 2621) | 21/1 | - |
| 3 | 29-08-17 | 0.1 | 58/3 | 1 (0.5) | 23/1 | - |
| 4 | 13-09-17 | - | 5/1 | 1 (0.05) | 26/1 | 1 (0.05) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzolari, M.; Carra, E.; Rugna, G.; Bonilauri, P.; Bergamini, F.; Bellini, R.; Varani, S.; Dottori, M. Isolation and Molecular Typing of Leishmania infantum from Phlebotomus perfiliewi in a Re-Emerging Focus of Leishmaniasis, Northeastern Italy. Microorganisms 2019, 7, 644. https://doi.org/10.3390/microorganisms7120644
Calzolari M, Carra E, Rugna G, Bonilauri P, Bergamini F, Bellini R, Varani S, Dottori M. Isolation and Molecular Typing of Leishmania infantum from Phlebotomus perfiliewi in a Re-Emerging Focus of Leishmaniasis, Northeastern Italy. Microorganisms. 2019; 7(12):644. https://doi.org/10.3390/microorganisms7120644
Chicago/Turabian StyleCalzolari, Mattia, Elena Carra, Gianluca Rugna, Paolo Bonilauri, Federica Bergamini, Romeo Bellini, Stefania Varani, and Michele Dottori. 2019. "Isolation and Molecular Typing of Leishmania infantum from Phlebotomus perfiliewi in a Re-Emerging Focus of Leishmaniasis, Northeastern Italy" Microorganisms 7, no. 12: 644. https://doi.org/10.3390/microorganisms7120644
APA StyleCalzolari, M., Carra, E., Rugna, G., Bonilauri, P., Bergamini, F., Bellini, R., Varani, S., & Dottori, M. (2019). Isolation and Molecular Typing of Leishmania infantum from Phlebotomus perfiliewi in a Re-Emerging Focus of Leishmaniasis, Northeastern Italy. Microorganisms, 7(12), 644. https://doi.org/10.3390/microorganisms7120644







