Isolation and Identification of Lactobacillus plantarum HFY05 from Natural Fermented Yak Yogurt and Its Effect on Alcoholic Liver Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Purification of LAB
2.2. Preliminary Identification of the LAB
2.3. Polymerase Chain Reaction (PCR) Amplification and Electrophoretic Detection of Genomic DNA from LAB
2.4. Resistance of LAB to 0.3% Bile Salts
2.5. Ability of LAB to Tolerate Artificial Gastric Juice
2.6. Experiment Strains
2.7. Establishment of the Alcoholic Liver Injury Model
2.8. Determination of Serum AST, ALT, ALP, TG, TC, BUN, ALB, SOD, NO, CAT, MDA, and GSH-Px Levels
2.9. Determination of Serum TNF-α, INF-γ, IL-6, and IL-12 Levels
2.10. Pathological Observations of Liver Tissue
2.11. qPCR Assay
2.12. Western Blot Analysis
2.13. Detection of Intestinal Microbiota in Feces
2.14. Statistical Analysis
3. Results
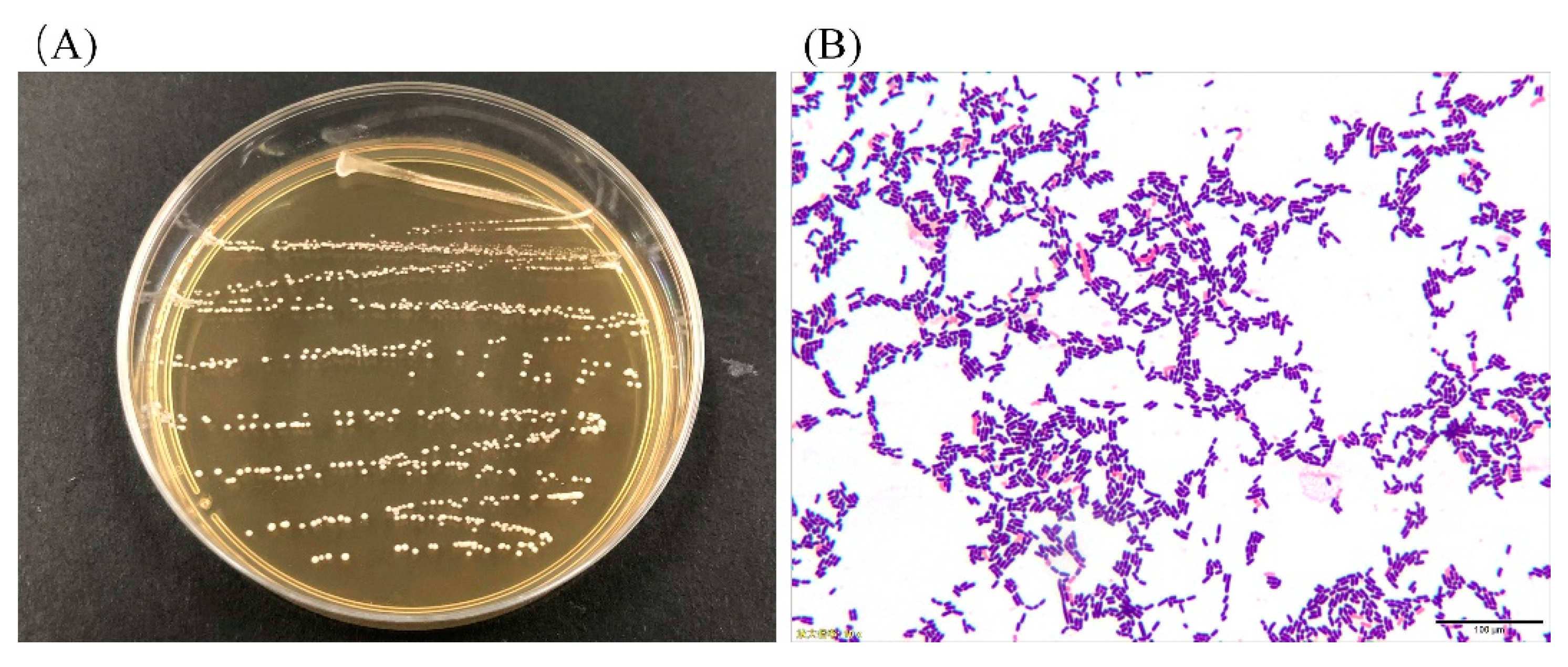
3.1. Colony Morphology and LAB Cell Morphology
3.2. Analysis of 16S rDNA of the LAB
3.3. Resistance of LAB to Artificial Gastric Juice and Bile Salts
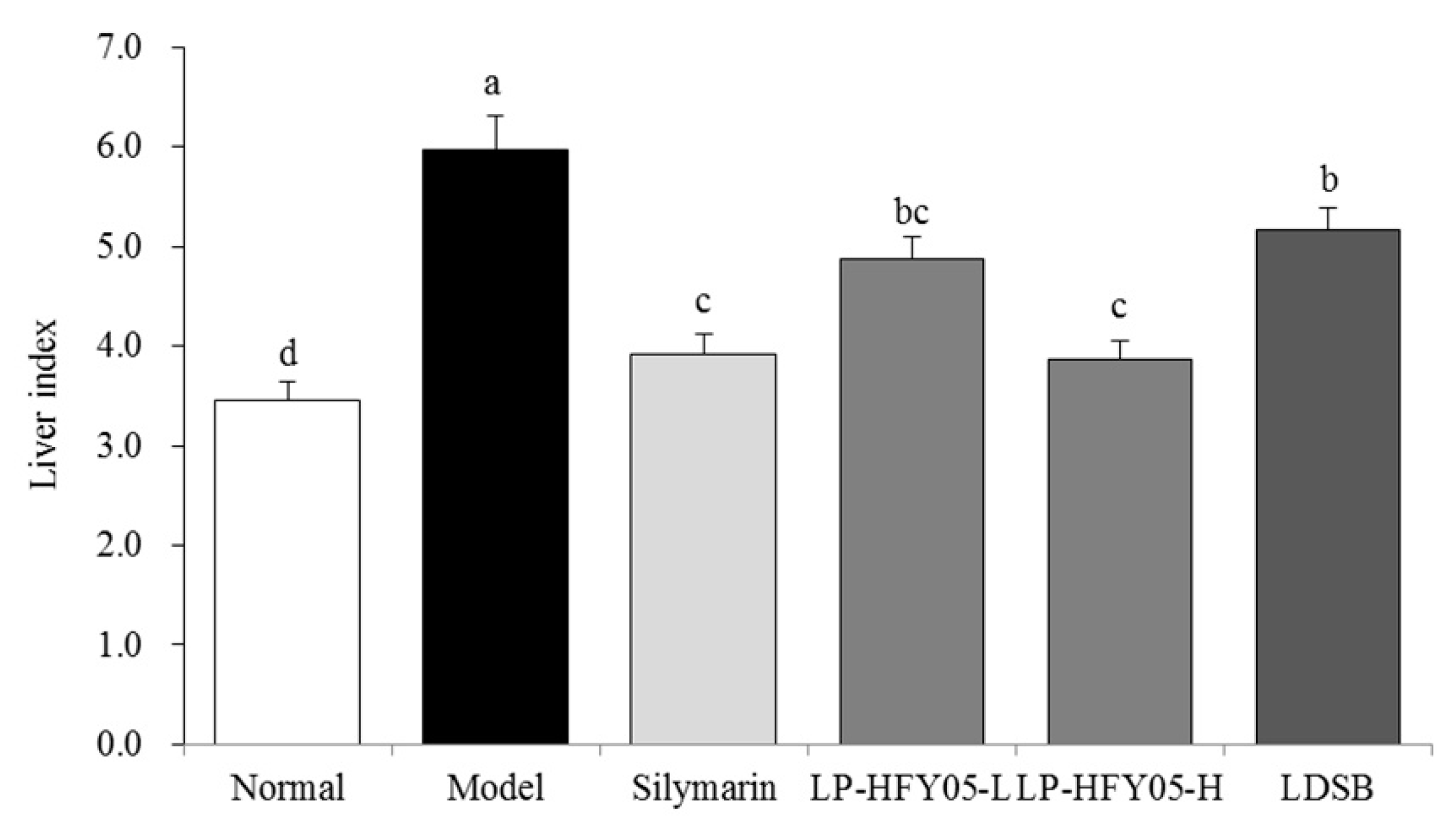
3.4. Mice Liver Indices
3.5. Serum Levels of AST, ALT, ALP, TG, TC, BUN, and ALB in the Mice
3.6. Serum Levels of SOD, NO, CAT, MDA, and GSH-Px in the Mice
3.7. Serum Levels of IL-6, IL-12, TNF-α, and IFN-γ in Mice
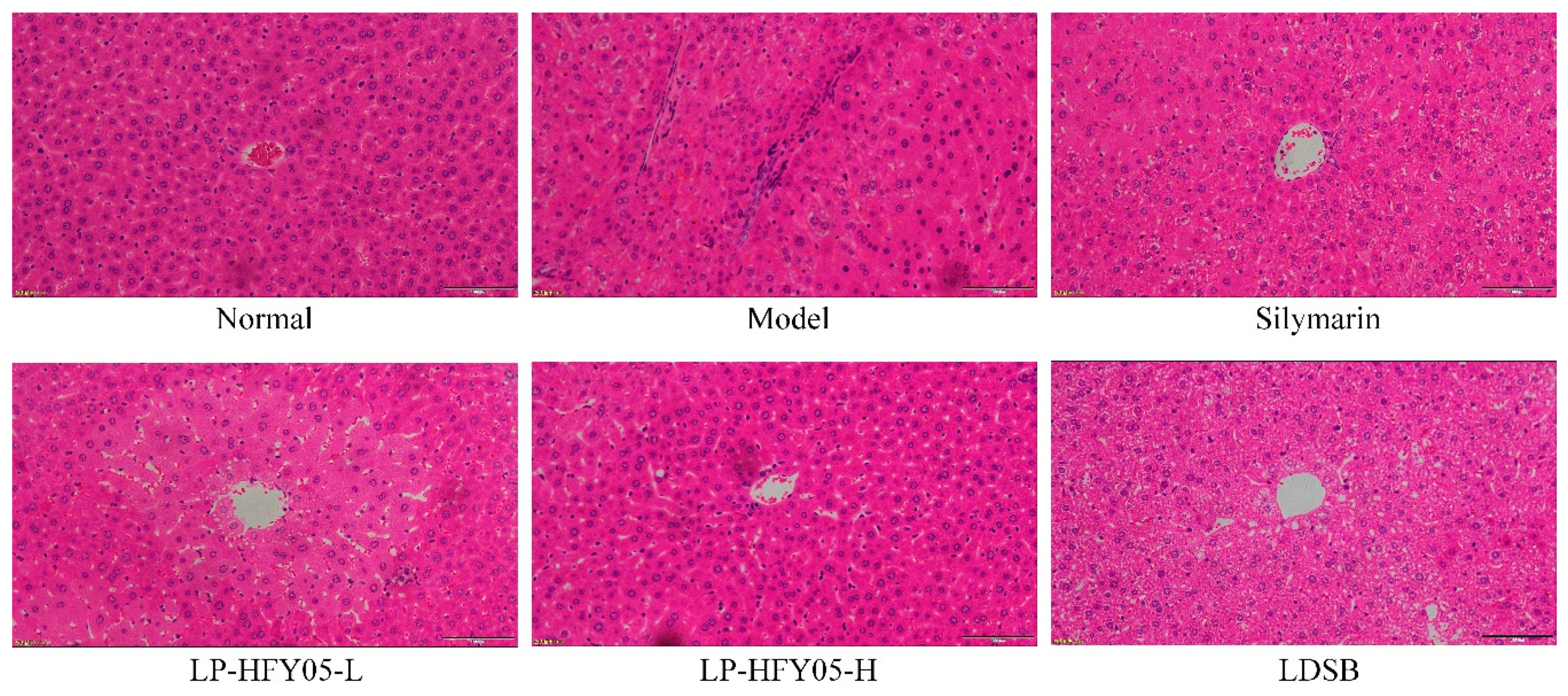
3.8. Pathological Observations of Mice Liver Tissue
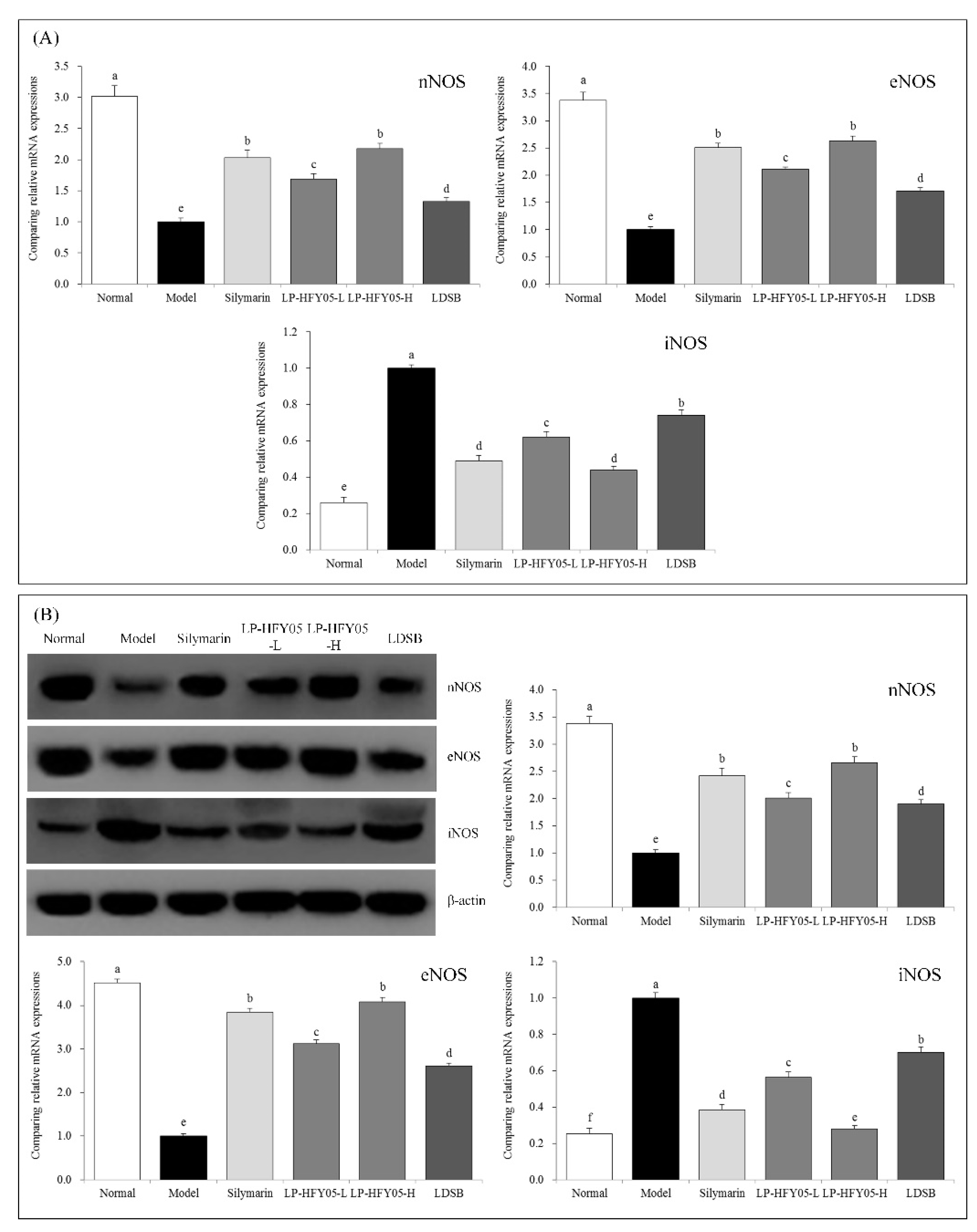
3.9. nNOS, eNOS, and iNOS mRNA and Protein Expression in Mouse Liver Tissue
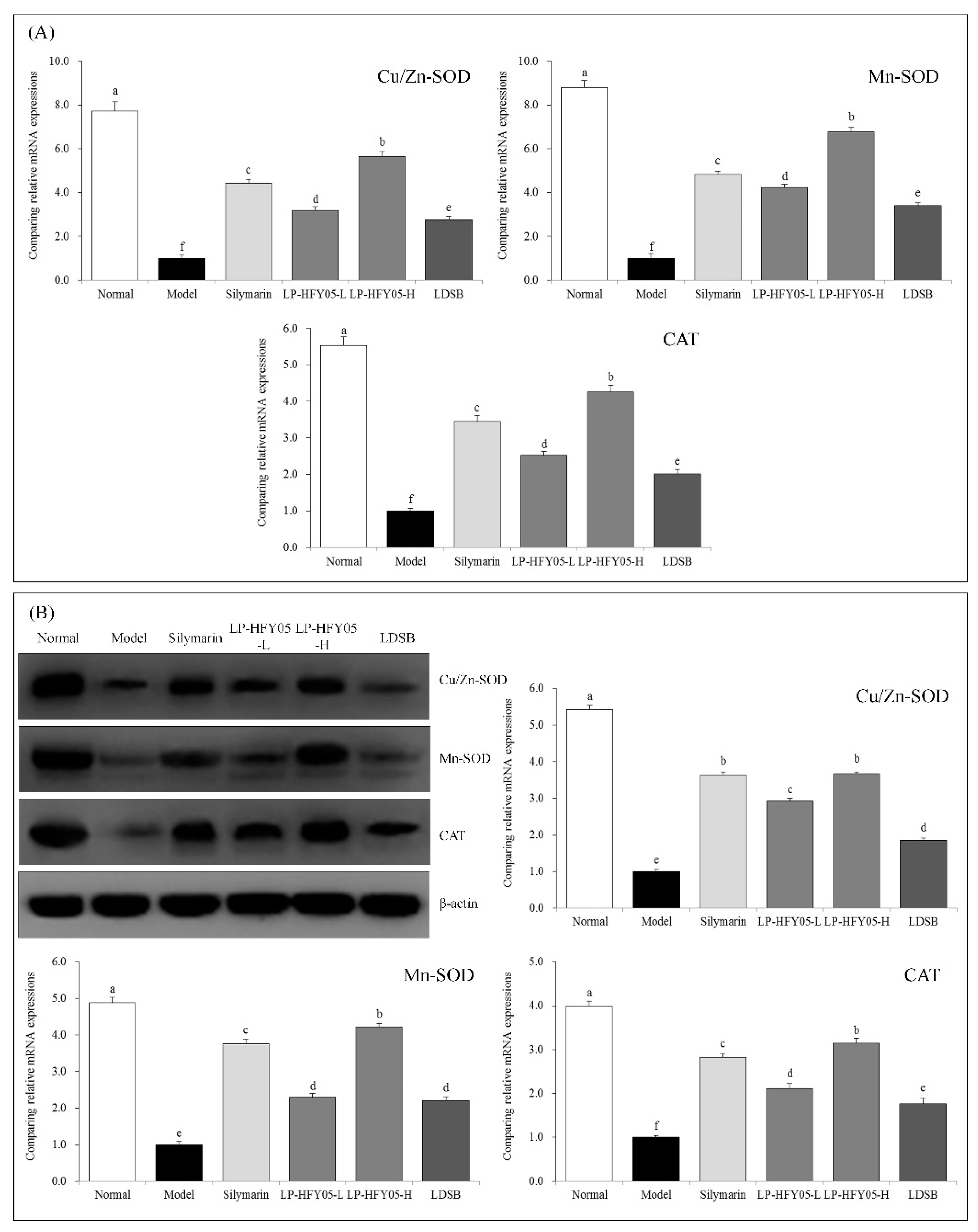
3.10. Cu/Zn-SOD, Mn-SOD, and CAT mRNA and Protein Expression in Mouse Liver Tissue
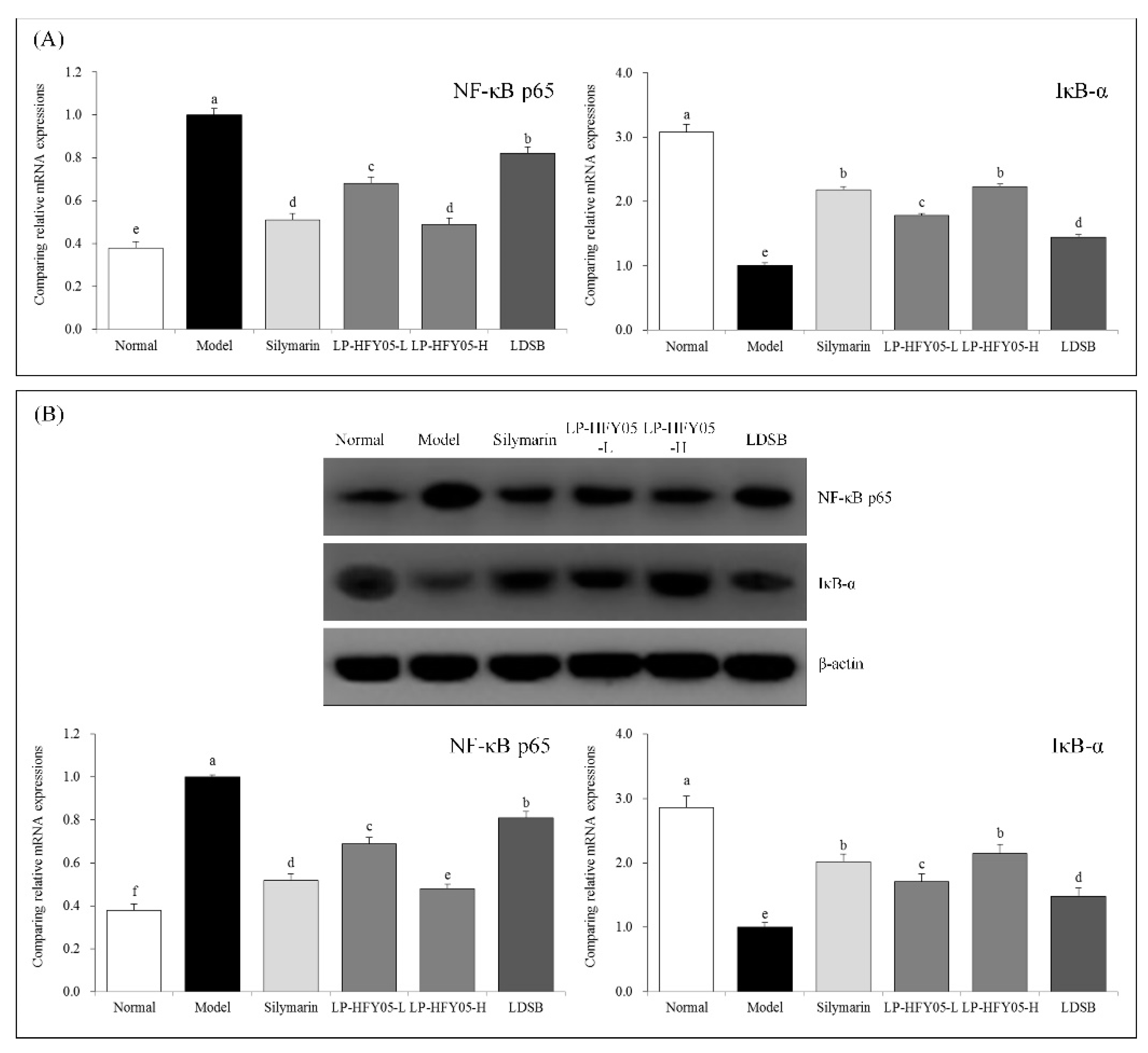
3.11. NF-κB p65 and IκB-α mRNA and Protein Expression in Mouse Liver Tissue
3.12. Intestinal Fecal Microbiota of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Zhao, X.; Wang, H.; Yang, Z.; Li, J.; Suo, H. Prevent effects of Lactobacillus fermentum HY01 on dextran sulfate sodium-induced colitis in mice. Nutrients 2017, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, X.H.; Chen, J.X.; Du, M.Y.; Kan, J.Q. Isolation and identification of lactic acid bacteria from natural yak yogurt in Tibet plateau pastoral areas of Tibet and western Sichuan. Food Sci. 2013, 34, 150–155. [Google Scholar]
- Liao, Y.T.; Wu, J.; Long, M.; Du, M.Y.; Kan, J.Q. Screening of dominant lactic acid bacteria from naturally fermented yak milk in Tibetan pastoral areas and optimization of fermentation conditions for yak yogurt production. Food Sci. 2015, 36, 140–144. [Google Scholar]
- Qian, Y.; Long, X.Y.; Pan, Y.N.; Li, G.J.; Zhao, X. Isolation and identification of lactic acid bacteria (Lactobacillus plantarum YS2) from yak yogurt and its probiotic properties. Biomed. Res. 2018, 29, 815–820. [Google Scholar] [CrossRef]
- Qin, H.; Liang, Q.; Zhang, W.B.; Zhang, Y.; Mi, L. Isolation and identification of lactic acid bacteria with high fermentation performance from naturally fermented yak yogurt in Tibetan pastoral areas of Gansu. Food Sci. 2013, 34, 241–246. [Google Scholar]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous food fermentations and potential risks for human health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Ripari, V.; Cecchi, T.; Berardi, E. Microbiological characterisation and volatiles profile of model, ex-novo, and traditional Italian white wheat sourdoughs. Food Chem. 2016, 205, 297–307. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini-review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Mutanda, T.; Olaniran, A.O. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr. Res. 2016, 60, 29630. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Aguirre, L.; Milton-Laskibar, I.; Fernández-Quintela, A.; Trepiana, J.; Kajarabille, N.; Mosqueda-Solís, A.; González, M.; Portillo, M.P. Relationship between changes in microbiota and liver steatosis induced by high-fat feeding—A review of rodent models. Nutrients 2019, 11, 2156. [Google Scholar] [CrossRef]
- Gupta, H.; Youn, G.S.; Shin, M.J.; Suk, K.T. Role of gut microbiota in hepatocarcinogenesis. Microorganisms 2019, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic fermentation as an efficient tool to enhance the antioxidant activity of tropical fruit juices and teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hu, T.; Qu, X.; Zhang, L.; Ding, Z.; Dong, A. Plasmids from food lactic acid bacteria: Diversity, similarity, and new developments. Int. J. Mol. Sci. 2015, 16, 13172–13202. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Alcoholic liver disease: Current mechanistic aspects with focus on their clinical relevance. Biomedicines 2019, 7, 68. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Oxidative stress and alcoholic liver disease. Semin. Liver Dis. 2009, 29, 141–154. [Google Scholar] [CrossRef]
- Jeong, H.M.; Kim, D.J. Bone diseases in patients with chronic liver disease. Int. J. Mol. Sci. 2019, 20, 4270. [Google Scholar] [CrossRef]
- Gómez-Bañuelos, E.; Mukherjee, A.; Darrah, E.; Andrade, F. Rheumatoid arthritis-associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J. Clin. Med. 2019, 8, 1309. [Google Scholar] [CrossRef] [PubMed]
- Drescher, H.K.; Weiskirchen, S.; Weiskirchen, R. Current status in testing for nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). Cells 2019, 8, 845. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Yi, S.; Li, X.; Guo, Z.; Zhou, X.; Mu, J.; Yi, R. Lactobacillus plantarum CQPC02 prevents obesity in mice through the PPAR-α signaling pathway. Biomolecules 2019, 9, 407. [Google Scholar]
- Liu, L.L.; Liu, W.S.; Han, B.Q.; Hu, J.B. Protective effects of glucosamine and chitooligosaccharide in alcohol liver injury mice. Period. Ocean Univ. China 2010, 40, 73–76. [Google Scholar]
- Sun, J.; Wang, F.; Li, H.; Zhang, H.; Jin, J.; Chen, W.; Pang, M.; Yu, J.; He, Y.; Liu, J.; et al. Neuroprotective effect of sodium butyrate against cerebral ischemia/reperfusion injury in mice. Biomed. Res. Int. 2015, 2015, 395895. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, F.; Yang, J.; Yang, Y.; Gou, Y.; Li, S.; Zhao, X. Antioxidant effects of Apocynum venetum tea extracts on d-galactose-induced aging model in mice. Antioxidants 2019, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Sun, P.; Yi, R.; Han, X.; Zhao, X. Raw Bowl tea (Tuocha) polyphenol prevention of nonalcoholic fatty liver disease by regulating intestinal function in mice. Biomolecules 2019, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Boccuto, L.; Abenavoli, L. Silymarin: An option to treat non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 8437–8438. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Niu, J.; Ou, L.; Deng, B.; Wang, Y.; Li, S. Zerumbone protects against carbon tetrachloride (CCl4)-induced acute liver injury in mice via inhibiting oxidative stress and the inflammatory response: Involving the TLR4/NF-κB/COX-2 pathway. Molecules 2019, 24, 1964. [Google Scholar] [CrossRef]
- Iweala, E.E.J.; Evbakhavbokun, W.O.; Maduagwu, E.N. Antioxidant and hepatoprotective effect of Cajanus cajan in N-nitrosodiethylamine-induced liver damage. Sci. Pharm. 2019, 87, 24. [Google Scholar] [CrossRef]
- Albasher, G.; Almeer, R.; Al-Otibi, F.O.; Al-Kubaisi, N.; Mahmoud, A.M. Ameliorative effect of Beta vulgaris root extract on chlorpyrifos-induced oxidative stress, inflammation and liver injury in rats. Biomolecules 2019, 9, 261. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Zhang, J.; Mu, J.; Zhou, X.; Zhao, X. Liver injury induced by carbon tetrachloride in mice is prevented by the antioxidant capacity of Anji White tea polyphenols. Antioxidants 2019, 8, 64. [Google Scholar] [CrossRef]
- Tang, B.B.; Hu, D.H. Effect of early bedside hemofiltration on systemic inflammatory state as well as liver and kidney function in patients with severe acute pancreatitis. J. Hainan Med. Univ. 2016, 22, 124–127. [Google Scholar]
- Farbiszewski, R.; Radecka, A.; Chwiecko, M.; Holownia, A. The effect of heparegen on antioxidant enzyme activities in ethanol-induced liver injury in rats. Alcohol 1992, 9, 403–407. [Google Scholar] [CrossRef]
- Huang, Q.H.; Xu, L.Q.; Liu, Y.H.; Wu, J.Z.; Wu, X.; Lai, X.P.; Li, Y.C.; Su, Z.R.; Chen, J.N.; Xie, Y.L. Polydatin protects rat liver against ethanol-induced injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-κB p65 pathway. Evid. Based Complement. Alternat. Med. 2017, 2017, 7953850. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Ji, D.F.; Chen, S.; Hu, G.Y. Protective effects of sericin protein on alcohol-mediated liver damage in mice. Alcohol Alcohol. 2008, 43, 246–253. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Zhang, C.; Li, S.; Yang, Q.; Zhang, J.; Gong, Z.; Han, J.; Jia, L. Antioxidant activity and protective effects of enzyme-extracted Oudemansiella radiata polysaccharides on alcohol-induced liver injury. Molecules 2018, 23, 481. [Google Scholar] [CrossRef]
- Sun, X.; Wang, P.; Yao, L.P.; Wang, W.; Gao, Y.M.; Zhang, J.; Fu, Y.J. Paeonol alleviated acute alcohol-induced liver injury via SIRT1/Nrf2/NF-κB signaling pathway. Environ. Toxicol. Pharmacol. 2018, 60, 110–117. [Google Scholar] [CrossRef]
- Clemens, M.G. Nitric oxide in liver injury. Hepatology 1999, 30, 1–5. [Google Scholar] [CrossRef]
- Arteel, G.E. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 2003, 124, 778–790. [Google Scholar] [CrossRef]
- An, L.; Wang, X.; Cederbaum, A.I. Cytokines in alcoholic liver disease. Arch. Toxicol. 2012, 86, 1337–1338. [Google Scholar] [CrossRef]
- Eipel, C.; Hardenberg, J.; Negendank, S.; Abshagen, K.; Vollmar, B. Thrombopoietin limits IL-6 release but fails to attenuate liver injury in two hepatic stress models. Eur. J. Gastroenterol. Hepatol. 2009, 21, 923–931. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, J.; Li, X.; Xing, Q.; Du, P.; Su, L.; Wang, S. Interleukin-6 induces Gr-1+CD11b+ myeloid cells to suppress CD8+ T cell-mediated liver injury in mice. PLoS ONE 2011, 6, e17631. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Du, X.; Wang, Z.; Ju, J.; Jia, M.; Huang, Q.; Xing, Q.; Xu, M.; Tan, Y.; Liu, M.; et al. Hyper-IL-15 suppresses metastatic and autochthonous liver cancer by promoting tumour-specific CD8+ T cell responses. J. Hepatol. 2014, 61, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Wang, H.; Shi, K.; Li, J.M.; Zong, Y.; Du, R. Hepatoprotective effect of baicalein against acetaminophen-induced acute liver injury in mice. Molecules 2019, 24, 131. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, Y.; Yi, R.; Zhao, X. Preventive effect of blueberry extract on liver injury induced by carbon tetrachloride in mice. Foods 2019, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.Y.; Li, H.W.; Liu, F.G.; Li, Y.C.; Tian, S.; Cao, L.H.; Hu, K.; Wu, X.X.; Miao, M.S. Effects of Portulaca Oleracea Extract on Acute Alcoholic Liver Injury of Rats. Molecules 2019, 24, 2887. [Google Scholar] [CrossRef] [PubMed]
- Alexaki, V.I.; Charalampopoulos, I.; Kampa, M.; Vassalou, H.; Theodoropoulos, P.; Stathopoulos, E.N.; Hatzoglou, A.; Gravanis, A.; Castanas, E. Estrogen exerts neuroprotective effects via membrane estrogen receptors and rapid Akt/NOS activation. FASEB J. 2004, 18, 1594–1596. [Google Scholar] [CrossRef] [PubMed]
- Wickman, A.; Jonsdottir, I.H.; Bergstrom, G.; Hedin, L. GH and IGF-I regulate the expression of endothelial nitric oxide synthase (eNOS) in cardiovascular tissues of hypophysectomized female rats. Eur. J. Endocrinol. 2002, 147, 523–533. [Google Scholar] [CrossRef]
- Hayashi, Y.; Abe, M.; Murai, A.; Shimizu, N.; Okamoto, I.; Katsuragi, T.; Tanaka, K. Comparison of effects of nitric oxide synthase (NOS) inhibitors on plasma nitrite/nitrate levels and tissue NOS activity in septic organs. Microbiol. Immunol. 2005, 49, 139–147. [Google Scholar] [CrossRef]
- Lin, H.I.; Wang, D.; Leu, F.J.; Chen, C.F.; Chen, H.I. Ischemia and reperfusion of liver induces eNOS and iNOS expression: Effects of a NO donor and NOS inhibitor. Chin. J. Physiol. 2004, 47, 121–127. [Google Scholar]
- Liu, B.; Li, J.; Yi, R.; Mu, J.; Zhou, X.; Zhao, X. Preventive effect of alkaloids from Lotus plumule on acute liver injury in mice. Foods 2019, 8, 36. [Google Scholar] [CrossRef]
- Kanai, S.; Okano, H. Mechanism of the protective effects of sumac gall extract and gallic acid on the progression of CCl4-induced acute liver injury in rats. Am. J. Chin. Med. 1998, 26, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.D.; Nakagami, M.; Bradford, B.U.; Uesugi, T.; Mason, R.P.; Connor, H.D.; Dikalova, A.; Kadiiska, M.; Thurman, R.G. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J. Biol. Chem. 2001, 276, 36664–36672. [Google Scholar] [CrossRef] [PubMed]
- Kobaek-Larsen, M.; Baatrup, G.; Notabi, M.K.; El-Houri, R.B.; Pipó-Ollé, E.; Christensen Arnspang, E.; Christensen, L.P. Dietary polyacetylenic oxylipins falcarinol and falcarindiol prevent inflammation and colorectal neoplastic transformation: A mechanistic and dose-response study in a rat model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Rusciano, M.R.; Sommariva, E.; Douin-Echinard, V.; Ciccarelli, M.; Poggio, P.; Maione, A.S. CaMKII activity in the inflammatory response of cardiac diseases. Int. J. Mol. Sci. 2019, 20, 4374. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Zhang, X.L.; Zhang, Y.X.; Song, Q.M.; Hua, X.M.; Jia, D.M.; Wang, Y.H. Ameliorating alcoholic liver injury by adjusting intestinal microbiota with probiotics. Microbiology 2015, 42, 1988–1993. [Google Scholar]
- Miao, Y.L.; Xiao, Y.L.; Duan, L.P.; Li, X.Y.; Chen, L.F.; Li, H.N. The study of oligomeric nucleotide chip to detect gene expression profile of ulcerative colitis patients. Chin. J. Digestol. 2009, 29, 775–777. [Google Scholar]
- Neuman, M.G.; Brenner, D.A.; Reherman, N.B.; Taieb, J.; Chollet-Martin, S.; Cohard, M.; Garaud, J.J.; Poynard, T.; Katz, G.G.; Cameron, R.G.; et al. Mechanisms of alcoholic liver disease: Cytokines. Alcohol Clin. Exp. Res. 2001, 25, 251–253. [Google Scholar] [CrossRef]
- Inokuchi, S.; Tsukamoto, H.; Park, E.; Liu, Z.X.; Brenner, D.A.; Seki, E. Toll-like receptor 4 mediates alcohol-induced steato hepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin. Exp. Res. 2011, 35, 1509–1518. [Google Scholar]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 675–685. [Google Scholar] [CrossRef]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef]
- Ripari, V. Techno-functional role of exopolysaccharides in cereal-based, yogurt-like beverages. Beverages 2019, 5, 16. [Google Scholar] [CrossRef]

| Gene Name | Sequence |
|---|---|
| nNOS | Forward: 5′-GAATACCAGCCTGATCCATGGAA-3′ |
| Reverse: 5′-TCCTCCAGGAGGGTGTCCACCGCATG-3′ | |
| eNOS | Forward: 5′-TCAGCCATCACAGTGTTCCC-3′ |
| Reverse: 5′-ATAGCCCGCATAGCGTATCAG-3′ | |
| iNOS | Forward: 5′-GTTCTCAGCCCAACAATACAAGA-3′ |
| Reverse: 5′-GTGGACGGGTCGATGTCAC-3′ | |
| Cu/Zn–SOD | Forward: 5′–AACCAGTTGTGTTGTCAGGAC–3′ |
| Reverse: 5′–CCACCATGTTTCTTAGAGTGAGG–3′ | |
| Mn–SOD | Forward: 5′-CAGACCTGCCTTACGACTATGG-3′ |
| Reverse: 5′-CTCGGTGGCGTTGAGATTGTT-3′ | |
| CAT | Forward: 5′-GGAGGCGGGAACCCAATAG-3′ |
| Reverse: 5′-GTGTGCCATCTCGTCAGTGAA-3′ | |
| IκB-α | Forward: 5′-CGCGGGATGGCCTCAAGAAGGA-3′ |
| Reverse: 5′-GCCAAGTGCAGGAACGAGTCT-3′ | |
| NF-κB p65 | Forward: 5′-GAGGCACGAGGCTCCTTTTCT-3′ |
| Reverse: 5′-GTAGCTGCATGGAGACTCGAACA-3′ | |
| GAPDH | Forward: 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse: 5′-GGGGTCGTTGATGGCAACA-3′ |
| Gene Name | Sequence |
|---|---|
| Firmicutes | Forward: 5′-GGAGYATGTGGTTTAATTCGAAGCA-3′ |
| Reverse: 5′-AGCTGACGACAACCARGCAC-3′ | |
| Bacteroides | Forward: 5′-GGARCATGTGGTTTAATTCGATGAT-3′ |
| Reverse: 5′-AGCTGACGACAACCATGCAG-3′ | |
| Actinobacteria | Forward: 5′-TACGGCCGCAAGGCTA-3′ |
| Reverse: 5′-TARTCCCCACCTTCCTCCG-3′ | |
| Proteobacteria | Forward: 5′–CATGACGTTACCCGCAGAAGAA–3′ |
| Reverse: 5′–CTCTACGAGACTCAAGCTTGC–3′ | |
| Bifidobacterium | Forward: 5′-TCGCGTC(C/T)GGTGTGAAAG-3′ |
| Reverse: 5′-CCACATCCAGC(A/G)TCCAC-3′ | |
| Lactobacillus | Forward: 5′-AGCAGTAGGGAATCTTCCA-3′ |
| Reverse: 5′-CACCGCTACACATGGAG-3′ | |
| Enterobacteriaceae | Forward: 5′-CATTGACGTTACCCGCAGAAGAAGC-3′ |
| Reverse: 5′-CTCTACGAGACTCAAGCTTGC-3′ | |
| Universal primer | Forward: 5′-ACTCCTACGGGAGGCAGCAG-3′ |
| Reverse: 5′-ATTACCGCGGCTGCTGG-3′ |
| Treatment | Survival Rate in Artificial Gastric Juice at pH 3.0 (%) | Survival Rate in 0.3% Bile Salt (%) |
|---|---|---|
| LP-HFY05 | 69.38 ± 4.62 | 20.77 ± 1.26 |
| LDSB | 34.57 ± 2.29 | 7.03 ± 0.38 |
| Group | ALT (U/L) | AST (U/L) | ALP (K-A) | TG (mmol/L) | TC (mmol/L) | BUN (mg/dL) | ALB (g/dL) |
|---|---|---|---|---|---|---|---|
| Normal | 15.82 ± 1.62 e | 11.10 ± 0.47 e | 6.88 ± 0.71 d | 0.42 ± 0.04 e | 1.42 ± 0.20 e | 20.38 ± 2.44 e | 3.83 ± 0.12 a |
| Model | 67.32 ± 3.23 a | 53.69 ± 2.91 a | 15.37 ± 2.42 a | 1.81 ± 0.13 a | 5.71 ± 0.48 a | 47.58 ± 3.91 a | 2.56 ± 0.13 e |
| Silymarin | 30.86 ± 1.79 d | 20.36 ± 1.21 d | 9.12 ± 1.05 c | 0.72 ± 0.07 d | 2.43 ± 0.27 d | 30.18 ± 2.23 d | 3.22 ± 0.08 b |
| LP-HFY05-L | 46.17 ± 2.88 c | 31.59 ± 1.93 c | 12.30 ± 1.12 b | 1.25 ± 0.10 c | 3.49 ± 0.31 c | 35.25 ± 1.98 c | 2.96 ± 0.10 c |
| LP-HFY05-H | 28.97 ± 2.15 d | 19.17 ± 1.33 d | 9.01 ± 0.82 c | 0.64 ± 0.06 d | 2.29 ± 0.25 d | 28.97 ± 2.12 d | 3.31 ± 0.10 b |
| LDSB | 53.17 ± 2.08 b | 35.07 ± 1.60 b | 13.57 ± 1.18 b | 1.49 ± 0.09 b | 4.12 ± 0.26 b | 40.39 ± 2.07 b | 2.71 ± 0.11 d |
| Group | SOD (U/mL) | NO (µmol/L) | CAT (U/mL) | MDA (µmol/L) | GSH-Px (U/mL) |
|---|---|---|---|---|---|
| Normal | 128.75 ± 8.12 a | 55.36 ± 4.12 e | 33.47 ± 2.51 a | 6.39 ± 0.46 e | 255.37 ± 25.05 a |
| Model | 72.06 ± 3.88 d | 133.62 ± 7.23 a | 11.86 ± 1.89 e | 15.19 ± 0.69 a | 134.09 ± 12.38 e |
| Silymarin | 100.32 ± 4.55 b | 71.23 ± 5.22 d | 26.30 ± 2.24 b | 8.02 ± 0.37 d | 202.17 ± 15.32 b |
| LP-HFY05-L | 84.21 ± 3.65 c | 91.08 ± 6.05 c | 18.32 ± 1.93 c | 11.03 ± 0.42 c | 171.05 ± 13.47 c |
| LP-HFY05-H | 103.14 ± 5.17 b | 67.48 ± 5.07 d | 27.92 ± 3.03 b | 7.78 ± 0.36 d | 208.64 ± 16.37 b |
| LDSB | 82.17 ± 3.38 c | 108.92 ± 5.40 b | 15.32 ± 1.69 d | 16.01 ± 0.12 b | 145.25 ± 12.28 d |
| Group | IL-6 (pg/mL) | IL-12 (pg/mL) | TNF-α (pg/mL) | IFN-γ (pg/mL) |
|---|---|---|---|---|
| Normal | 36.77 ± 3.45 e | 205.67 ± 16.27 e | 22.34 ± 3.06 e | 19.87 ± 2.36 e |
| Model | 221.80 ± 18.92 a | 805.62 ± 31.20 a | 92.54 ± 5.28 a | 80.11 ± 4.62 a |
| Silymarin | 75.36 ± 5.30 d | 415.28 ± 14.69 d | 48.36 ± 3.62 d | 32.58 ± 2.87 d |
| LP-HFY05-L | 128.75 ± 11.03 c | 581.24 ± 25.33 c | 68.20 ± 3.74 c | 46.67 ± 3.56 c |
| LP-HFY05-H | 71.39 ± 4.92 d | 401.87 ± 18.31 d | 44.58 ± 4.02 d | 29.87 ± 3.12 d |
| LDSB | 178.45 ± 15.27 b | 677.80 ± 22.36 b | 80.52 ± 3.68 b | 61.02 ± 4.02 b |
| Group | Normal | Model | Silymarin | LP-HFY05-L | LP-HFY05-H | LDSB |
|---|---|---|---|---|---|---|
| Firmicutes | 6.35 ± 0.31 e | 8.52 ± 0.47 a | 8.12 ± 0.28 b | 7.64 ± 0.31 c | 7.01 ± 0.21 d | 8.05 ± 0.23 b |
| Bacteroides | 11.39 ± 0.42 a | 7.91 ± 0.35 e | 8.26 ± 0.28 d | 8.77 ± 0.22 c | 9.40 ± 0.42 b | 8.23 ± 0.20 d |
| Actinobacteria | 1.21 ± 0.15 f | 4.32 ± 0.18 a | 3.89 ± 0.12 b | 2.34 ± 0.11 d | 1.81 ± 0.17 e | 2.97 ± 0.13 c |
| Proteobacteria | 2.36 ± 0.17 f | 5.92 ± 0.20 a | 4.98 ± 0.18 b | 3.69 ± 0.21 d | 2.91 ± 0.30 e | 4.02 ± 0.19 c |
| Bifidobacterium | 2.26 ± 0.11 a | 0.35 ± 0.04 d | 0.42 ± 0.05 d | 0.82 ± 0.08 c | 1.89 ± 0.15 b | 0.78 ± 0.09 c |
| Lactobacillus | 5.23 ± 0.21 c | 2.15 ± 0.16 e | 2.87 ± 0.19 d | 9.41 ± 0.22 b | 14.83 ± 0.24 a | 4.92 ± 0.24 c |
| Enterobacteriaceae | 2.30 ± 0.21 e | 6.98 ± 0.31 a | 6.34 ± 0.28 b | 5.23 ± 0.20 d | 2.42 ± 0.25 e | 5.51 ± 0.18 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, R.; Tan, F.; Liao, W.; Wang, Q.; Mu, J.; Zhou, X.; Yang, Z.; Zhao, X. Isolation and Identification of Lactobacillus plantarum HFY05 from Natural Fermented Yak Yogurt and Its Effect on Alcoholic Liver Injury in Mice. Microorganisms 2019, 7, 530. https://doi.org/10.3390/microorganisms7110530
Yi R, Tan F, Liao W, Wang Q, Mu J, Zhou X, Yang Z, Zhao X. Isolation and Identification of Lactobacillus plantarum HFY05 from Natural Fermented Yak Yogurt and Its Effect on Alcoholic Liver Injury in Mice. Microorganisms. 2019; 7(11):530. https://doi.org/10.3390/microorganisms7110530
Chicago/Turabian StyleYi, Ruokun, Fang Tan, Wei Liao, Qiang Wang, Jianfei Mu, Xianrong Zhou, Zhennai Yang, and Xin Zhao. 2019. "Isolation and Identification of Lactobacillus plantarum HFY05 from Natural Fermented Yak Yogurt and Its Effect on Alcoholic Liver Injury in Mice" Microorganisms 7, no. 11: 530. https://doi.org/10.3390/microorganisms7110530
APA StyleYi, R., Tan, F., Liao, W., Wang, Q., Mu, J., Zhou, X., Yang, Z., & Zhao, X. (2019). Isolation and Identification of Lactobacillus plantarum HFY05 from Natural Fermented Yak Yogurt and Its Effect on Alcoholic Liver Injury in Mice. Microorganisms, 7(11), 530. https://doi.org/10.3390/microorganisms7110530





