Relative Abundance of Ammonia Oxidizing Archaea and Bacteria Influences Soil Nitrification Responses to Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Data Description
2.2. Model Construction
2.3. DNA Extraction and Quantification of Ammonia Oxidizer Populations
2.4. Statistical Analysis
3. Results
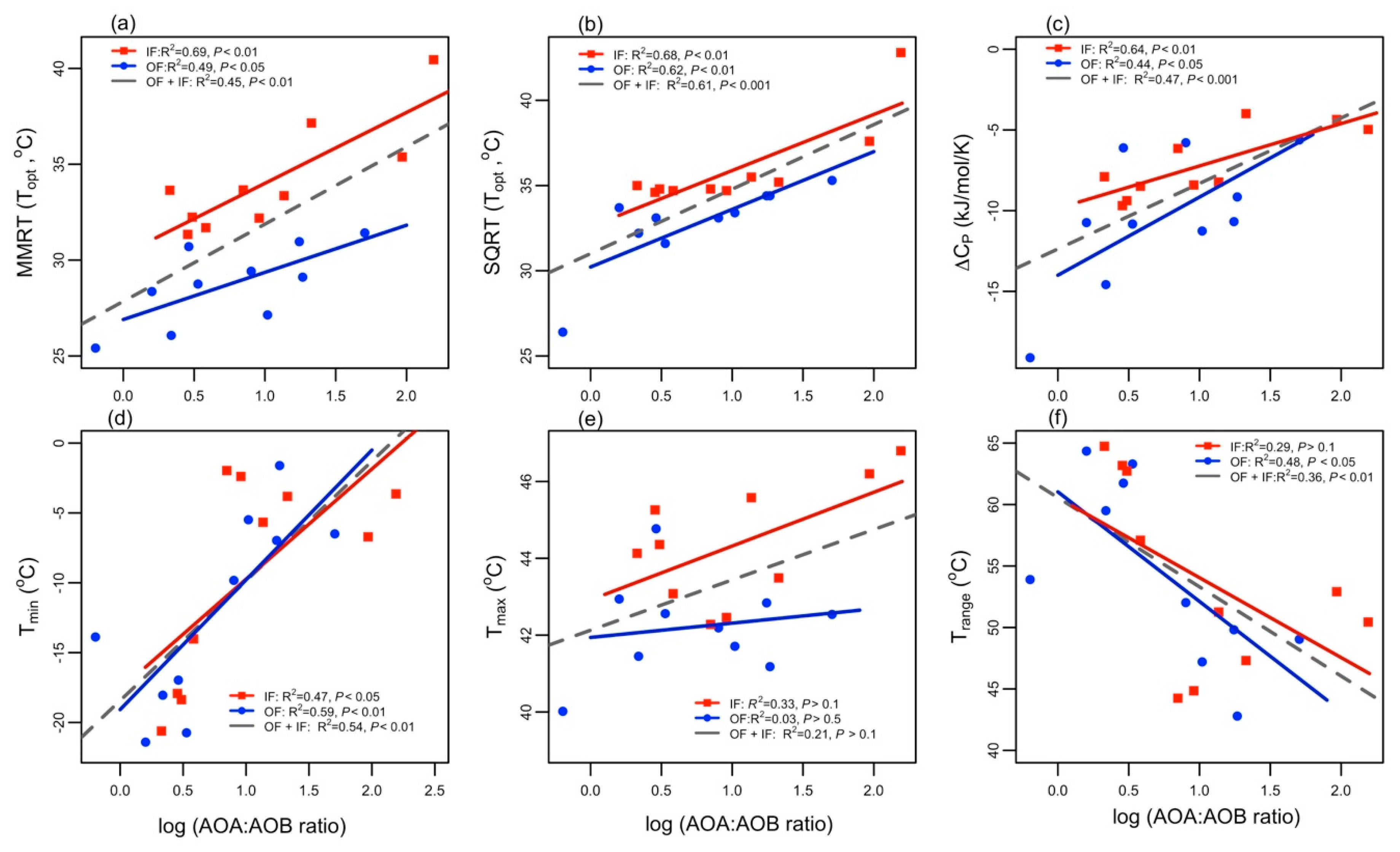
3.1. Effect of AOA to AOB Ratios on Nitrification Potential over Temperature Gradient
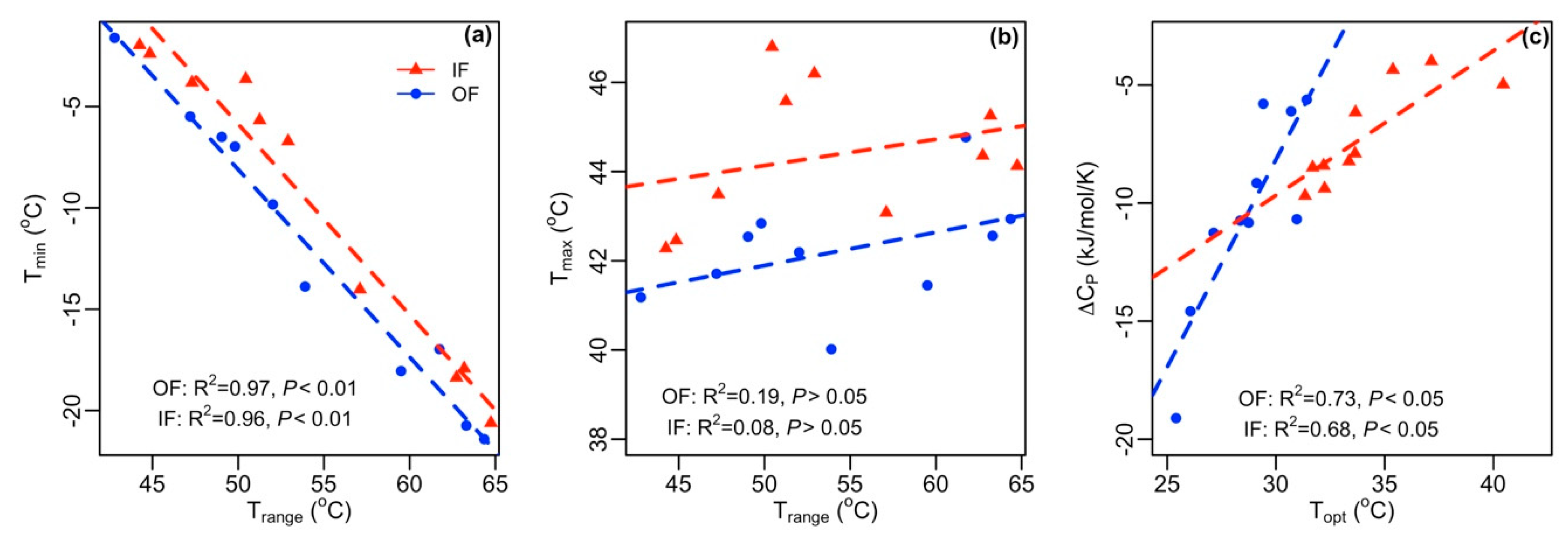
3.2. Variation in Temperature Sensitivity Traits
4. Discussion
4.1. Nitrification Responses to Temperature Vary among AOA to AOB Ratios
4.2. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niu, S.; Classen, A.T.; Dukes, J.S.; Kardol, P.; Liu, L.; Luo, Y.; Rustad, L.; Sun, J.; Tang, J.; Templer, P.H. Global patterns and substrate-based mechanisms of the terrestrial nitrogen cycle. Ecol. Lett. 2016, 19, 697–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Guo, X.; Wang, D.; Chu, H. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. 2015, 95, 171–178. [Google Scholar] [CrossRef]
- Erguder, T.H.; Boon, N.; Wittebolle, L.; Marzorati, M.; Verstraete, W. Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol. Rev. 2009, 33, 855–869. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Zhang, L.; Yuan, C.; Zheng, Y.; Wang, J.; Chen, D.; He, J. The large-scale distribution of ammonia oxidizers in paddy soils is driven by soil pH, geographic distance, and climatic factors. Front. Microbiol. 2015, 6, 938. [Google Scholar] [CrossRef]
- Shen, J.-P.; Zhang, L.-M.; Di, H.J.; He, J.-Z. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front. Microbiol. 2012, 3, 296. [Google Scholar] [CrossRef] [Green Version]
- Szukics, U.; Abell, G.C.J.; Hödl, V.; Mitter, B.; Sessitsch, A.; Hackl, E.; Zechmeister-Boltenstern, S. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Ecol. 2010, 72, 395–406. [Google Scholar] [CrossRef]
- Taylor, A.E.; Zeglin, L.H.; Wanzek, T.A.; Myrold, D.D.; Bottomley, P.J. Dynamics of ammonia-oxidizing archaea and bacteria populations and contributions to soil nitrification potentials. ISME J. 2012, 6, 2024. [Google Scholar] [CrossRef]
- Taylor, A.E.; Giguere, A.T.; Zoebelein, C.M.; Myrold, D.D.; Bottomley, P.J. Modeling of soil nitrification responses to temperature reveals thermodynamic differences between ammonia-oxidizing activity of archaea and bacteria. ISME J. 2017, 11, 896–908. [Google Scholar] [CrossRef]
- Duan, P.; Wu, Z.; Zhang, Q.; Fan, C.; Xiong, Z. Thermodynamic responses of ammonia-oxidizing archaea and bacteria explain N2O production from greenhouse vegetable soils. Soil Biol. Biochem. 2018, 120, 37–47. [Google Scholar] [CrossRef]
- Lehtovirta-Morley, L.E.; Ross, J.; Hink, L.; Weber, E.B.; Gubry-Rangin, C.; Thion, C.; Prosser, J.I.; Nicol, G.W. Isolation of ‘Candidatus Nitrosocosmicus franklandus’, a novel ureolytic soil archaeal ammonia oxidiser with tolerance to high ammonia concentration. FEMS Microbiol. Ecol. 2016, 92, fiw057. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Norton, J.M.; Stark, J.M. Ammonium availability and temperature control contributions of ammonia oxidizing bacteria and archaea to nitrification in an agricultural soil. Soil Biol. Biochem. 2017, 113, 161–172. [Google Scholar] [CrossRef]
- Jiang, Q.Q.; Bakken, L.R. Comparison of Nitrosospira strains isolated from terrestrial environments. FEMS Microbiol. Ecol. 1999, 30, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.D.; Morita, R.Y. Low-temperature growth and whole-cell kinetics of a marine ammonium oxidizer. Mar. Ecol. Prog. Ser. 1985, 239–243. [Google Scholar] [CrossRef]
- Tourna, M.; Stieglmeier, M.; Spang, A.; Könneke, M.; Schintlmeister, A.; Urich, T.; Engel, M.; Schloter, M.; Wagner, M.; Richter, A. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. USA 2011, 108, 8420–8425. [Google Scholar] [CrossRef]
- Lehtovirta-Morley, L.E.; Ge, C.; Ross, J.; Yao, H.; Nicol, G.W.; Prosser, J.I. Characterisation of terrestrial acidophilic archaeal ammonia oxidisers and their inhibition and stimulation by organic compounds. FEMS Microbiol. Ecol. 2014, 89, 542–552. [Google Scholar] [CrossRef]
- Lehtovirta-Morley, L.E.; Stoecker, K.; Vilcinskas, A.; Prosser, J.I.; Nicol, G.W. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc. Natl. Acad. Sci.USA 2011, 108, 15892–15897. [Google Scholar] [CrossRef] [Green Version]
- De la Torre, J.R.; Walker, C.B.; Ingalls, A.E.; Könneke, M.; Stahl, D.A. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 2008, 10, 810–818. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, L.; Zhu, Y.; Zhang, J.; He, J. Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ. Microbiol. 2008, 10, 1601–1611. [Google Scholar] [CrossRef]
- Ying, J.; Zhang, L.; He, J. Putative ammonia-oxidizing bacteria and archaea in an acidic red soil with different land utilization patterns. Environ. Microbiol. Rep. 2010, 2, 304–312. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, L.; Wang, B.; Lin, X.; Zhu, J.; Cai, Z.; Yan, X.; Jia, Z. Long-term field fertilization significantly alters community structure of ammonia-oxidizing bacteria rather than archaea in a paddy soil. Soil Sci. Soc. Am. J. 2011, 75, 1431–1439. [Google Scholar] [CrossRef]
- Wessén, E.; Söderström, M.; Stenberg, M.; Bru, D.; Hellman, M.; Welsh, A.; Thomsen, F.; Klemedtson, L.; Philippot, L.; Hallin, S. Spatial distribution of ammonia-oxidizing bacteria and archaea across a 44-hectare farm related to ecosystem functioning. ISME J. 2011, 5, 1213. [Google Scholar] [CrossRef]
- Wessén, E.; Nyberg, K.; Jansson, J.K.; Hallin, S. Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Appl. Soil Ecol. 2010, 45, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Bottomley, P.J.; Myrold, D.D. Contributions of ammonia-oxidizing archaea and bacteria to nitrification in Oregon forest soils. Soil Biol. Biochem. 2015, 85, 54–62. [Google Scholar] [CrossRef]
- Alves, R.J.E.; Wanek, W.; Zappe, A.; Richter, A.; Svenning, M.M.; Schleper, C.; Urich, T. Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J. 2013, 7, 1620. [Google Scholar] [CrossRef]
- Taylor, A.E.; Zeglin, L.H.; Dooley, S.; Myrold, D.D.; Bottomley, P.J. Evidence for different contributions of archaea and bacteria to the ammonia-oxidizing potential of diverse Oregon soils. Appl. Environ. Microbiol. 2010, 76, 7691–7698. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J.M.; Stark, J.M.; Reeve, J.R.; Habteselassie, M.Y. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil Biol. Biochem. 2016, 96, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Ratkowsky, D.A.; Lowry, R.K.; McMeekin, T.A.; Stokes, A.N.; Chandler, R.E. Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J. Bacteriol. 1983, 154, 1222–1226. [Google Scholar] [Green Version]
- Van Gestel, N.C.; Reischke, S.; Bååth, E. Temperature sensitivity of bacterial growth in a hot desert soil with large temperature fluctuations. Soil Biol. Biochem. 2013, 65, 180–185. [Google Scholar] [CrossRef]
- Alster, C.J.; Koyama, A.; Johnson, N.G.; Wallenstein, M.D.; Fischer, J.C. Temperature sensitivity of soil microbial communities: An application of macromolecular rate theory to microbial respiration. J. Geophys. Res. Biogeosc. 2016, 121, 1420–1433. [Google Scholar] [CrossRef]
- Stark, J.M. Modeling the temperature response of nitrification. Biogeochemistry 1996, 35, 433–445. [Google Scholar] [CrossRef]
- Liang, L.L.; Arcus, V.L.; Heskel, M.A.; O’Sullivan, O.S.; Weerasinghe, L.K.; Creek, D.; Egerton, J.J.G.; Tjoelker, M.G.; Atkin, O.K.; Schipper, L.A. Macromolecular rate theory (MMRT) provides a thermodynamics rationale to underpin the convergent temperature response in plant leaf respiration. Glob. Chang. Biol. 2017, 24, 1538–1547. [Google Scholar] [CrossRef] [Green Version]
- Schipper, L.A.; Hobbs, J.K.; Rutledge, S.; Arcus, V.L. Thermodynamic theory explains the temperature optima of soil microbial processes and high Q10 values at low temperatures. Glob. Chang. Biol. 2014, 20, 3578–3586. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Jiao, W.; Easter, A.D.; Parker, E.J.; Schipper, L.A.; Arcus, V.L. Change in heat capacity for enzyme catalysis determines temperature dependence of enzyme catalyzed rates. ACS Chem. Biol. 2013, 8, 2388–2393. [Google Scholar] [CrossRef]
- Alster, C.J.; Baas, P.; Wallenstein, M.D.; Johnson, N.G.; Von Fischer, J.C. Temperature Sensitivity as a Microbial Trait Using Parameters from Macromolecular Rate Theory. Front. Microbiol. 2016, 7, 1821. [Google Scholar] [CrossRef] [Green Version]
- Klappenbach, J.A.; Saxman, P.R.; Cole, J.R.; Schmidt, T.M. RRNDB: The ribosomal RNA operon copy number database. Nucleic Acids Res. 2001, 29, 181–184. [Google Scholar] [CrossRef]
- Kozlowski, J.A.; Kits, K.D.; Stein, L.Y. Complete genome sequence of Nitrosomonas ureae strain Nm10, an Oligotrophic Group 6a Nitrosomonad. Genome Announc. 2016, 4, e00094-16. [Google Scholar] [CrossRef]
- Norton, J.M.; Low, J.M.; Klotz, M.G. The gene encoding ammonia monooxygenase subunit A exists in three nearly identical copies in Nitrosospira sp. NpAV. FEMS Microbiol. Lett. 1996, 139, 181–188. [Google Scholar] [CrossRef]
- Norton, J.M.; Alzerreca, J.J.; Suwa, Y.; Klotz, M.G. Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch. Microbiol. 2002, 177, 139–149. [Google Scholar] [CrossRef]
- Stein, L.Y.; Arp, D.J.; Berube, P.M.; Chain, P.S.G.; Hauser, L.; Jetten, M.S.M.; Klotz, M.G.; Larimer, F.W.; Norton, J.M.; Op den Camp, H.J.M. Whole-genome analysis of the ammonia-oxidizing bacterium, Nitrosomonas eutropha C91: Implications for niche adaptation. Environ. Microbiol. 2007, 9, 2993–3007. [Google Scholar] [CrossRef]
- Hermansson, A.; Lindgren, P.-E. Quantification of ammonia-oxidizing bacteria in arable soil by real-time PCR. Appl. Environ. Microbiol. 2001, 67, 972–976. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models part I—A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Mukhtar, H.; Lin, Y.-P.; Lin, C.-M.; Petway, J.R. Assessing Thermodynamic Parameter Sensitivity for Simulating Temperature Response of Soil Nitrification. Environ. Sci. Process. Impacts 2019, 21, 1596–1608. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Li, Y.; Ran, Y.; Liu, Y.; Zhang, Q.; Li, Z.; He, Y.; Xu, J.; Di, H. High temperatures inhibited the growth of soil bacteria and archaea but not that of fungi and altered nitrous oxide production mechanisms from different nitrogen sources in an acidic soil. Soil Biol. Biochem. 2017, 107, 168–179. [Google Scholar] [CrossRef]
- Taylor, A.E.; Taylor, K.; Tennigkeit, B.; Palatinszky, M.; Stieglmeier, M.; Myrold, D.D.; Schleper, C.; Wagner, M.; Bottomley, P.J. Inhibitory properties of C2–C10 1-alkynes on ammonia oxidation in two Nitrososphaera species. Appl. Environ. Microbiol. 2015, 81, 1942–1948. [Google Scholar] [CrossRef]
- Corkrey, R.; Olley, J.; Ratkowsky, D.; McMeekin, T.; Ross, T. Universality of thermodynamic constants governing biological growth rates. PLoS ONE 2012, 7, e32003. [Google Scholar] [CrossRef]
- Fan, F.; Yang, Q.; Li, Z.; Wei, D.; Cui, X.; Liang, Y. Impacts of organic and inorganic fertilizers on nitrification in a cold climate soil are linked to the bacterial ammonia oxidizer community. Microb. Ecol. 2011, 62, 982–990. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhtar, H.; Lin, Y.-P.; Lin, C.-M.; Lin, Y.-R. Relative Abundance of Ammonia Oxidizing Archaea and Bacteria Influences Soil Nitrification Responses to Temperature. Microorganisms 2019, 7, 526. https://doi.org/10.3390/microorganisms7110526
Mukhtar H, Lin Y-P, Lin C-M, Lin Y-R. Relative Abundance of Ammonia Oxidizing Archaea and Bacteria Influences Soil Nitrification Responses to Temperature. Microorganisms. 2019; 7(11):526. https://doi.org/10.3390/microorganisms7110526
Chicago/Turabian StyleMukhtar, Hussnain, Yu-Pin Lin, Chiao-Ming Lin, and Yann-Rong Lin. 2019. "Relative Abundance of Ammonia Oxidizing Archaea and Bacteria Influences Soil Nitrification Responses to Temperature" Microorganisms 7, no. 11: 526. https://doi.org/10.3390/microorganisms7110526
APA StyleMukhtar, H., Lin, Y.-P., Lin, C.-M., & Lin, Y.-R. (2019). Relative Abundance of Ammonia Oxidizing Archaea and Bacteria Influences Soil Nitrification Responses to Temperature. Microorganisms, 7(11), 526. https://doi.org/10.3390/microorganisms7110526





