Oral Microbiota Composition and Antimicrobial Antibody Response in Patients with Recurrent Aphthous Stomatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Humoral Response to Bacterial and Fungal Antigens
Preparation of Lysates
2.3. ELISA
2.4. Analysis of Microbiota Composition
2.4.1. DNA Extraction
2.4.2. PCR Amplification
2.4.3. Sequencing Data Analysis
2.4.4. Bacteria–Fungi Correlation
3. Results
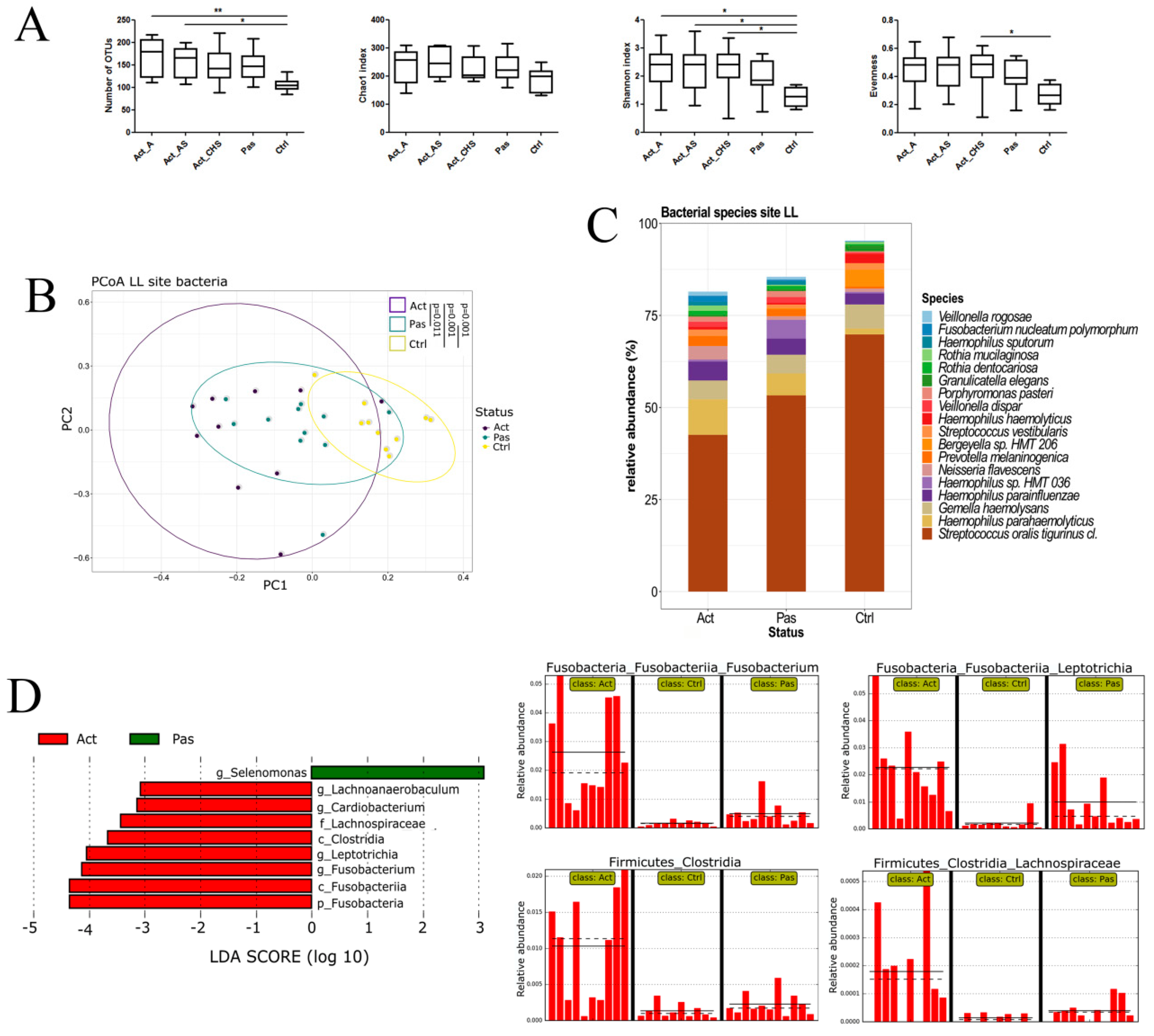
3.1. Sampling Site and Disease Status Have an Effect on the Observed Microbiota Diversity
3.2. Descriptive Analysis of Bacterial Microbiota in the Oral Cavity of RAS Patients with Special Emphasis on the Lower Labial Mucosa
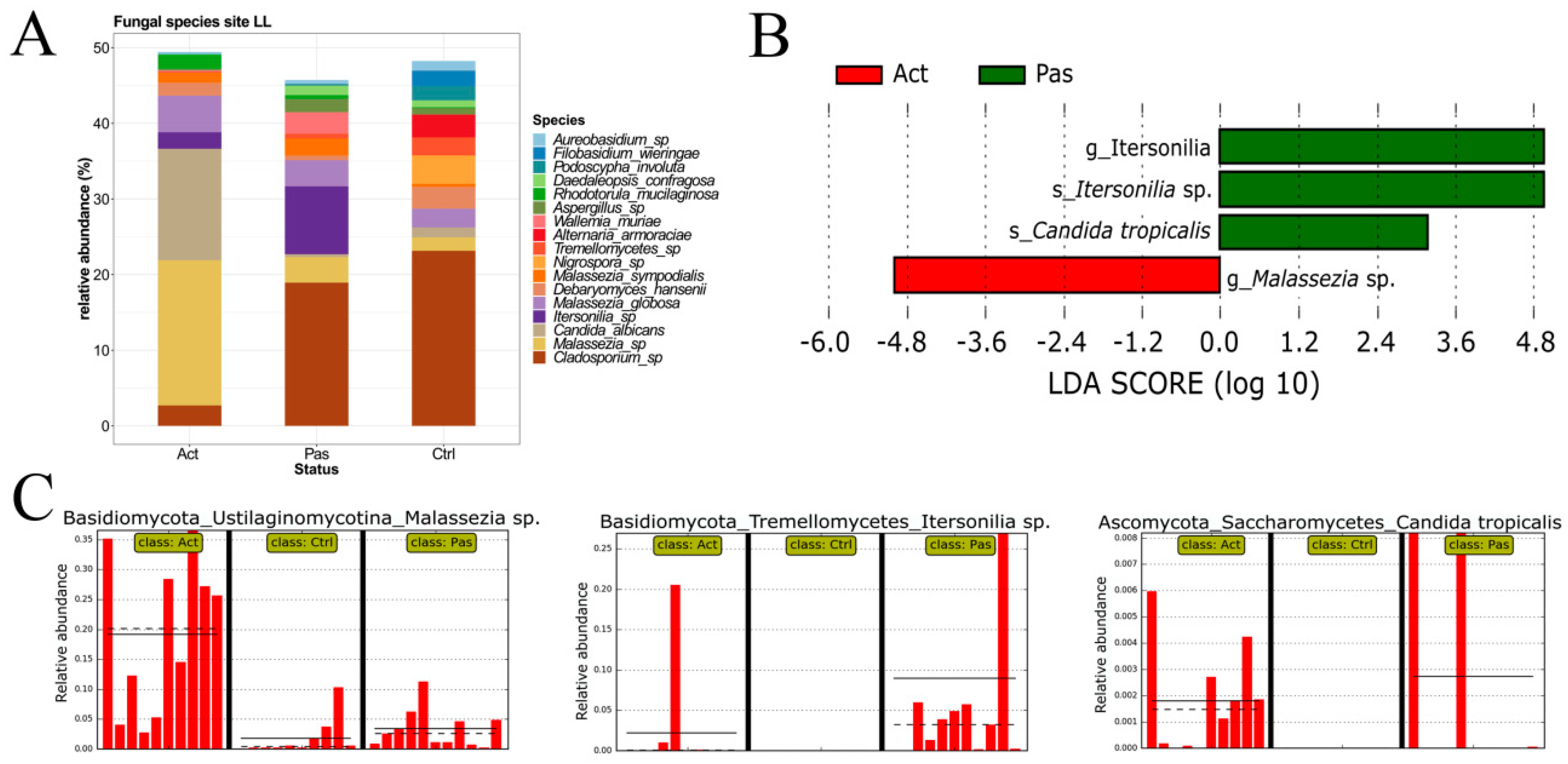
3.3. Descriptive Analysis of Fungal Microbiota in the Oral Cavity of RAS Patients with Special Emphasis on the Lower Labial Mucosa
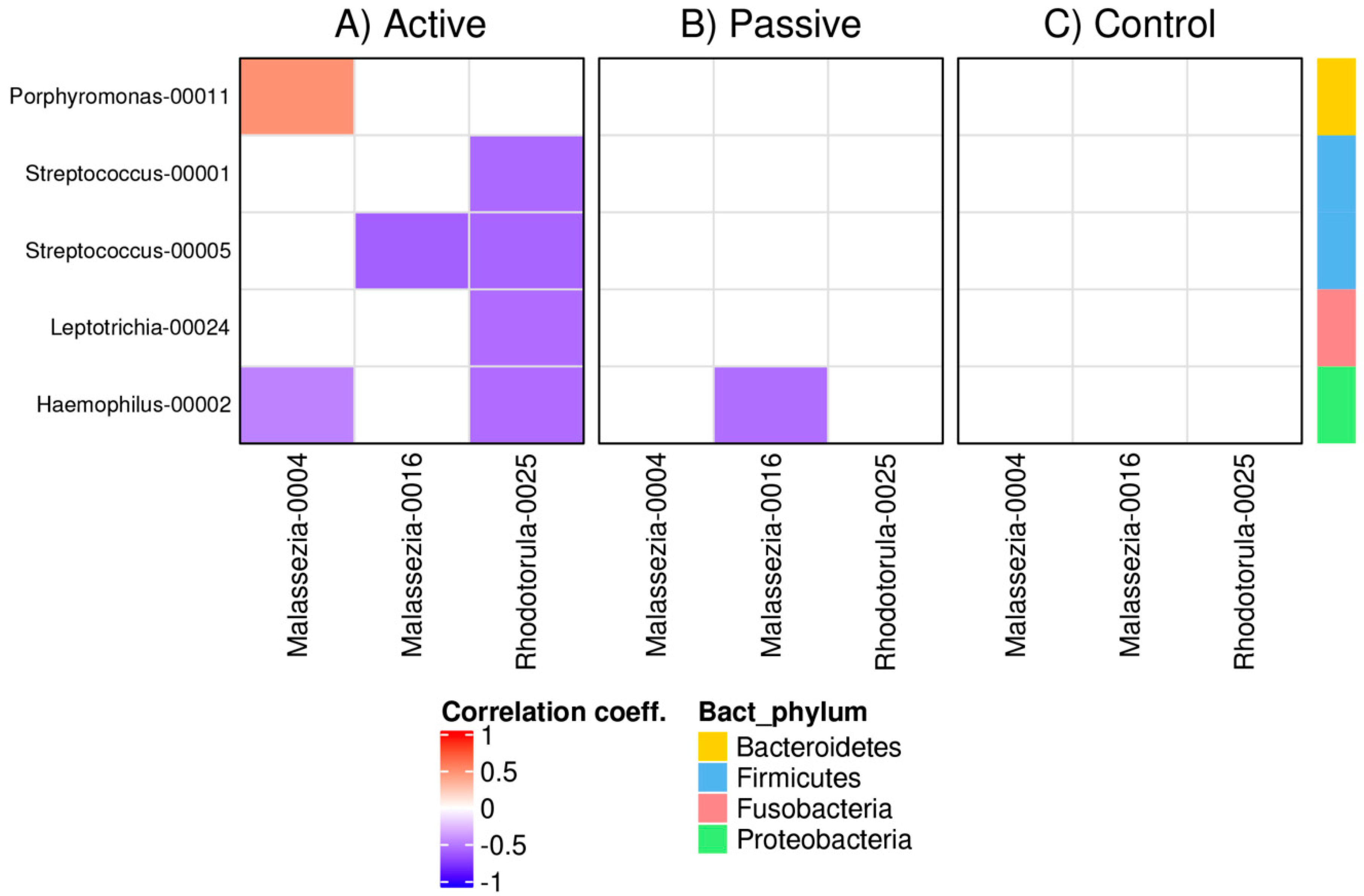
3.4. Bacterial and Fungal Correlation Networks in RAS
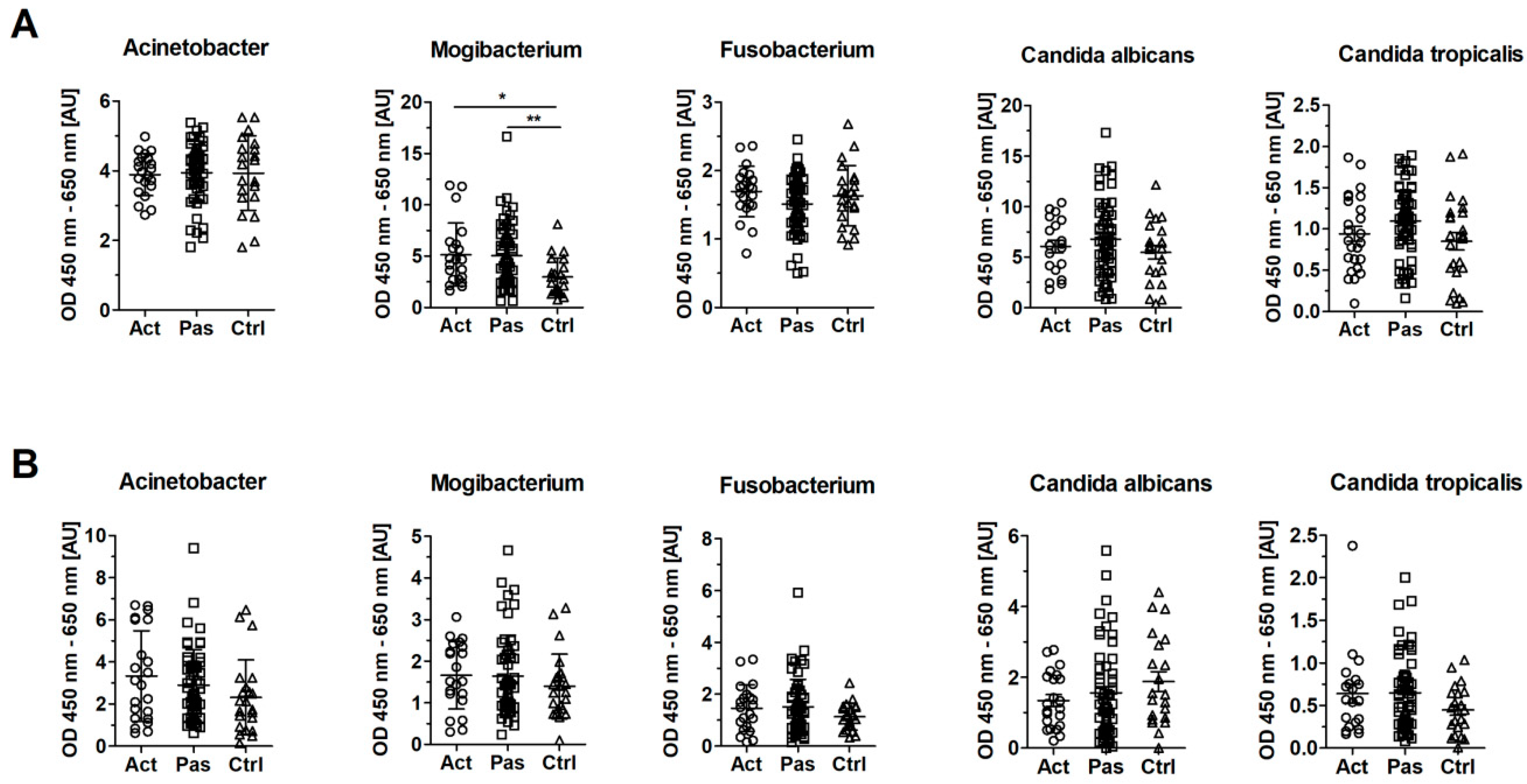
3.5. Serum Levels of IgG Against Mogibacterium timidum are Elevated in Patients with RAS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Porter, S.R.; Scully, C.; Pedersen, A. Recurrent aphthous stomatitis. Crit. Rev. Oral Biol. Med. 1998, 9, 306–321. [Google Scholar] [CrossRef]
- Scully, C.; Porter, S. Oral mucosal disease: Recurrent aphthous stomatitis. Br. J. Oral Maxillofac. Surg. 2008, 46, 198–206. [Google Scholar] [CrossRef]
- Edgar, N.R.; Saleh, D.; Miller, R.A. Recurrent aphthous stomatitis: A review. J. Clin. Aesthet. Derm. 2017, 10, 26–36. [Google Scholar]
- Akintoye, S.O.; Greenberg, M.S. Recurrent aphthous stomatitis. Dent. Clin. N. Am. 2014, 58, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.; Jain, H.; Diwan, N.; Khedkar, S.; Shete, A.; Durkar, S. Recurrent aphthous stomatitis: A review. J. Oral Pathol. Med. 2012, 41, 577–583. [Google Scholar] [CrossRef]
- Bankvall, M.; Sjoberg, F.; Gale, G.; Wold, A.; Jontell, M.; Ostman, S. The oral microbiota of patients with recurrent aphthous stomatitis. J. Oral Microbiol. 2014, 6, 25739. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, Y.S.; Baek, K.J.; Yoon, S.H.; Park, H.K.; Choi, Y. Mucosal and salivary microbiota associated with recurrent aphthous stomatitis. BMC Microbiol. 2016, 16, 57. [Google Scholar] [CrossRef]
- Adler, I.; Muino, A.; Aguas, S.; Harada, L.; Diaz, M.; Lence, A.; Labbrozzi, M.; Muino, J.M.; Elsner, B.; Avagnina, A.; et al. Helicobacter pylori and oral pathology: Relationship with the gastric infection. World J. Gastroenterol. 2014, 20, 9922–9935. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Asmar, M.; Bagherzadeh, A.H.; Ekbataninezhad, S. Helicobacter pylori infection in oral lesions of patients with recurrent aphthous stomatitis. Med. Sci. Monit. 2005, 11, 576–579. [Google Scholar]
- Tas, D.A.; Yakar, T.; Sakalli, H.; Serin, E. Impact of Helicobacter pylori on the clinical course of recurrent aphthous stomatitis. J. Oral Pathol. Med. 2013, 42, 89–94. [Google Scholar] [CrossRef]
- Hasan, A.; Childerstone, A.; Pervin, K.; Shinnick, T.; Mizushima, Y.; Van der Zee, R.; Vaughan, R.; Lehner, T. Recognition of a unique peptide epitope of the mycobacterial and human heat shock protein 65-60 antigen by T cells of patients with recurrent oral ulcers. Clin. Exp. Immunol. 1995, 99, 392–397. [Google Scholar] [CrossRef]
- Gadekar, N.B.; Hosmani, J.V.; Bhat, K.G.; Kotrashetti, V.S.; Nayak, R.S.; Babji, D.V.; Pattanshetty, S.M.; Joshi, V.M.; Bansode, R.A. Detection of antibodies against Aggregatibacter actinomycetemcomitans in serum and saliva through ELISA in periodontally healthy individuals and individuals with chronic periodontitis. Microb. Pathog. 2018, 125, 438–442. [Google Scholar] [CrossRef]
- Ship, J.A.; Chavez, E.M.; Doerr, P.A.; Henson, B.S.; Sarmadi, M. Recurrent aphthous stomatitis. Quintessence Int. 2000, 31, 95–112. [Google Scholar] [CrossRef]
- Consortium, T.H.M.P. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Souza, M.N.; Ortiz, S.O.; Mello, M.M.; Oliveira Fde, M.; Severo, L.C.; Goebel, C.S. Comparison between four usual methods of identification of Candida species. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 281–287. [Google Scholar] [CrossRef]
- Zakostelska, Z.; Kverka, M.; Klimesova, K.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Hornova, M.; Srutkova, D.; Hudcovic, T.; Ridl, J.; et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE 2011, 6, e27961. [Google Scholar] [CrossRef]
- Vetrovsky, T.; Baldrian, P.; Morais, D.; Berger, B. SEED 2: A user-friendly platform for amplicon high-throughput sequencing data analyses. Bioinformatics 2018. [Google Scholar] [CrossRef]
- Aronesty, E. Comparison of sequencing utility programs. Open Bioinform. J. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; Wit, P.D.; Sánchez-García, M.; Ebersberger, I.; Sousa, F.D.; et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, K.; Lowe, T.; Meharg, C.; Berry, S.H.; Foley, J.; Hold, G.L. Mucosal microbiome in patients with recurrent aphthous stomatitis. J. Dent. Res. 2015, 94, 87S–94S. [Google Scholar] [CrossRef]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Riggio, M.P.; Lennon, A.; Ghodratnama, F.; Wray, D. Lack of association between Streptococcus oralis and recurrent aphthous stomatitis. J. Oral Pathol. Med. 2000, 29, 26–32. [Google Scholar] [CrossRef]
- Wang, X.; Du, L.; You, J.; King, J.B.; Cichewicz, R.H. Fungal biofilm inhibitors from a human oral microbiome-derived bacterium. Org. Biomol. Chem. 2012, 10, 2044–2050. [Google Scholar] [CrossRef]
- Morales, D.K.; Grahl, N.; Okegbe, C.; Dietrich, L.E.; Jacobs, N.J.; Hogan, D.A. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. MBio 2013, 4, e00526-12. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Hall, M.W.; Singh, N.; Ng, K.F.; Lam, D.K.; Goldberg, M.B.; Tenenbaum, H.C.; Neufeld, J.D.; Beiko, R.G.; Senadheera, D.B. Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. NPJ Biofilms Microbiomes 2017, 3, 2. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Socransky, S.S. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000 1994, 5, 78–111. [Google Scholar] [CrossRef] [PubMed]
- Chenicheri, S.; Usha, R.; Ramachandran, R.; Thomas, V.; Wood, A. Insight into Oral Biofilm: Primary, Secondary and Residual Caries and Phyto-Challenged Solutions. Open Dent. J. 2017, 11, 312–333. [Google Scholar] [CrossRef] [PubMed]
- Klimesova, K.; Jiraskova Zakostelska, Z.; Tlaskalova-Hogenova, H. Oral bacterial and fungal microbiome impacts colorectal carcinogenesis. Front. Microbiol. 2018, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.K.; David, M.S.; Li, L.; Heider, T.N.; Peterson, J.D.; Montano, E.A.; Dongari-Bagtzoglou, A.; Diaz, P.I.; Strausbaugh, L.D. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: Discovery of Malassezia as a prominent commensal. PLoS ONE 2014, 9, e90899. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome. Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Hiruma, M.; Cho, O.; Kurakado, S.; Sugita, T.; Ikeda, S. Genotype analyses of human commensal scalp fungi, Malassezia globosa, and Malassezia restricta on the scalps of patients with dandruff and healthy subjects. Mycopathologia 2014, 177, 263–269. [Google Scholar] [CrossRef]
- Tajima, M.; Sugita, T.; Nishikawa, A.; Tsuboi, R. Molecular analysis of Malassezia microflora in seborrheic dermatitis patients: Comparison with other diseases and healthy subjects. J. Investig. Dermatol. 2008, 128, 345–351. [Google Scholar] [CrossRef]
- Saxena, R.; Mittal, P.; Clavaud, C.; Dhakan, D.B.; Hegde, P.; Veeranagaiah, M.M.; Saha, S.; Souverain, L.; Roy, N.; Breton, L.; et al. Comparison of healthy and dandruff scalp microbiome reveals the role of commensals in scalp health. Front. Cell. Infect. Microbiol. 2018, 8, 346. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Farina, S.; Truong, D.T.; Asnicar, F.; Zolfo, M.; Beghini, F.; Armanini, F.; Jousson, O.; De Sanctis, V.; et al. Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. NPJ Biofilms Microbiomes 2017, 3, 14. [Google Scholar] [CrossRef]
- Stehlikova, Z.; Kostovcik, M.; Kostovcikova, K.; Kverka, M.; Juzlova, K.; Rob, F.; Hercogova, J.; Bohac, P.; Pinto, Y.; Uzan, A.; et al. Dysbiosis of skin microbiota in psoriatic Patients: Co-occurrence of fungal and bacterial communities. Front. Microbiol. 2019, 10, 438. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef]
- Adedeji, A.R.; Adeniyi, D.O. Comparative fungal profile of tea leaves from highland and lowland in Nigeria. Afr. J. Agric. Res. 2014, 10, 1531–1535. [Google Scholar] [CrossRef][Green Version]
- McGovern, R.J.; Horita, H.; Stiles, C.M.; Seijo, T.E. Host range of Itersonilia perplexans and management of itersonilia petal blight of china aster. Plant Health Prog. 2006. [Google Scholar] [CrossRef]
- Bubici, G. First report of Itersonilia perplexans on Anethum graveolens in Italy. J. Plant Pathol. 2015, 97, 221. [Google Scholar] [CrossRef]
- Zuza-Alves, D.L.; Silva-Rocha, W.P.; Chaves, G.M. An Update on Candida tropicalis based on basic and clinical approaches. Front. Microbiol 2017, 8, 1927. [Google Scholar] [CrossRef]
- Albandar, J.M.; DeNardin, A.M.; Adesanya, M.R.; Diehl, S.R.; Winn, D.M. Associations between serum antibody levels to periodontal pathogens and early-onset periodontitis. J. Periodontol. 2001, 72, 1463–1469. [Google Scholar] [CrossRef]
- Bartova, J.; Krejsa, O.; Sirova, M.; Tlaskalova, H.; Prochazkova, J.; Duskova, J. Local antibodies and cytokine responses in crevicular fluid of patients with juvenile periodontitis. Adv. Exp. Med. Biol. 1995, 371B, 1109–1112. [Google Scholar]
- Moore, W.E.; Holdeman, L.V.; Cato, E.P.; Smibert, R.M.; Burmeister, J.A.; Palcanis, K.G.; Ranney, R.R. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985, 48, 507–519. [Google Scholar]
- Stoitsova, S.; Ivanova, R.; Dimova, I. Lectin-binding epitopes at the surface of Escherichia coli K-12: Examination by electron microscopy, with special reference to the presence of a colanic acid-like polymer. J. Basic Microbiol. 2004, 44, 296–304. [Google Scholar] [CrossRef]
- Colmer-Hamood, J.A.; Dzvova, N.; Kruczek, C.; Hamood, A.N. In vitro analysis of pseudomonas aeruginosa virulence using conditions that mimic the environment at specific infection sites. Prog. Mol. Biol. Transl. Sci. 2016, 142, 151–191. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G. The microbiota, a necessary element of immunity. CR Biol. 2018, 341, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liao, C.; Zhang, B.; Tolbert, W.D.; He, W.; Dai, Z.; Zhang, W.; Yuan, W.; Pazgier, M.; Liu, J.; et al. Human enteric alpha-defensin 5 promotes shigella infection by enhancing bacterial adhesion and invasion. Immunity 2018, 48, 1233–1244.e6. [Google Scholar] [CrossRef] [PubMed]
- Porat, R.; Clark, B.D.; Wolff, S.M.; Dinarello, C.A. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science 1991, 254, 430–432. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stehlikova, Z.; Tlaskal, V.; Galanova, N.; Roubalova, R.; Kreisinger, J.; Dvorak, J.; Prochazkova, P.; Kostovcikova, K.; Bartova, J.; Libanska, M.; et al. Oral Microbiota Composition and Antimicrobial Antibody Response in Patients with Recurrent Aphthous Stomatitis. Microorganisms 2019, 7, 636. https://doi.org/10.3390/microorganisms7120636
Stehlikova Z, Tlaskal V, Galanova N, Roubalova R, Kreisinger J, Dvorak J, Prochazkova P, Kostovcikova K, Bartova J, Libanska M, et al. Oral Microbiota Composition and Antimicrobial Antibody Response in Patients with Recurrent Aphthous Stomatitis. Microorganisms. 2019; 7(12):636. https://doi.org/10.3390/microorganisms7120636
Chicago/Turabian StyleStehlikova, Zuzana, Vojtech Tlaskal, Natalie Galanova, Radka Roubalova, Jakub Kreisinger, Jiri Dvorak, Petra Prochazkova, Klara Kostovcikova, Jirina Bartova, Marketa Libanska, and et al. 2019. "Oral Microbiota Composition and Antimicrobial Antibody Response in Patients with Recurrent Aphthous Stomatitis" Microorganisms 7, no. 12: 636. https://doi.org/10.3390/microorganisms7120636
APA StyleStehlikova, Z., Tlaskal, V., Galanova, N., Roubalova, R., Kreisinger, J., Dvorak, J., Prochazkova, P., Kostovcikova, K., Bartova, J., Libanska, M., Cermakova, R., Schierova, D., Fassmann, A., Borilova Linhartova, P., Coufal, S., Kverka, M., Izakovicova-Holla, L., Petanova, J., Tlaskalova-Hogenova, H., & Jiraskova Zakostelska, Z. (2019). Oral Microbiota Composition and Antimicrobial Antibody Response in Patients with Recurrent Aphthous Stomatitis. Microorganisms, 7(12), 636. https://doi.org/10.3390/microorganisms7120636





